Abstract
Objective
Gender is a risk factor in the onset of osteoarthritis (OA). The aim of the study was to investigate gender differences in contact area (CA) and congruity index (CI) in the medial tibiofemoral (MTF) joint in 2 different cohorts, quantified automatically from magnetic resonance imaging (MRI).
Design
The CA and CI markers were validated on 2 different data sets from Center for Clinical and Basic Research (CCBR) and Osteoarthritis Initiative (OAI). The CCBR cohort consisted of 159 subjects and the OAI subcohort consisted of 1,436 subjects. From the MTF joint, the contact area was located and quantified using Euclidean distance transform. Furthermore, the CI was quantified over the contact area by assessing agreement of the first- and second-order general surface features. Then, the gender differences between CA and CI values were evaluated at different stages of radiographic OA.
Results
Female CAs were significantly higher than male CAs after normalization, male CIs were significantly higher than female CIs after correcting with age and body mass index (P < 0.05), consistent across the 2 data sets. For the OAI data set, the gender differences were present at all stages of radiographic OA.
Conclusion
This study demonstrated the gender differences in CA and CI in MTF joints. The higher normalized CA and lower CI values in female knees may be linked with the increased risk of incidence of radiographic OA in females. These differences may help further understand the gender differences and/or to establish gender specific treatment strategies.
Keywords: knee, radiographic osteoarthritis, gender, congruity index, osteoarthritis initiative, MRI
Introduction
Osteoarthritis (OA) is a major health concern worldwide causing pain and limited range of motion in load-bearing joints, particularly for the elderly.1 There exist several systemic and nonsystemic risk factors that contribute toward the development and progression of the OA.2,3 Gender is one of the systemic risk factors during the onset of OA.4,5 The various factors that contribute to the predisposition of OA in men and/or women could be cartilage structure, hormonal imbalance, biomechanics, malalignment, age, and exercise. Biomechanical factors in general play a significant role in the onset of OA6 and previous research showed that there existed gender differences in the biomechanics of the OA knees.7 Age also plays a critical role making women more susceptible to OA than men, generally from the onset of menopause.8
The contact area (CA) in the medial tibiofemoral (MTF) joint is the region where the articular cartilage surfaces covering the bone ends are in close proximity. In the CA, the 2 surfaces interact and transfer the local stresses, ideally causing no or insignificant degeneration to the cartilage in a joint with no radiographic OA. The “congruity” could physically be defined as how well any 2 surfaces fit together when superimposed one on another. In an MTF joint with no radiographic OA, the smooth femoral cartilage surface articulates well with the smooth tibial cartilage surface and is congruent in association with the meniscus.
Several studies assessed the gender differences from the longitudinal volume change, gait analysis, pain, and correlation of clinical OA with Kellgren and Lawrence (KL) score.9 Starting with non-invasive studies from cadavers, the gender differences in patellofemoral joint biomechanics were explored10 and concluded that women had less contact areas and greater contact pressures. In Atheshian et al.,11 the gender differences in congruity for thumb carpometacarpal (CMC) were explored and concluded that male joints were more congruent than female joints; and the lower congruity may be a risk factor for development of CMC joint OA in females more frequently.
Magnetic resonance imaging (MRI) has become a major imaging modality in OA research12,13 since it allows noninvasive visualization of all the tissues present in the joint, especially the cartilage.14,15 In the literature,16-18 knee cartilage volume and bone mineral density differences from MRI were validated and it was observed that men have significantly more cartilage than women after adjustment for confounding factors such as age and body mass index (BMI). Women had smaller joint surfaces and thinner cartilage as compared with men after adjusting for height and weight;19 however, there were no differences in tibial and patellar surface pressures. With gender differences in morphometric and biological measurements from radiographs, MRI, and biochemical markers; there is a need for research characterizing the gender differences in articular measurements and mechanics. Such differences may have implications for the development of gender based treatment options.20,21
Here we investigated the gender differences in CA and congruity index (CI) in the MTF joint stratified according to KL index using the data sets from the Center for Clinical and Basic Research (CCBR), and the Osteoarthritis Initiative (OAI). We hypothesized that male joints show lower normalized CA values and higher CI values compared with females at all stages of the KL index, indicating a significant role of gender in altering the biomechanical properties in the MTF joint.
