Abstract
Background
Evidence has been systematically assessed comparing robotic with standard laparoscopy for treatment of endometrial cancer.
Methods
A search of Medline, Embase and Cochrane databases was performed until 30th October 2016.
Results
Thirty‐six papers including 33 retrospective studies, two matched case–control studies and one randomized controlled study were used in a meta‐analysis. Information from a further seven registry/database studies were assessed descriptively. There were no differences in the duration of surgery but days stay in hospital were shorter in the robotic arm (0.46 days, 95%CI 0.26 to 0.66). A robotic approach had less blood loss (57.74 mL, 95%CI 38.29 to 77.20), less conversions to laparotomy (RR = 0.41, 95%CI 0.29 to 0.59), and less overall complications (RR = 0.82, 95%CI 0.72 to 0.93). A robotic approach had higher costs ($1746.20, 95%CI $63.37 to $3429.03).
Conclusion
A robotic approach has favourable clinical outcomes but is more expensive.
1. INTRODUCTION
Evidence from randomised controlled trials support the use of laparoscopic techniques over open surgery for endometrial cancer.1 Standard laparoscopy for endometrial cancer is often possible but can be difficult to perform due to co‐morbidities such as obesity that can be associated with uterine malignancy.2 It has been proposed that robotic surgery is easier to learn than standard laparoscopy,3 and a number of studies have demonstrated improved ergonomics and outcomes in vitro. 3, 4 Furthermore, it has been suggested that the in vitro benefits for robotics might be paralleled by improved clinical outcomes for endometrial cancer patients. To date, a number of studies have demonstrated a higher proportion of women having a laparoscopic approach instead of open surgery when a robot is available.5, 6 Furthermore, they have suggested that this would improve the overall rate of conversion to laparotomy, operative complications and costs.5, 6 The aim of this study is to systematically assess comparative cohort studies from single institutions that compare standard laparoscopy with robot assisted laparoscopy for the treatment of endometrial cancer.
2. METHOD
A systematic search of Medline, Embase and the Cochrane database was performed for the period 1st January 1991 until 30th October 2016. No start date was used for the search. The search criteria included a search of titles, abstracts, and Medical Subject Headings for the words (‘uterine’ or ‘uterus’ or ‘endometrial’ or ‘endometrium’) and (‘carcinoma’ or ‘cancer’ or ‘neoplasia’ or ‘neoplasm’) and (‘robot’ or ‘robotic’ or ‘DaVinci’). Studies that compared a standard laparoscopic approach to endometrial cancer with a robotic approach within a discrete cohort were included. Papers were eliminated from the analysis if there was no such comparison or if it was not possible to extract data for endometrial cancer patients from other diagnoses. If two papers were published from the same institution, only the most recent manuscript was used to avoid duplication. The exception was when different outcomes were reported in separate papers. It was not possible to include papers that looked at outcomes from large registries as many patients from the other studies would have been included in national and regional databases resulting in duplication. However, registry papers were retrieved from the search and assessed descriptively in the discussion of this paper.
Data were taken from the text and tables of the published papers. The presentation of data depended on that reported in individual papers. For example, if a study reported both the pelvic and para‐aortic lymph node yields, it was only possible to include this data in total lymph node counts if that data was reported. A similar situation was applied to the reporting of operative complications. To avoid a complication being counted twice and potentially prejudicing one arm, a conversion to a laparotomy in it's own right was not reported in the complication fields but treated separately. The same applied to blood transfusions. Where possible, complications were reported as ‘total’ but divided into ‘major’ and ‘minor’ in nature if reported as well as ‘intra‐operative’ and ‘post‐operative’ if separated in a paper's text. If the Clavien‐Dindo classification was used in a paper, post‐operative complications classed as III or above were defined as ‘major’. Additional information clarifying data was sought from three authors and in one case this was provided.7
Costs and charges were presented in United States Dollars. If this was reported in another currency then this was converted to Dollars using the exchange rate published for the middle year of the recruitment period from the Bank of England website (www.bankofengland.co.uk). The data were recorded using Review Manager.8 Dichotomous data were presented as Risk Ratios using the Mantel–Haenszel method with random effects.9 Continuous data were presented as means with standard deviations and analysed using the Inverse Variance method using random effects.10 When continuous data were presented as medians with ranges, the data were converted for inclusion into the meta‐analysis using the method described by Hozo et al.. 11 When only interquartile ranges were reported, the data could not be included into the meta‐analysis.
3. RESULTS
A flowchart of how papers were selected is given in Figure 1. This revealed 35 papers that were included in the study.5, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 A further hand‐search of review article references included one additional paper.45 Therefore, a total of 36 papers were included in the analysis and these involved 8075 patients (3830 robotic and 4245 laparoscopic). A list of papers included in the meta‐analysis and the outcomes included are detailed in Table 1. This included 35 retrospective cohort studies of which two contained matched case‐controls.19, 31 In addition, there was one randomised controlled study32 (Table 1). Furthermore, seven papers reporting data from registries were carefully read and used for comparative discussion in the relevant section of this paper.46, 47, 48, 49, 50, 51
Figure 1.
