Abstract
Aim
To evaluate the long‐term safety and efficacy of canagliflozin as add‐on therapy in patients with type 2 diabetes mellitus (T2DM) who had inadequate glycaemic control with teneligliptin monotherapy.
Methods
This open‐label 52‐week study was conducted in Japan. Patients received canagliflozin 100 mg added to teneligliptin 20 mg orally once daily for 52 weeks. The safety endpoint was the incidence of adverse events (AEs). The efficacy endpoints included changes in glycated haemoglobin (HbA1c), fasting plasma glucose (FPG) and body weight from baseline to week 52 (with last observation carried forward).
Results
Overall, 153 patients entered the treatment period and 142 completed the study. The overall incidence rates of AEs and drug‐related AEs were 69.9% and 22.9%, respectively. Most AEs and drug‐related AEs were mild or moderate in severity. There were no previously undescribed safety signals. The mean changes in HbA1c, FPG and body weight were −0.99% (95% confidence interval [CI] −1.12 to −0.85), −38.6 mg/dL (95% CI −43.4 to −33.9) and −3.92% (95% CI −4.53 to −3.31), respectively. These effects were maintained for 52 weeks without attenuation. HbA1c and body weight were both decreased in 82.24% of patients at the end of the treatment period. Reductions in postprandial glucose were observed at weeks 24 and 52.
Conclusions
No new safety risks with this combination were identified, and sustained improvements in HbA1c, FPG and body weight were observed. The findings suggest that long‐term co‐administration of canagliflozin with teneligliptin is well tolerated and effective in Japanese patients with T2DM who have inadequate glycaemic control on teneligliptin alone.
Keywords: canagliflozin, DPP‐4 inhibitor, SGLT2 inhibitor, teneligliptin, type 2 diabetes mellitus
1. INTRODUCTION
Over the past decade, several classes of oral glucose‐lowering agents have been launched, including dipeptidyl peptidase‐4 (DPP‐4) inhibitors and sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors.1 DPP‐4 inhibitors increase levels of the active forms of glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide after food intake, which in turn promote insulin secretion and suppress glucagon secretion. Because these mechanisms are glucose‐dependent, the risk of hypoglycaemia is low.1, 2 SGLT2 inhibitors suppress glucose reabsorption in the renal tubules and exert antihyperglycaemic effects in an insulin‐independent manner. This class of drug reduces both blood glucose levels and body weight1, 3; however, it has been reported that SGLT2 inhibitors cause a rise in glucagon levels and enhance gluconeogenesis.3, 4, 5 Owing to these differences in their mechanisms of action, the combined use of both types of inhibitor has been reported to be beneficial because they act in a complementary manner,6, 7 and 2 fixed‐dose combination products have been launched in Western countries.7
Diabetes mellitus is caused by impaired insulin secretion and insulin resistance8; however, there are differences in the pathology of type 2 diabetes mellitus (T2DM) between Japanese and Caucasian subjects. The dominant pathology in Japanese individuals is impaired insulin secretion, while that in Caucasian individuals is insulin resistance.2, 9 Although metformin is the first‐line drug in Western countries,10 the use of DPP‐4 inhibitors has been increasing in Japan: according to an analysis of the Japan Medical Data Centre Claims Database (Japan Medical Data Centre Co. Ltd, Tokyo, Japan), ~70% of Japanese patients with T2DM use DPP‐4 inhibitors, and ~60% of patients receiving DPP‐4 inhibitors are drug‐naïve.2 In some cases, however, it is difficult to control blood glucose using a DPP‐4 inhibitor alone.11, 12, 13 In such cases, combining a DPP‐4 inhibitor with an SGLT2 inhibitor is a possible option, but this combination has yet to be fully evaluated in Japanese patients. Owing to the differences in T2DM pathology and treatment algorithms between Japanese and Western populations, the results of studies examining combination therapy with a DPP‐4 inhibitor and an SGLT2 inhibitor in Western patients may not be generalizable to Japanese patients; therefore, it is important to examine the efficacy and safety of this combination in Japanese patients.
The DPP‐4 inhibitor teneligliptin has been approved in Japan and Korea for the treatment of T2DM.14, 15 The 1‐year efficacy and safety of teneligliptin as monotherapy and in combination with oral antidiabetic drugs (other than SGLT2 inhibitors) in Japanese patients with T2DM have been evaluated.16, 17 In addition, its combination with metformin was evaluated in a European multicentre study.18 Canagliflozin is an SGLT2 inhibitor approved for the treatment of T2DM in North America, Latin America, Europe and the Asia‐Pacific region, including Japan.19 A recently completed 24‐week, double‐blind study demonstrated the safety and efficacy, including during a mixed‐meal tolerance test, of canagliflozin add‐on therapy to teneligliptin in Japanese patients with T2DM who had inadequate glycaemic control on teneligliptin monotherapy.20 In the present study, we report the safety and efficacy of teneligliptin and canagliflozin combination therapy, including the results of mixed‐meal tolerance tests and subgroup analyses according to patient characteristics, over the longer term (52 weeks) in patients with inadequate glycaemic control on teneligliptin alone. In addition, because of the increasing recognition of the importance of composite endpoints in T2DM trials,21 the percentage of patients with decreases in both glycated haemoglobin (HbA1c) and body weight at the end of treatment was evaluated in a post hoc analysis.