Methods
Study Population
The CCBR study population consisted of 82 male and 77 female subjects recruited from the greater Copenhagen area. The OAI subjects consisted of 580 male and 856 female subjects selected from publicly available OAI dataset at baseline (https://oai.epi-ucsf.org). The population consisted of individuals with no radiographic OA as well as individuals with varying degrees of radiographic OA. Refer to Table 1 for detailed characterization of the study populations. Subjects with a history of previous knee injury or trauma or contradiction to image acquisition were excluded from both the studies. More details on the CCBR study population are also described elsewhere.22
Table 1.
Number of Subjects (N1) and Knees (N2), Age (Years), and BMI (kg/m2) for Male and Female Subjects for CCBR and OAI Data Sets with Respect to KL Index.a
| CCBR |
OAI |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male |
Female |
Male |
Female |
|||||||||
| N1/N2 | Age (Mean) | BMI (Mean) | N1/N2 | Age (Mean) | BMI (Mean) | N1/N2 | Age (Mean) | BMI (Mean) | N1/N2 | Age (Mean) | BMI (Mean) | |
| All KL | 74/148 | 23-77 (57) | 20-38 (27) | 70/140 | 21-81 (56) | 18-37 (26)* | 580/580 | 45-79 (61) | 20-42 (29) | 856/856 | 45-79 (61) | 18-49 (29) |
| KL 0 | 48/79 | 23-77 (49) | 20-38 (25) | 46/66 | 21-78 (47) | 18-36 (24) | 106/106 | 45-78 (59) | 21-37 (28) | 127/127 | 45-77 (57) | 18-38 (25)** |
| KL 1 | 35/40 | 46- 77 (64) | 20-34 (27) | 33/48 | 37-81 (61) | 19-37 (26) | 62/62 | 45-78 (62) | 22-37 (29) | 85/85 | 45-79 (61) | 18-41 (28) |
| KL 2 | 10/16 | 56-70 (65) | 24-37 (31) | 13/15 | 47-78 (67) | 22-34 (28)* | 187/187 | 45-79 (61) | 20-41 (30) | 379/379 | 45-79 (61) | 19-47 (30) |
| KL 3/4 | 9/13 | 61-72 (68) | 23-34 (29) | 10/11 | 58-78 (67) | 23-34 (28) | 225/225 | 45-79 (62) | 22-42 (30) | 265/265 | 45-79 (64) | 18-49 (31)* |
BMI = body mass index; CCBR = Center for Clinical and Basic Research; KL = Kellgren and Lawrence; OAI = Osteoarthritis Initiative.
Subject that has different KL between the knees was added to the both KL groups that the knees belong. Asterisks indicate the significance of difference between the genders for age and BMI for that specific KL index. The significance of difference between genders was given at the female demographic.
P < 0.05, **P < 0.01, ***P < 0.001.
Image Acquisition
The CCBR study had 318 knees. Five out of 318 knees were excluded due to insufficient image quality in either MRI or radiograph. Another 25 knees used for training of classifier for automatic cartilage segmentation were excluded from the evaluation. The radiographs of both the knees for each subject were taken using an X-ray scanner in anterior-posterior load bearing position. The film distance and tube angulation for the scanner were 1.0 m and 10°, respectively. The radiographs were used to grade the severity of OA from the KL index and also to measure the joint space width (JSW) by an experienced radiologist (P. C. Pettersen, who has 5 years of experience in semiquantitative grading).23 Furthermore, the MRI scans for all the subjects were acquired in a non-loadbearing supine position using a sagittal Turbo 3D T1 sequence at 0.18 T from an Esaote C-span scanner dedicated to scan the lower extremities. The parameters of the scanner were 40° flip angle, 50 ms repetition time (RT), and 16 ms echo time (ET) with scan time of approximately 10 minutes. The in-plane resolution was 0.7 mm × 0.7 mm with slice thickness ranging from 0.7 mm to 0.9 mm.