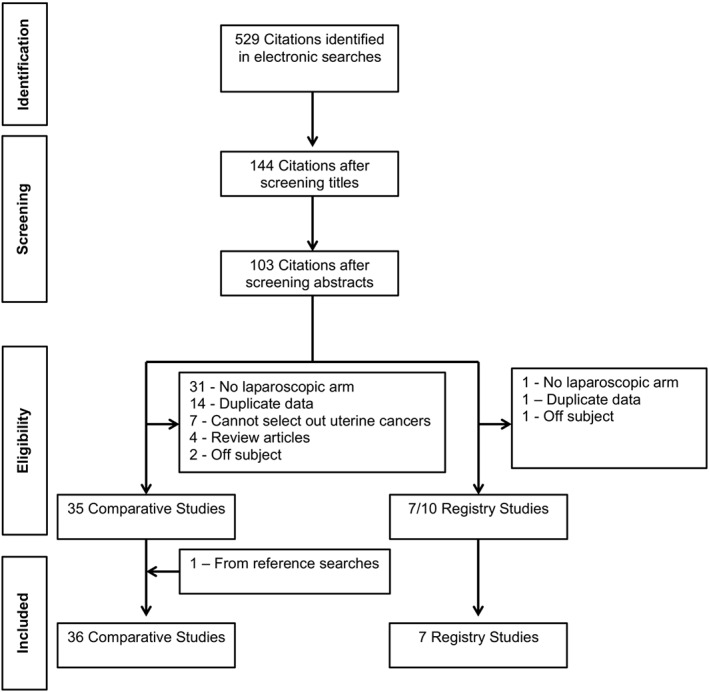
PRISMA flow chart for trial identification and selection
Table 1.
Studies selected for inclusion into the meta‐analyses
| First author | Year | Design | Countries | Period of recruitment | N‐rob | N‐lap | Outcomes included in meta‐analysis |
|---|---|---|---|---|---|---|---|
| Bell | 2008 | RCC | USA | May 2000 to Jun 2009 | 40 | 30 | OT, Los, RNA, TLN, BL, BT, ac, AMC, C, Tc |
| Boggess | 2008 | RCC | USA |
Jun 2005 to Dec 2007 – Rob Apr 2000 to Sep 2004 ‐ lap |
103 | 81 | OT, Los, BL, BT, TLN, PLN, PALN, cl, ac, AIC, mic, APC |
| Hoekstra | 2009 | RCC | USA | Jul 2007 to Jul 2008 | 24 | 7 | BT, cl, Ra, ac, AMC, AIC |
| Seamon | 2009 | RCC | USA | Jan 2006 to Apr 2008 | 105 | 76 | OT, ort, Orit, Los, BT, PLN, PALN, BL, cl, ac |
| Holtz | 2010 | RCC | USA | Jul 2007 to Jul 2008 | 13 | 20 | OT, LOS, PLN, PALN, BL, DHb, CL, AC, AMC, AIC, MIC, APC, MPC, TC |
| Jung | 2010 | RCC | Korea | May 2006 to Jan 2009 | 28 | 25 | OT, Los, PLN, PALN, BT, cl, ac, AMC, AIC, mic, APC, MPC |
| Lim | 2011 | mRCC | USA | Mar 2008 to Jul 2010 | 122 | 122 | OT, Los, TLN, PLN, PALN, BL, BT, cl, Ra, ac, AMC, AIC, mic |
| Magrina | 2011 | RCC | USA |
Mar 2004 to Dec 2007 ‐ rob; Nov 1999 to Aug 2006 ‐ lap |
37 | 67 | OT, LOS, PLN, PALN, BL, BT, CL, RA, AC, AIC, APC, rec |
| Martino | 2011 | RCC | USA | Sep 2005 to Jun 2010 | 101 | 114 | PPS |
| Shah | 2011 | RCC | USA | Jan 2009 to Dec 2009 | 43 | 118 | OT, Los, BL, cl, ac, AIC, mic, APC |
| Coronado | 2012 | RCC | Spain | 2003 to Jun 2011 | 71 | 84 | OT, LOS, PLN, BL, BT, DHb, CL, AC, AIC, MIC, APC, TC |
| Escobar | 2012 | mRCC | USA | Apr 2009 to Sep 2010 | 30 | 60 | OT, Los, PLN, PALN, BL, BT, cl, ac, AMC, AIC, mic |
| Estape | 2012 | RCC | USA | 2002 to 2009; robot from 2006 | 102 | 104 | OT, Los, TLN, BL, BT, cl, RI, Ra, ac, AMC, AIC, mic |
| Fagotti | 2012 | RCC | Italy | Feb 2009 to Jun 2011 | 75 | 75 | OT, Los, TLN, BL, cl, ac, AMC, AIC, APC, MPC |
| Fleming | 2012 | RCC | USA | Jun 2008 to Sep 2010 | 23 | 43 | OT, Los, ort, PLN, PALN, BL, cl, ac, AMC, AIC, mic, MPC, PPS, INU, PNU |
| Leitao MM Jr | 2012 | RCC | USA | May 2007 to Dec 2010 | 347 | 302 | OT, ort, Los, BT, TLN, PLN, PALN, BL, cl, ac, AMC, APC |
| Nevadunsky | 2012 | RCC | USA | Aug 2006 to Jan 2009 | 102 | 115 | OT, Los, BL, BT, cl, ac, APC |
| Venkat | 2012 | RCC | USA | 2008–2010 | 27 | 27 | OT, ort, Los, TLN, BL |
| Cardenas‐Goicoechea | 2013 | RCC | USA |
Dec 2007 to Apr 2010 – Rob Jan 2003 to Dec 2007 ‐ lap |