2. MATERIALS AND METHODS
2.1. Study design
This 52‐week, open‐label study was performed in 24 institutions in Japan. The participating institutions/investigators are listed in Appendix S1.
2.2. Patients
The study included Japanese patients with T2DM with inadequate glycaemic control on teneligliptin and diet and exercise therapy. The inclusion criteria were as follows: age ≥20 years; glycated haemoglobin (HbA1c) ≥7.0% and <10.5%; fasting plasma glucose (FPG) concentration ≤270 mg/dL; and diet and exercise therapy for diabetes for >12 weeks before the treatment period. Patients meeting the following criteria at the screening visit were excluded from the study: type 1 diabetes; diabetes mellitus resulting from a pancreatic disorder, or secondary diabetes; serious complications of diabetes; hereditary glucose‐galactose malabsorption or primary renal glucosuria; class III/IV heart failure symptoms according to the New York Heart Association functional classification; and severe hepatic or severe renal disorder.
2.3. Interventions
Patients underwent a washout period of >12 weeks in which they stopped all antidiabetic drugs, except for teneligliptin, before the treatment period. Patients continued their fixed programme of diet and exercise therapy during the washout period. Teneligliptin and canagliflozin were orally administered at doses of 20 and 100 mg, respectively (the approved doses in Japan), once daily before breakfast for 52 weeks. Diet and exercise therapy continued unchanged during the treatment period. A 2‐week observation period followed the treatment period. Mixed‐meal tolerance tests were performed at baseline, week 24 and week 52 using a method similar to that reported in a previous study.20 Visits were scheduled every 4 weeks during the treatment period, and at 2 weeks after study completion or at study withdrawal (if before week 52).
2.4. Outcomes
The safety evaluation included assessments of adverse events (AEs), hypoglycaemia, laboratory values, ECG, and vital signs. AEs and safety assessments were recorded throughout the study by the study investigators, and were not limited to the time of hospital visits. AEs were listed using Medical Dictionary for Regulatory Activities (Version 18.1) system organ class and preferred terms, and their potential relationships to the study drug (no causal relationship or a possible causal relationship, ie, adverse drug reactions; ADRs) and severity (mild, moderate or severe) were assessed.
The efficacy evaluation included the following assessments: change from baseline in HbA1c, FPG, body weight, proinsulin/C‐peptide ratio and homeostatis model assessment 2 steady state beta‐cell function (HOMA2‐%B) values; and evaluation of postprandial glucose, glucagon and C‐peptide at weeks 24 and 52 in the mixed‐meal tolerance test. HbA1c was measured by high‐performance liquid chromatography using a reference standard approved by the US National Glycohemoglobin Standardization Program. Glucagon was measured by radioimmunoassay (SCETI K.K., Tokyo, Japan). All efficacy and safety laboratory measurements were assayed at a central laboratory (LSI Medience Corporation, Tokyo, Japan). Prespecified subgroup analyses were performed to evaluate the safety and efficacy of this combination therapy in patients subdivided on the basis of the following background factors: HbA1c <8% and ≥8%; body mass index (BMI) <25 and ≥25 kg/m2; and estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, ≥60 to <90 mL/min/1.73 m2 and ≥90 mL/min/1.73 m2.
2.5. Statistical methods
Safety analyses were performed in the safety analysis set, which comprised all patients, excluding those who did not receive a dose of the study drug or who lacked safety data after starting administration of the study drug. Efficacy analyses were performed in the full analysis set, which comprised all patients, excluding those who did not receive a dose of the study drug or who had no efficacy data after starting administration of the study drug. Descriptive statistics of values measured at each time point and at the end of the treatment period were calculated for each variable. Changes in efficacy variables from baseline to week 52 were determined using the last observation carried forward (LOCF) to impute missing values. Descriptive statistics and 95% confidence intervals (CIs) were calculated for changes or percent changes from baseline to each measurement time point and the end of the treatment period. HbA1c <7.0% and <8.0% success rates and 95% CI (based on the F distribution) at the end of the treatment period were calculated. Using individual patient data, the correlation between changes in HbA1c and body weight from baseline and a composite endpoint (the percentage of patients with decreases in both HbA1c and body weight) at the end of the treatment period were evaluated in post hoc analyses. Owing to the descriptive nature of the analyses, P values were not calculated. All statistical analyses were performed using Windows SAS version 9.2 or later.
2.6. Ethical considerations
The trial was registered at clinicaltrials.gov (identifier NCT02220907), and was carried out in accordance with the ethical principles of the Declaration of Helsinki, the Law for Ensuring the Quality, Efficacy, and Safety of Drugs and Medical Devices, Good Clinical Practice guidelines, and the approved study protocol practice. The study was approved by the ethics committee/institutional review boards at all participating institutions (Appendix S1). All patients provided written informed consent.