The OAI study consisted of 1436 scans. The data set was from 0.E.1. We selected this subcohort for this study since the MTF cartilage segmentations were available only for it from Dam et.al.13 The KL index was graded using the radiograph acquired in anterior-posterior loadbearing position. The MRI images of OAI sub-cohort were acquired using 3.0 T 3D dual-echo steady-state water excitation Siemens Trio scanner with 25° flip angle, 16 ms RT, 4.7 ms ET, in-plane resolution of 0.36 mm × 0.36 mm, slice thickness of 0.7 mm, and scanning time of approximately 10 minutes.
The CCBR study protocol was approved by the local ethical committee and was carried out in accordance with the principles of the Helsinki Declaration II and European Guidelines for good Clinical Practice. Also, the OAI study protocol was approved by local ethics committees at all the participating sites. All participants signed and approved the written information consent prior to the study.
The MTF cartilage compartments of the scans from both studies were segmented fully automatically using a voxel classification in a supervised learning approach described elsewhere.13,24
Contact Area Quantification
The CA in a knee was defined as the region where the tibial superior surface and the femoral inferior surface were less than a voxel width apart. We refer to the CA as the cartilage-cartilage contact area ( Fig. 1 ) but not the cartilage-meniscus contact area. First, the tibial surface that was less than a voxel width from femoral surface was estimated and called TibProx. Second, the femoral surface that was less than a voxel width from the tibial surface was computed and denoted as FemProx. The areas of TibProx and FemProx were quantified by converting the estimated region into a triangulated surface. The CA was defined as the mean of the area of TibProx and the area of FemProx since the cartilage surfaces were not symmetric. Then to account for differences in knee sizes, the CA values were normalized. For OAI scans, The CA values were normalized using tibial bone surface area (tAB) (CA = CA/tAB × 100). Since we do not have tAB measures for CCBR scans, we normalized the CA values for these scans by dividing with square of the corresponding tibial bone width (following the methodology from Dam et al.25). Below, CA refers to normalized CA.
Figure 1.
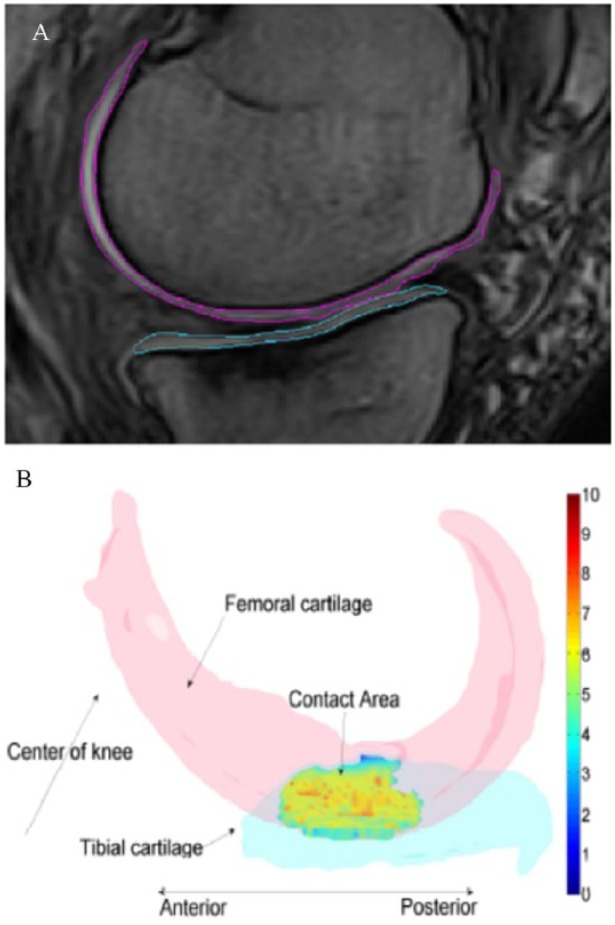
(A) Illustration of medial tibial and femoral cartilage tracings on a slice of MRI. (B) Illustration of location of contact region where the CI was quantified in the medial tibiofemoral joint. Color bar indicates values of CI.
MRI= magnetic resonance imaging; CI= congruity index.
Congruity Index Quantification
Using the estimated tibial and femoral proximity surfaces, the point-by-point CI in the MTF joint was quantified by assessing and combining the first- and second-order general surface features in TibProx and FemProx ( Fig. 1 ). We proposed that the MTF joint was locally congruent if the distance between the local surface normal vectors (first-order features) scaled by local surface normal curvatures (second-order features) was small. Since the number of points in TibProx most likely was not equal to the number of points in FemProx, we computed the CI from TibProx to FemProx and vice versa. The methods to compute the CA and point-by-point CI were detailed and validated previously.26
Statistical Methods
The computations and statistics were performed in MATLAB (Mathworks Inc). Whether the measures on any 2 groups were different was evaluated using the independent-sample t tests. When the data was normally distributed, the Student t test was used otherwise we used Mann-Whitney U tests. The differences between groups were corrected for age and BMI, whenever appropriate. A P value of less than 0.05 was used to establish statistical significance.
Since the study contained data from both knees, we explicitly modeled the interknee correlations within subjects for CA and CI values using generalized estimation equations (GEE). The GEEQBOX package implemented in MATLAB was used to compute the GEE P values (PGEE).27
Results
The age and BMI of the populations were evenly distributed for both the data sets ( Table 1 ). We arranged the populations according to gender and further stratified with respect to KL index.
Gender Differences in CA
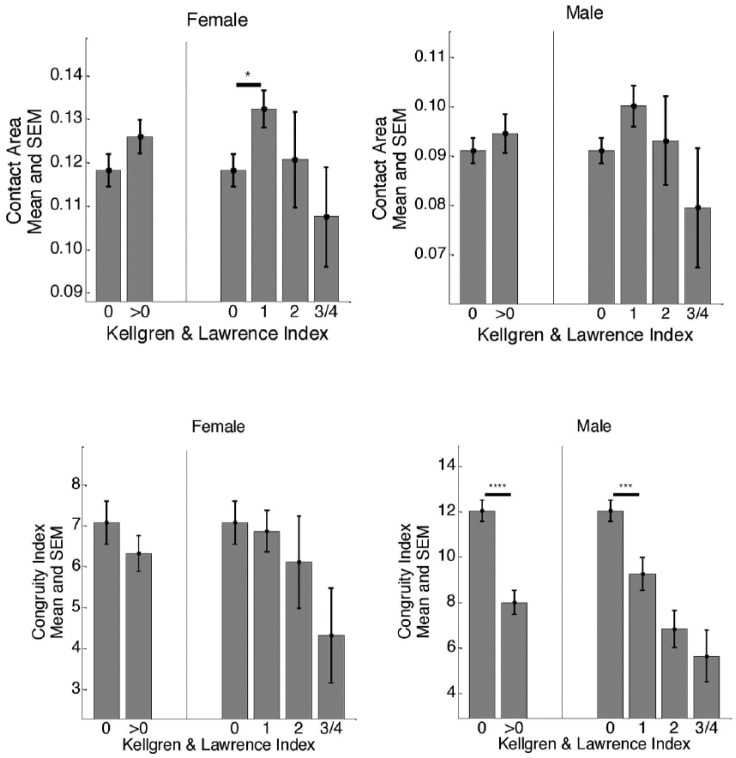
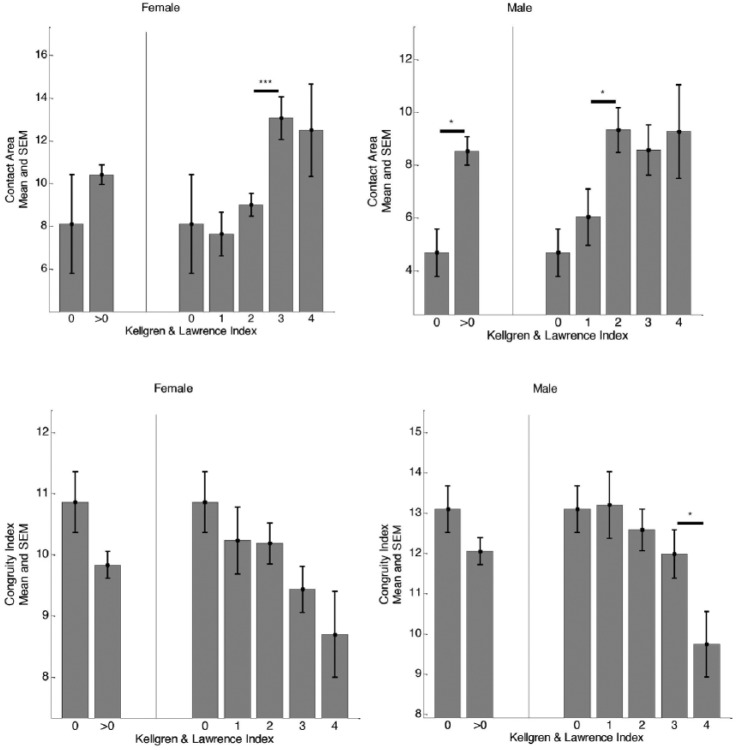
Stratification of CA according to KL index for the male and female subjects is shown in Figures 2 and 3 . For the CCBR scans, the CA values for male subjects were not significantly different with respect to KL index whereas for female joints, the CA values were significantly different between no radiographic OA (KL 0) and presence of radiographic OA (KL 1) subjects (P < 0.05). However, for OAI scans, significant increases in CA values were observed at mild/moderate radiographic OA (KL 0 to KL2/KL3) in both genders. In general, for both studies, the CA values of the female subjects were larger than the male subjects from no radiographic OA to all stages of radiographic OA ( Table 2 ). Also, radiographic OA knees demonstrated higher CA values for both genders, which was also consistent across the 2 studies.
Figure 2.