3. RESULTS
3.1. Patients
Of the 200 patients who consented to participate, 153 entered the treatment period and 142 completed the study. All 153 patients were included in the full analysis set and safety analysis set. Patient characteristics are described in Table 1. Of the 153 patients, 108 (70.6%) were men. The mean ± standard deviation (s.d.) age and BMI were 56.1 ± 10.4 years and 26.52 ± 4.47 kg/m2, respectively. The mean ± s.d. baseline HbA1c and FPG were 8.14% ± 0.94% and 177.3 ± 34.8 mg/dL, respectively.
Table 1.
Demographics and baseline characteristics (safety analysis set/full analysis set; N = 153)
| Variable | Value |
|---|---|
| Sex, n (%) | |
| Men | 108 (70.6) |
| Women | 45 (29.4) |
| Age(years), mean(s.d) | 56.1 (10.4) |
| Duration of diabetes(years), mean(s.d.) | 9.07 (5.55) |
| Body weight (kg), mean(s.d.) | 72.12 (14.87) |
| BMI (kg/m2), mean(s.d.) | 26.52 (4.47) |
| Diabetic complications, n (%) | |
| Total | 68 (44.4) |
| Diabetic retinopathy | 24 (15.7) |
| Diabetic neuropathy | 16 (10.5) |
| Diabetic nephropathy | 51 (33.3) |
| Non‐diabetic complications, n (%) | |
| Hypertension | 89 (58.2) |
| Hyperlipidaemia | 128 (83.7) |
| HbA1c (%), mean(s.d.) | 8.14 (0.94) |
| Fasting plasma glucose(mg/dL), mean(s.d.) | 177.3 (34.8) |
| eGFR(mL/min/1.73m2), mean(s.d.) | 86.7 (19.2) |
aFasting plasma glucose: 1 mg/dL = 0.0555 mmol/L.
3.2. Safety
A summary of the AEs is shown in Table 2. The overall incidence of AEs and ADRs at 52 weeks was 69.9% (107/153 patients; 269 events) and 22.9% (35/153 patients; 45 events), respectively. There were 13 serious AEs in 11 patients, including one ADR in one patient. The serious AEs observed were cataract, myocardial infarction, ankle fracture, influenza, pneumonia, disseminated herpes zoster, rectal cancer, hepatocellular carcinoma, atrial fibrillation and sinus node dysfunction. A serious ADR of myocardial infarction occurred in 1 patient.
Table 2.
Adverse events occurring during the study (safety analysis set, N = 153)
| AEs | n (%) |
|---|---|
| AEs | 107 (69.9) |
| Drug‐related AEs | 35 (22.9) |
| Serious AEs | 11 (7.2) |
| Serious drug‐related AEs | 1 (0.7) |
| AEs leading to discontinuation | 7 (4.6) |
| Drug‐related AEs leading to discontinuation | 3 (2.0) |
| AEs of special interest | |
| Documented hypoglycaemia | 4 (2.6) |
| Osmotic diuresis | 15 (9.8) |
| Volume depletion | 2 (1.3) |
| Genital infection (males) | 1/108 (0.9) |
| Genital infection (females) | 5/45 (11.1) |
| Urinary tract infection | 2 (1.3) |
| Fracture | 3 (2.0) |
| Blood ketone body increased | 3 (2.0) |
| Hepatic function impairment | 2 (1.3) |
| Skin and subcutaneous tissue disorders | 13 (8.5) |
| Cardiovascular‐related events | 3 (2.0) |
| Malignant neoplasm | 2 (1.3) |
| Gastrointestinal disorders | 23 (15.0) |
A total of 9 AEs, including 4 ADRs, led to discontinuation in 7 and 3 patients, respectively. AEs that led to discontinuation were myocardial infarction, vulvar vaginal candidiasis, rectal cancer, hepatocellular carcinoma, atrial fibrillation, sinus node dysfunction, eczema and balanoposthitis. ADRs that led to discontinuation were vulvar vaginal candidiasis, myocardial infarction, eczema and balanoposthitis. No AEs resulted in death during the study.
Table 2 shows the AEs of special interest. No other AEs of special interest were observed. For canagliflozin, the AEs included ketoacidosis and sepsis. For teneligliptin, the AEs were intestinal obstruction and interstitial pneumonia. Laboratory values are presented in Table S1, Appendix S1.
3.3. Efficacy
A decrease in HbA1c from baseline to week 52 (LOCF) was observed, with a mean change of −0.99% (95% CI −1.12 to −0.85; Table 3). The decrease in HbA1c was apparent at week 4; HbA1c then continued to decrease to week 12, gradually decreased thereafter, and its level was maintained until week 52 (Figure 1A). The proportions of patients who achieved HbA1c <7% and HbA1c <8% at week 52 were 43.75% (95% CI 35.51–52.26) and 72.37% (95% CI 60.91–82.01), respectively. A decrease in FPG was detected by week 4 and was maintained until week 52 (Figure 1B). The mean change in FPG from baseline to week 52 (LOCF) was −38.6 mg/dL (95% CI −43.4 to −33.9; Table 3). A decrease in body weight was detected by week 4. Body weight continued to decrease until week 20 and was maintained until week 52 (Figure 1C). The change and percent change in body weight from baseline to week 52 (LOCF) were −2.86 kg (95% CI −3.42 to −2.31) and −3.92% (95% CI −4.53 to −3.31), respectively (Table 3). Figure 1D is a scatter plot of the change in HbA1c vs the percent change in body weight from baseline to week 52 (LOCF). As indicated in this figure, decreases in both HbA1c and body weight occurred in 82.24% of patients. The change in HbA1c was not correlated with the percent change in body weight (r = 0.2778).