Cross-sectional separation across different KL grades for male and female subjects based on CA and CI for CCBR scans. The asterisks indicate the statistical significance computed from appropriate t test. *P <0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
KL= Kellgren and Lawrence; CA= contact area; CI= congruity index; CCBR= Center for Clinical and Basic Research; SEM= standard error mean.
Figure 3.
Cross-sectional separation across different KL grades for male and female subjects based on CA and CI for OAI scans. The asterisks indicate the statistical significance computed from appropriate t test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
KL= Kellgren and Lawrence; CA= contact area; CI= congruity index; OAI= Osteoarthritis Initiative; SEM= standard error mean.
Table 2.
CA Values of Male and Female Subjects for Both Data Sets.a
| CCBR |
OAI |
|||
|---|---|---|---|---|
| CA Male |
CA Female |
CA Male |
CA Female |
|
| No radiographic OA (KL 0) | 0.091 ± 0.02 | 0.118 ± 0.03**** | 4.7 ± 5.0 | 8.1 ± 11.6* |
| Possible radiographic OA (KL 1) | 0.101 ± 0.03 | 0.132 ± 0.03**** | 6.1 ± 7.5 | 7.6 ± 7.1* |
| Definite radiographic OA (KL 2 and above) | 0.087 ± 0.04 | 0.114 ± 0.04** | 9.0 ± 9.8 | 10.8 ± 10.4*** |
CA = contact area; CCBR = Center for Clinical and Basic Research; KL = Kellgren and Lawrence; OA = osteoarthritis; OAI = Osteoarthritis Initiative.
The significance of difference between genders computed as P value from a statistical test is given in terms of asterisks.
P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
For both studies, the gender differences were retained at all stages of KL after intrasubject adjustment using GEE (e.g., for KL 0 in CCBR, PGEE = 4 × e−9).
Gender Differences in CI
For both male and female, similar trends were observed for cross-sectional separation of CI according to KL index ( Figs. 2 and 3 ) across both studies. In the CCBR study, the CIs for male joints were significantly different between KL 0 and KL 1 (P < 0.0001). In both studies, we also found that male joints with no radiographic OA were more congruent than male joints with definite radiographic OA (P < 0.0001). The CIs of the female joints were generally lower, but not different between no radiographic OA and definite OA (P = 0.40). See Figures 2 and 3 for comparison of mean CI values for male and female subjects with respect to KL index. The male joints were more congruent than female joints at no radiographic OA and at possible presence of radiographic OA (P < 0.01) for both OAI and CCBR populations. However, at moderate to advanced stages of radiographic OA, the differences were significant only in the OAI population (P < 0.0001, see Table 3 for more details).
Table 3.
CI Values of Male and Female Subjects from Both Data Sets.a
| CCBR |
OAI |
|||
|---|---|---|---|---|
| CI Male |
CI Female |
CI Male |
CI Female |
|
| No radiographic OA (KL 0) | 12.0 ± 4.1 | 7.1 ± 4.3**** | 13.1 ± 5.3 | 10.9 ± 5.0** |
| Possible radiographic OA (KL 1) | 9.3 ± 4.6 | 6.9 ± 3.5*** | 13.2 ± 6.3 | 10.2 ± 4.5** |
| Definite radiographic OA (KL 2 and above) | 6.3 ± 3.7 | 5.3 ± 3.9 | 11.8 ± 6.1 | 9.8 ± 5.1**** |
CCBR = Center for Clinical and Basic Research; CI = congruity index; KL = Kellgren and Lawrence; OA = osteoarthritis; OAI = Osteoarthritis Initiative.
The significance of difference between genders computed as P value from a t test is given in terms of asterisks.
P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Also, for both studies, the gender differences in CI were retained at all stages of KL after intrasubject adjustment using GEE (e.g., for KL 0 for CCBR, PGEE = 4 × e−6).
Discussion
We investigated the gender difference in MTF joint CA and CI by focusing on their ability to separate subjects with no radiographic OA from definite radiographic OA. These results were from 2 independent cohorts and supported that there may be gender differences in the onset of radiographic OA from a biomechanical point of view. For proper biomechanics of synovial joints, there are different tissues (cartilage, meniscus) involved in transmitting the load effectively during all daily activities. The normalized CA values representing the cartilage-cartilage CA were higher in radiographic OA compared with no radiographic OA. This trend is consistent between genders and across the 2 studies. In the CCBR scans, there was an increase in cartilage-cartilage CA from no radiographic OA to early radiographic OA in both male and female joints suggesting that the meniscus likely plays a role in the onset of biomechanical instability in the joint. However, for the OAI cohort, this trend was observed between no radiographic OA and mild/moderate radiographic OA. Therefore, we speculate that these differences may be due to usage of different normalization procedures in scaling out the knee size differences. The higher CA values of the female subjects after adjusting for knee sizes suggests that more cartilage-cartilage CA may be involved in load transmission for them compared with male joints at all stages of radiographic OA. The lower CA values at advanced stages of radiographic OA for both genders and even across the studies may be due to loss of cartilage.
Similar to CA, significant differences in CI values were evident between the genders from both the studies at all stages of KL index. Malalignment (Q-angle) may be one of the determining factors responsible for variation of local congruency in the joint. However, it was also hypothesized that local incongruity plays a role in determining the alignment.28 Male joints demonstrated higher local congruence compared with female joints at no radiographic OA and definite OA indicating higher risk of females to develop radiographic OA. The differences between genders were not significant for CCBR scans at moderate to advanced stages of radiographic OA; we feel that it may be due to low sample size of the scans. Therefore, since male joints with no radiographic OA were more locally congruent, this may be responsible for higher malalignment in females compared with males. In this study, we computed the CI values only in the cartilage-cartilage CA, and by including the cartilage-meniscus region in the analysis, we would possibly be able to draw more concrete conclusions.
The quantification of CA and CI values was based on fully automatic segmentations from 2 different data sets. This supports that the observed gender differences were likely due to actual differences in CA and CI values and not due to algorithmic or acquisition artifacts. Female MTF joint CI values were significantly lower than the male joint CI values irrespective of source of data. The CI values were also comparable between the datasets at no radiographic OA stage and early stages of radiographic OA. The CA values of the female joints were greater than male joints after normalization. On the other hand, interestingly from CCBR study, we found that females who were older and have higher BMI have more CA. Young male and female joints were also more congruent than older individuals. However, these differences were corrected while doing the KL index comparison between the genders.