Table 3.
Effects of canagliflozin and teneligliptin combination therapy on efficacy endpoints (full analysis set, N = 153)
| Variable | Value |
|---|---|
| HbA1c, % (n = 153) | |
| Baseline, mean (s.d.) | 8.14 (0.94) |
| Change from baseline (LOCF), mean (s.d.) | −0.99 (0.84) |
| 95% CI | −1.12 to −0.85 |
| FPG, mg/dL (n = 152) | |
| Baseline, mean (s.d.) | 177.1 (34.8)a |
| Change from baseline (LOCF), mean (s.d.) | −38.6 (1.66) |
| 95% CI | −2.14 to −1.88 |
| Body weight, kg (n = 152) | |
| Baseline, mean (s.d.) | 72.08 (14.91)a |
| Change from baseline (LOCF), mean (s.d.) | −2.86 (3.46) |
| 95% CI | −3.42 to −2.31 |
| Percent change from baseline (LOCF), mean (s.d.) | −3.92 (3.81) |
| 95% CI | −4.53 to −3.31 |
| Fasting proinsulin/C‐peptide ratio (n = 152) | |
| Baseline, mean (s.d.) | 0.0197 (0.0114)a |
| Change from baseline (LOCF), mean (s.d.) | −0.0047 (0.0073) |
| 95% CI | −0.0059 to −0.0036 |
| HOMA2‐%B, % (n = 152) | |
| Baseline, mean (s.d.) | 35.00 (16.53)a |
| Change from baseline (LOCF), mean (s.d.) | 10.91 (13.23) |
| 95% CI | 8.79 to 13.03 |
| Fasting glucagon, pg/mL (n = 152) | |
| Baseline, mean (s.d.) | 125.3 (21.8)a |
| Change from baseline (LOCF), mean (s.d.) | −0.6 (22.1) |
| 95% CI | −4.1 to 2.9 |
Baseline mean (s.d.) values for the population with LOCF data.
bFPG: 1 mg/dL = 0.0555 mmol/L. Glucagon: 1 pg/mL = 1 ng/L.
Figure 1.
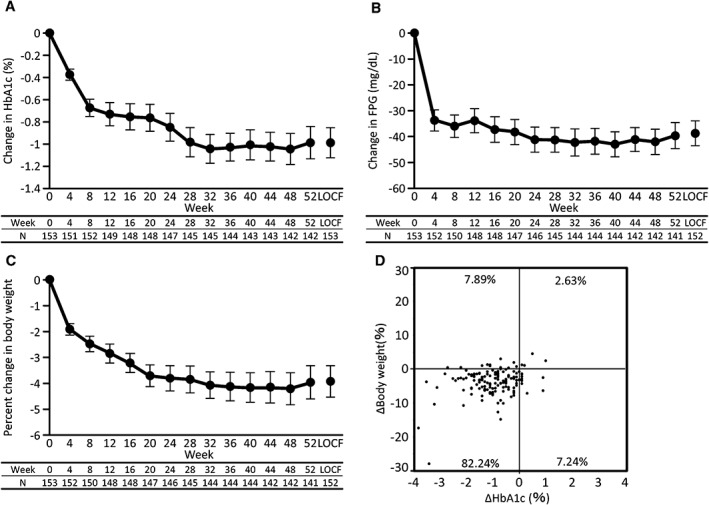
Changes in A, HbA1c; B, FPG and C, body weight from baseline during the study period. Data represent mean (95% CI). D, Scatter plot of the change in HbA1c vs percent change in body weight from baseline to the end of treatment in individual patients. FPG: 1 mg/dL = 0.0555 mmol/L.
Regarding other efficacy endpoints, the mean changes in the fasting proinsulin/C‐peptide ratio and HOMA2‐%B values from baseline to week 52 (LOCF), as markers of β‐cell function, were −0.0047 (95% CI −0.0059 to −0.0036) and 10.91% (95% CI 8.79‐13.03), respectively (Table 3). Other efficacy endpoints, including the changes from baseline to week 52 (LOCF) for systolic and diastolic blood pressure, triglycerides and HDL cholesterol, are summarized in Table S2, Appendix S1. Systolic and diastolic blood pressure was decreased and HDL cholesterol increased during this time. Figure 2 shows the results of the mixed‐meal tolerance test, with actual values of glucose, C‐peptide and glucagon after the meal at baseline, week 24 and week 52. As shown in Figure 2A, plasma glucose values measured at 0, 0.5, 1 and 2 hours were substantially lower at weeks 24 and 52 than at baseline. The changes in 2‐hour postprandial glucose from baseline to weeks 24 and 52 were −63.3 mg/dL (95% CI −71.5 to −55.0) and −60.7 mg/dL (95% CI −69.9 to −51.5), respectively (Table S3, Appendix S1). The time courses of plasma C‐peptide and glucagon were similar after treatment compared with baseline except that glucagon at each time point was lower at week 24 (Figure 2B,C). The mixed‐meal tolerance test also showed decreases from baseline in area under the curve (AUC)0‐2h at weeks 24 and 52 in postprandial plasma glucose (Table S3, Appendix S1). Although the postprandial C‐peptide AUC0‐2h did not increase after treatment, the C‐peptide AUC0‐2h/blood glucose AUC0‐2h ratio showed an increase from baseline at weeks 24 and 52. The postprandial glucagon AUC0‐2h was decreased at week 24, and the difference became smaller at week 52. The incremental 2‐hour postprandial plasma glucose and incremental postprandial plasma glucose AUC0‐2h decreased, while the incremental C‐peptide AUC0‐2h/blood glucose AUC0‐2h ratio increased at weeks 24 and 52 compared with baseline (Table S3, Appendix S1).
Figure 2.
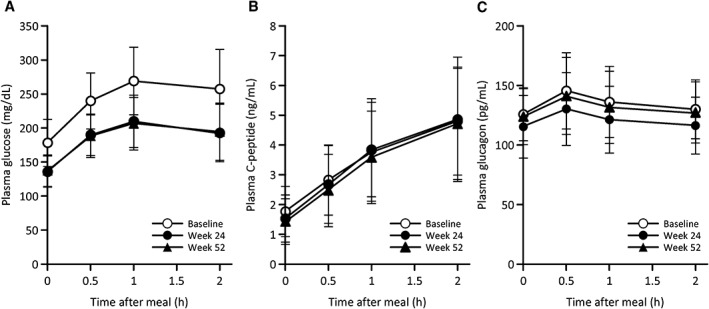
Changes in A, blood glucose; B, C‐peptide and C, glucagon levels in the mixed‐meal tolerance tests at baseline, week 24 and week 52. Data represent mean ± standard deviation. Glucose: 1 mg/dL = 0.0555 mmol/L; C‐peptide: 1 ng/mL = 0.333 nmol/L; Glucagon: 1 pg/mL = 1 ng/L.
3.4. Subgroup analyses of safety and efficacy according to background characteristics
The results of the prespecified subgroup analysis according to baseline HbA1c, BMI and eGFR are shown in Tables S4 and S5, Appendix S1. Because a small number of patients with eGFR <60 mL/min/1.73 m2 were enrolled, no further analysis was possible in this group. The extent of the decrease in HbA1c was greater in subgroups with higher baseline HbA1c (≥8%) and higher baseline eGFR (≥90 mL/min/1.73 m2), but the change in HbA1c did not differ among the BMI subgroups. The body weight change (%) did not differ among the subgroups (Table S4, Appendix S1). Baseline HbA1c, BMI and eGFR had no effect on the overall incidence of AEs or ADRs (Table S5, Appendix S1).
4. DISCUSSION
This study examined the long‐term safety and efficacy of canagliflozin added on to teneligliptin in Japanese patients with T2DM. In particular, we observed no new safety concerns after administering this combination of drugs compared with previous studies of canagliflozin and teneligliptin.16, 17, 22 We also observed reductions in HbA1c, FPG and body weight during the study. These effects were apparent within ~4 weeks of starting treatment, and were maintained for 52 weeks without attenuation. The reduction in postprandial glucose levels was also maintained for 52 weeks.
A recent 24‐week randomized, placebo‐controlled trial examined the safety and efficacy of canagliflozin added on to teneligliptin in Japanese patients with T2DM, and showed that this combination was well tolerated and effective.20 In that study, the addition of canagliflozin was associated with significant improvements in HbA1c, FPG, body weight and postprandial plasma glucose‐related variables. The present study not only confirms the results of this previous study, but also shows that the improvements in HbA1c, FPG, body weight and postprandial glucose were maintained over a longer time period (52 weeks vs 24 weeks). Similarly to the present study, Inagaki et al.22 also conducted an open‐label, long‐term (52‐week) study of the safety and efficacy of canagliflozin (100 or 200 mg) as monotherapy or added on to other oral antihyperglycaemic drugs, including DPP‐4 inhibitors (sitagliptin, vildagliptin or alogliptin) in Japan. Other 52‐week studies in Japanese patients examined the safety and efficacy of the SGLT2 inhibitors empagliflozin and dapagliflozin added on to other oral antihyperglycaemic drugs, including DPP‐4 inhibitors. Although some data (eg, postprandial glucose) were not reported, the authors mentioned that an SGLT2 inhibitor in combination with a DPP‐4 inhibitor achieved clinically relevant reductions in HbA1c, and was generally well tolerated.23, 24 The safety and efficacy of combinations of SGLT2 inhibitors and DPP‐4 inhibitors have also been investigated in Western patients. For example, a sub‐analysis of the CANVAS trial revealed improvements in HbA1c and body weight when canagliflozin was added on to a DPP‐4 inhibitor or GLP‐1 receptor agonist for 18 weeks.25 Some longer‐term Western studies have also been conducted in which dapagliflozin was added on to sitagliptin (with or without metformin) for 48 weeks,26 or saxagliptin was added on to dapagliflozin plus metformin for 52 weeks.27 The results of those studies in Western patients, together with the results of the present study in Japanese patients, indicate that the combination of an SGLT2 inhibitor with a DPP‐4 inhibitor is likely to be effective in terms of improving and maintaining glycaemic control for the long term in patients with T2DM, and ethnic differences are unlikely to confound the effects of this combination.