There were some limitations in this study. The quantifications of CA and CI values heavily depend on the knee angle, and with the available data, we were not able to validate the gender differences on other flexion angles. However, for consistency across knees with varying degrees of radiographic OA, we feel that the non-loadbearing supine position was a good posture. Therefore, we were also not able to optimize the knee angle at which the maximum gender differences in CA and CI could be extracted. Also, we did not include the cartilage-meniscus contact region in the analysis, even if this is a vital region that could be included along with cartilage-cartilage contact region. Even though we may be able to extract this from the OAI scans, we want to leave it to a future study. Moreover, we could not validate our methods with an ex vivo model, which is a further limitation.
In conclusion, we conducted a validation study to explore the gender differences in the MTF joint CA and CI values from both low- and high-field MRI. Similar results were found between the datasets with significant gender differences found in CA and CI values at all stages of radiographic OA. The existing differences may be helpful to understand the gender dissimilarities in subjects with no radiographic OA and those with definite radiographic OA. Further, the results may provide implications for making gender specific interventions or treatment strategies to treat radiographic OA.
Footnotes
Acknowledgments and Funding: The authors would like to thank the Center for Clinical and Basic Research and the Osteoarthritis Initiative for providing the MRI scans and radiographic readings. The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been funded by the Danish Strategic Research Council through the grant “Learning Imaging Biomarkers” (Grant No. 09-065145). Finally, this research has received funding from the D-BOARD consortium, a European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement number 305815. We also acknowledge the funding from the Danish Research Foundation (“Den Danske Forskningsfond”).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Sudhakar Tummala has received a PhD scholarship partly funded by Biomediq A/S. Dieuwke Schiphof was a PhD student at Erasmus MC, Rotterdam. Inger Byrjalsen is an employee of Nordic Bioscience A/S. Erik Dam is an employee and shareholder of Biomediq A/S. The intellectual and commercial property rights to the investigated MRI markers belong to Biomediq A/S.
Ethical Approval: The CCBR study protocol was approved by the local ethical committee and was carried out in accordance with the principles of the Helsinki Declaration II and European Guidelines for good Clinical Practice. Also, the OAI study protocol was approved by local ethics committees at all the participating sites.
Informed Consent: All participants signed and approved the written information consent prior to the study.
Trial Registration: Not applicable.
References
- 1. Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. The number of persons with symptomatic knee osteoarthritis in the United States: Impact of race/ethnicity, age, sex, and obesity. Arthritis Care Res (Hoboken). 2016;68:1743-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:507-15. [DOI] [PubMed] [Google Scholar]
- 3. Alizai H, Roemer FW, Hayashi D, Crema MD, Felson DT, Guermazi A. An update on risk factors for cartilage loss in knee osteoarthritis assessed using MRI-based semiquantitative grading methods. Eur Radiol. 2015;25:883-93. [DOI] [PubMed] [Google Scholar]
- 4. Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24-33. [DOI] [PubMed] [Google Scholar]
- 5. Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769-81. [DOI] [PubMed] [Google Scholar]
- 6. Jackson BD, Wluka AE, Teichtahl AJ, Morris ME, Cicuttini FM. Reviewing knee osteoarthritis—a biomechanical perspective. J Sci Med Sport. 2004;7:347-57. [DOI] [PubMed] [Google Scholar]
- 7. McKean KA, Landry SC, Hubley-Kozey CL, Dunbar MJ, Stanish WD, Deluzio KJ. Gender differences exist in osteoarthritic gait. Clin Biomech (Bristol, Avon). 2007;22:400-9. [DOI] [PubMed] [Google Scholar]