The common AEs observed in the present study (ie, those occurring in >5% of patients) were osmotic diuresis, genital infection (in women), skin disorders and gastrointestinal dysfunction. These AEs have already been reported for canagliflozin added on to teneligliptin20 and for other DPP‐4 inhibitors.22, 25 Nevertheless, almost all of these AEs were mild in severity, and the safety profile for the combination used here did not differ from the known safety profiles of teneligliptin and canagliflozin; therefore, no new risks requiring additional precautions were found during long‐term treatment as compared with monotherapy.
In the mixed‐meal tolerance test conducted in the present study, the decrease in incremental glucose levels suggests that the combination decreased postprandial glucose excursions. No increase in postprandial glucagon was observed, which was consistent with a previous double‐blind study.20 Although the acute effect of increasing postprandial glucagon was attributed to canagliflozin (300 mg),28 this finding may have resulted from the use of low doses of canagliflozin (100 mg) or the suppressive effect of teneligliptin, which was reported to supress postprandial glucagon levels.29 The present study also revealed improvements in HOMA2‐%B and the proinsulin/C‐peptide ratio, which were maintained until week 52, consistent with a recent 24‐week double‐blind study.20 These results suggest that the combination had sustained effects on β‐cell function. The post‐meal improvement in β‐cell function, in terms of the C‐peptide AUC0‐2h/blood glucose AUC0‐2h ratio, was also maintained until week 52. Overall, these findings suggest that the combination decreased postprandial glucose by enhancing glucose excretion and improving β‐cell function, probably by alleviating glucotoxicity.
Another possible mechanism underlying the effect of the combination on postprandial glucose is that it might enhance GLP‐1 secretion. Oral administration of 100 mg of canagliflozin increased plasma total GLP‐1 after breakfast in Japanese patients with T2DM,30 probably because of a weak inhibitory effect of canagliflozin against SGLT1.31 Although we did not measure GLP‐1 levels in the present study, the combination of teneligliptin and canagliflozin may increase active GLP‐1 levels, representing a beneficial effect of this combination.
In the present study, which is the first to report subgroup analyses in patients treated with canagliflozin in combination with a DPP‐4 inhibitor, we assessed the safety and efficacy of the combination in patients subdivided into subgroups on the basis of baseline HbA1c, BMI and eGFR. We found that the decrease in HbA1c was greater in subgroups with higher baseline HbA1c and higher baseline eGFR, but did not differ among the BMI subgroups. Moreover, the incidence of AEs was similar in each group. These results are consistent with a previous subgroup analysis of canagliflozin.32, 33
It has been reported that DPP‐4 inhibitors are potentially more effective in Asian than in non‐Asian patients,34 and in Japanese rather than in non‐Japanese patients,35 and Asian people are often characterized by a lower BMI than other groups. Meanwhile, DPP‐4 inhibitors may show decreased efficacy in patients with higher BMI.34 Considering these concepts, SGLT2 inhibitors may be a useful addition to the treatment regimen in patients with higher BMI who respond poorly to a DPP‐4 inhibitor. It has also been reported that body weight management is important for maintaining good long‐term glycaemic control with DPP‐4 inhibitors.36, 37 In the present study, the reductions in HbA1c and body weight were maintained for 52 weeks without attenuation, and 82.24% of patients showed decreases in both HbA1c and body weight at the end of the treatment period.
Based on these findings, a DPP‐4 inhibitor combined with an SGLT2 inhibitor represents a useful therapeutic option for Japanese patients with T2DM.
The present study has some limitations that must be acknowledged, including its single‐arm, open‐label design, and the fact that it only included Japanese patients. Long‐term data on the use of this combination in Japanese patients are important, however, particularly when we consider the differences in the pathophysiology, between Japanese and non‐Japanese patients. Importantly, we showed that this combination was effective and well tolerated over a longer period than that in a previous 24‐week randomized controlled trial.20 Finally, we did not perform statistical hypothesis testing; however, the changes in variables observed are likely to be clinically relevant when we consider the 95% CIs did not cross 0.
In conclusion, the results in this cohort of Japanese patients with T2DM suggest that canagliflozin added on to teneligliptin is tolerable and effective in individuals whose blood glucose levels cannot be sufficiently controlled by teneligliptin monotherapy. This study showed that the improvements in glycaemic control were maintained for the 52‐week study duration.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
The authors thank Helen Roberton, Nicholas D. Smith PhD, and Sarah Williams PhD (Edanz Medical Writing) for providing medical writing support, which was funded by Mitsubishi Tanabe Pharma Corp.
Conflict of interest
T. K. has received consulting fees and/or speakersʼ bureau fees from Astellas Pharma Inc., AstraZeneca K.K., MSD K.K., Mitsubishi Tanabe Pharma Corp., Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi K.K. and Takeda Pharmaceutical Co., Ltd, research support from Daiichi Sankyo Co., Ltd and Takeda Pharmaceutical Co., Ltd, scholarship grants from Astellas Pharma Inc., Daiichi Sankyo Co., Ltd, Mitsubishi Tanabe Pharma Corp., Sumitomo Dainippon Pharma Co., Ltd, Taisho Toyama Pharmaceutical Co., Ltd and Takeda Pharmaceutical Co., Ltd, and belongs to courses endowed by MSD K.K., Nippon Boehringer Ingelheim Co., Ltd, Novo Nordisk Pharma Ltd, and Takeda Pharmaceutical Co., Ltd. N. I. has received consulting fees and/or speakersʼ bureau fees from Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd, Sanofi K.K. and Takeda Pharmaceutical Co., Ltd, research support from Eli Lilly Japan K.K., MSD K.K. and Mitsubishi Tanabe Pharma Corp., and scholarship grants from Astellas Pharma Inc., AstraZeneca K.K., Daiichi Sankyo Co., Ltd, Japan Diabetes Foundation, Japan Tobacco Inc., Kissei Pharmaceutical Co., Ltd, Kyowa Hakko Kirin Co., Ltd, MSD K.K., Mitsubishi Tanabe Pharma Corp., Nippon Boehringer Ingelheim Co., Ltd, Novartis Pharma K.K., Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Pfizer Japan Inc., Sanwa Kagaku Kenkyusho Co., Ltd, Sanofi K.K., Sumitomo Dainippon Pharma Co., Ltd, Takeda Pharmaceutical Co., Ltd and Taisho Toyama Pharmaceutical Co., Ltd. K. K., K. N., G. K., N. M., N. N., Y. W., M. G. and H. I. are employees of Mitsubishi Tanabe Pharma Corp.
Author contributions
T. K., N. I. and K. K. were the medical advisors for this study and contributed to the study design. K. N., G. K. and N. M. contributed to study design, and performed the data collection. N. N. was involved in data analysis. Y. W., M. G. and H. I. contributed to the writing of the manuscript. All authors contributed to interpretation of data and reviewing the manuscript, and approved this manuscript for submission.
Kadowaki T, Inagaki N, Kondo K, et al. Long‐term safety and efficacy of canagliflozin as add‐on therapy to teneligliptin in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:77–84. https://doi.org/10.1111/dom.13038
Funding information The study was supported by Mitsubishi Tanabe Pharma Corp
REFERENCES
- 1. Wilding JP, Rajeev SP, DeFronzo RA. Positioning SGLT2 inhibitors/incretin‐based therapies in the treatment algorithm. Diabetes Care. 2016;39(suppl 2):S154–S164. [DOI] [PubMed] [Google Scholar]
- 2. Seino Y, Kuwata H, Yabe D. Incretin‐based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(suppl 1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium‐glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care. 2015;38:2344–2353. [DOI] [PubMed] [Google Scholar]
- 4. Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium‐glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merovci A, Solis‐Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma MD. Potential for combination of dipeptidyl peptidase‐4 inhibitors and sodium‐glucose co‐transporter‐2 inhibitors for the treatment of type 2 diabetes. Diabetes Obes Metab. 2015;17:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scheen AJ. DPP‐4 inhibitor plus SGLT‐2 inhibitor as combination therapy for type 2 diabetes: from rationale to clinical aspects. Expert Opin Drug Metab Toxicol. 2016;12:1407–1417. [DOI] [PubMed] [Google Scholar]
- 8. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Møller JB, Pedersen M, Tanaka H, et al. Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care. 2014;37:796–804. [DOI] [PubMed] [Google Scholar]
- 10. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetologia. 2015;58:429–442. [DOI] [PubMed] [Google Scholar]
- 11. Berkowitz SA, Krumme AA, Avorn J, et al. Initial choice of oral glucose‐lowering medication for diabetes mellitus: a patient‐centered comparative effectiveness study. JAMA Intern Med. 2014;174:1955–1962. [DOI] [PubMed] [Google Scholar]
- 12. Fujihara K, Igarashi R, Matsunaga S, et al. Comparison of baseline characteristics and clinical course in Japanese patients with type 2 diabetes among whom different types of oral hypoglycemic agents were chosen by diabetes specialists as initial monotherapy (JDDM 42). Medicine (Baltimore). 2017;96:e6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esposito K, Chiodini P, Maiorino MI, et al. Glycaemic durability with dipeptidyl peptidase‐4 inhibitors in type 2 diabetes: a systematic review and meta‐analysis of long‐term randomised controlled trials. BMJ Open. 2014;4:e005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goda M, Kadowaki T. Teneligliptin for the treatment of type 2 diabetes. Drugs Today (Barc). 2013;49:615–629. [DOI] [PubMed] [Google Scholar]
- 15. Morishita R, Nakagami H. Teneligliptin: expectations for its pleiotropic action. Expert Opin Pharmacother. 2015;16:417–426. [DOI] [PubMed] [Google Scholar]
- 16. Kadowaki T, Marubayashi F, Yokota S, Katoh M, Iijima H. Safety and efficacy of teneligliptin in Japanese patients with type 2 diabetes mellitus: a pooled analysis of two phase III clinical studies. Expert Opin Pharmacother. 2015;16:971–981. [DOI] [PubMed] [Google Scholar]