- 8. Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res 2004;(427 Suppl):S6-S15. [DOI] [PubMed] [Google Scholar]
- 9. Hanna FS, Teichtahl AJ, Wluka AE, Wang Y, Urquhart DM, English DR, et al. Women have increased rates of cartilage loss and progression of cartilage defects at the knee than men: a gender study of adults without clinical knee osteoarthritis. Menopause. 2009;16:666-70. [DOI] [PubMed] [Google Scholar]
- 10. Csintalan RP, Schulz MM, Woo J, McMahon PJ, Lee TQ. Gender differences in patellofemoral joint biomechanics. Clin Orthop Relat Res. 2002;(402):260-9. [DOI] [PubMed] [Google Scholar]
- 11. Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25:591-607. [DOI] [PubMed] [Google Scholar]
- 12. Schwaiger BJ, Gersing AS, Mbapte Wamba J, Nevitt MC, McCulloch CE, Link TM. Can signal abnormalities detected with MR imaging in knee articular cartilage be used to predict development of morphologic cartilage defects? 48-month data from the Osteoarthritis Initiative. Radiology. 2016;281:158-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dam EB, Lillholm M, Marques J, Nielsen M. Automatic segmentation of high- and low-field knee MRIs using knee image quantification with data from the osteoarthritis initiative. J Med Imaging (Bellingham). 2015;2:024001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayashi D, Guermazi A, Kwoh CK. Clinical and translational potential of MRI evaluation in knee osteoarthritis. Curr Rheumatol Rep. 2014;16:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guermazi A, Hayashi D, Roemer FW, Felson DT. Osteoarthritis: a review of strengths and weaknesses of different imaging options. Rheum Dis Clin North Am. 2013;39:567-91. [DOI] [PubMed] [Google Scholar]
- 16. Berry PA, Wluka AE, Davies-Tuck ML, Wang Y, Strauss BJ, Dixon JB, et al. Sex differences in the relationship between bone mineral density and tibial cartilage volume. Rheumatology (Oxford). 2011;50:563-8. [DOI] [PubMed] [Google Scholar]
- 17. Cicuttini F, Forbes A, Morris K, Darling S, Bailey M, Stuckey S. Gender differences in knee cartilage volume as measured by magnetic resonance imaging. Osteoarthritis Cartilage. 1999;7:265-71. [DOI] [PubMed] [Google Scholar]
- 18. Ding C, Cicuttini F, Scott F, Glisson M, Jones G. Sex differences in knee cartilage volume in adults: role of body and bone size, age and physical activity. Rheumatology (Oxford). 2003;42:1317-23. [DOI] [PubMed] [Google Scholar]
- 19. Otterness IG, Eckstein F. Women have thinner cartilage and smaller joint surfaces than men after adjustment for body height and weight. Osteoarthritis Cartilage. 2007;15:666-72. [DOI] [PubMed] [Google Scholar]
- 20. Maleki-Fischbach M, Jordan JM. New developments in osteoarthritis. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Res Ther. 2010;12:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O’Connor MI. Osteoarthritis of the hip and knee: sex and gender differences. Orthop Clin North Am. 2006;37:559-68. [DOI] [PubMed] [Google Scholar]
- 22. Tummala S, Bay-Jensen AC, Karsdal MA, Dam EB. Diagnosis of osteoarthritis by cartilage surface smoothness quantified automatically from knee MRI. Cartilage. 2011;2:50-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folkesson J, Dam EB, Olsen OF, Pettersen PC, Christiansen C. Segmenting articular cartilage automatically using a voxel classification approach. IEEE Trans Med Imaging. 2007;26:106-15. [DOI] [PubMed] [Google Scholar]
- 25. Dam EB, Folkesson J, Pettersen PC, Christiansen C. Automatic morphometric cartilage quantification in the medial tibial plateau from MRI for osteoarthritis grading. Osteoarthritis Cartilage. 2007;15:808-18. [DOI] [PubMed] [Google Scholar]
- 26. Tummala S, Nielsen M, Lillholm M, Christiansen C, Dam EB. Automatic quantification of tibio-femoral contact area and congruity. IEEE Trans Med Imaging. 2012;31:1404-12. [DOI] [PubMed] [Google Scholar]
- 27. Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17:1643-58. [DOI] [PubMed] [Google Scholar]
- 28. Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(Suppl 1):85-9. [DOI] [PubMed] [Google Scholar]