- 17. Kadowaki T, Kondo K. Efficacy and safety of teneligliptin in combination with pioglitazone in Japanese patients with type 2 diabetes mellitus. J Diabetes Invest. 2013;4:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bryson A, Jennings PE, Deak L, Paveliu FS, Lawson M. The efficacy and safety of teneligliptin added to ongoing metformin monotherapy in patients with type 2 diabetes: a randomized study with open label extension. Expert Opin Pharmacother. 2016;17:1309–1316. [DOI] [PubMed] [Google Scholar]
- 19. Rosenthal N, Meininger G, Ways K, et al. Canagliflozin: a sodium glucose co‐transporter 2 inhibitor for the treatment of type 2 diabetes mellitus. Ann N Y Acad Sci. 2015;1358:28–43. [DOI] [PubMed] [Google Scholar]
- 20. Kadowaki T, Inagaki N, Kondo K, et al. Efficacy and safety of canagliflozin as add‐on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: results of a 24‐week, randomised, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2017;19:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Einarson TR, Garg M, Kaur V, Hemels ME. Composite endpoints in trials of type‐2 diabetes. Diabetes Obes Metab. 2014;16:492–499. [DOI] [PubMed] [Google Scholar]
- 22. Inagaki N, Kondo K, Yoshinari T, Kuki H. Efficacy and safety of canagliflozin alone or as add‐on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: a 52‐week open‐label study. J Diabetes Invest. 2015;6:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Araki E, Tanizawa Y, Tanaka Y, et al. Long‐term treatment with empagliflozin as add‐on to oral antidiabetes therapy in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:665–674. [DOI] [PubMed] [Google Scholar]
- 24. Kaku K, Maegawa H, Tanizawa Y, et al. Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: an open‐label study. Diabetes Ther. 2014;5:415–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fulcher G, Matthews DR, Perkovic V, et al. Efficacy and safety of canagliflozin when used in conjunction with incretin‐mimetic therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:82–91. [DOI] [PubMed] [Google Scholar]
- 26. Jabbour SA, Hardy E, Sugg J, Parikh S, Study 10 Group . Dapagliflozin is effective as add‐on therapy to sitagliptin with or without metformin: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study. Diabetes Care. 2014;37:740–750. [DOI] [PubMed] [Google Scholar]
- 27. Mathieu C, Herrera Marmolejo M, González González JG, et al. Efficacy and safety of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin over 52 weeks in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:1134–1137. [DOI] [PubMed] [Google Scholar]
- 28. Stein P, Berg JK, Morrow L, et al. Canagliflozin, a sodium glucose co‐transporter 2 inhibitor, reduces post‐meal glucose excursion in patients with type 2 diabetes by a non‐renal mechanism: results of a randomized trial. Metabolism. 2014;63:1296–1303. [DOI] [PubMed] [Google Scholar]
- 29. Eto T, Inoue S, Kadowaki T. Effects of once‐daily teneligliptin on 24‐h blood glucose control and safety in Japanese patients with type 2 diabetes mellitus: a 4‐week, randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2012;14:1040–1046. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka H, Takano K, Iijima H, et al. Factors affecting canagliflozin‐induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther. 2017;34:436–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oguma T, Kuriyama C, Nakayama K, et al. The effect of combined treatment with canagliflozin and teneligliptin on glucose intolerance in Zucker diabetic fatty rats. J Pharmacol Sci. 2015;127:456–461. [DOI] [PubMed] [Google Scholar]
- 32. Inagaki N, Goda M, Yokota S, Maruyama N, Iijima H. Safety and efficacy of canagliflozin in Japanese patients with type 2 diabetes mellitus: post hoc subgroup analyses according to body mass index in a 52‐week open‐label study. Expert Opin Pharmacother. 2015;16:1577–1591. [DOI] [PubMed] [Google Scholar]
- 33. Gilbert RE, Weir MR, Fioretto P, et al. Impact of age and estimated glomerular filtration rate on the glycemic efficacy and safety of canagliflozin: a pooled analysis of clinical studies. Can J Diabetes. 2016;40:247–257. [DOI] [PubMed] [Google Scholar]
- 34. Kim YG, Hahn S, TJ O, Kwak SH, Park KS, Cho YM. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia. 2013;56:696–708. [DOI] [PubMed] [Google Scholar]
- 35. Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase‐4 inhibitors in type 2 diabetes: meta‐analysis. Ann Pharmacother. 2012;46:1453–1469. [DOI] [PubMed] [Google Scholar]
- 36. Kanamoria A, Matsuba I. Factors associated with reduced efficacy of sitagliptin therapy: analysis of 93 patients with type 2 diabetes treated for 1.5 years or longer. J Clin Med Res. 2013;5:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubota A, Yabe D, Kanamori A, et al. Factors influencing the durability of the glucose‐lowering effect of sitagliptin combined with a sulfonylurea. J Diabetes Invest. 2014;5:445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