187 | 245 | OT, Los, TLN, PLN, PALN, BL, BT, cl, RI, Ra, ac, AIC, mic, APC |
| Desille‐Gbaguidi | 2013 | RCC | France | 2008 to Dec 2011 | 20 | 15 | OT, Los, TLN, BL, Ra, Tc |
| Leitao MM Jr | 2013 | RCC | USA | May 2007 to Jun 2010 | 239 | 236 | PPS, D1PS |
| Turunen | 2013 | RCC | Finland | May 2009 to Feb 2013 | 67 | 150 | OT, PLN, BL, cl |
| Leitao MM Jr | 2014 | RCC | USA | Jan 2009 to Dec 2010 | 262 | 132 | TC |
| Mendivil | 2014 | RCC | USA | Sep 2008 to Dec 2011 | 13 | 16 | OT, Los, TLN, BL, BT, cl, Ra, ac, AIC, mic, APC |
| Pakish | 2014 | RCC | USA & Brazil | Jan 2007 to Nov 2012 | 52 | 142 | OT, PLN, PALN, BL, BT, cl, Ra, AIC, mic |
| Seror | 2014 | RCC | France | Jan 2002 to Dec 2011. (robotics started in 2008) | 40 | 106 | BT, cl, ac, AMC, AIC, mic, APC, MPC |
| Chiou | 2015 | RCC | Taiwan | 2011 to 2013 ‐ rob; 2005–2013 ‐ lap | 86 | 150 | OT, Los, DFD, TLN, PLN, BL, ac, AMC, PPS, D1PS |
| Corrado | 2015 | RCC | Italy | Jan 2001 to Dec 2013 | 72 | 277 | OT, LOS, PLN, BL, BT, CL, RI, AC, AMC, AIC, MIC, APC, MPC, rec |
| Frey | 2015 | RCC | UA | May 2006 to Oct 2010 | 77 | 45 | OT, Los, TLN, PLN, PALN, BL, cl |
| Ind | 2015 | RCC | UK | Jan 2010 to Dec 2013; (robot from 2012) | 24 | 77 | OT, LOS, BL, BT, DHb, CL, AL, AC, AMC, AIC, MIC, APC, MPC, TC |
| Manchana | 2015 | RCC | Thailand | Jan 2011 ro Dec 20014 | 28 | 47 | BT, cl, AIC, mic, APC |
| Turner | 2015 | RCC | USA | Jan 2008 to may 2012 | 122 | 213 | Ort, BL, cl, INU, PNU |
| Barrie | 2016 | RCC | USA | Jan 2009 to Jan 2014 | 745 | 688 | Ac, AMC, AIC, mic, APC, MPC, cl, BT |
| Johnson | 2016 | RCC | USA | Oct 2008 to Sep 2012 | 353 | 187 | OT, ort, Los, PLN, PALN, BL, cl, Ra, ac, AIC, APC |
| Maenpaa | 2016 | RCT | Finland | Dec 2010 to Oct 2013 | 50 | 49 | OT, ORT, LOS, TLN, PLN, BL, BT, PHb, DHb, CL, AC, AMC, AIC, MIC, APC, MPC, D1PS, D2PS |
| Pilka | 2016 | RCC | Czech Republic | Oct 2012 to Jun 2015 | 64 | 13 | TLN, BL, DHb, PPS |
Abbreviations
RCC – Retrospective Cohort Comparison, mRCC – Matched Retrospective Cohort Comparison, RCT – Randomised Controlled Trial
OT – Operative Time; ORT ‐ Operating Room Time; LOS – Length Of Stay; ORIT – Operating Room to Incision Time; DFD – Days to Full Diet; RNA – Days Return to Normal Activity
TLN – Total Lymph Node count; PLN – Pelvic Lymph Node count; PALN – Para‐Aortic Lymph Node count
BL – Blood Loss; BT – Blood transfusion; PHb – Post‐operative Haemaglobin, DHb – Drop in Haemaglobin
CL – Conversion to Laparotomy; RI – Re‐Intervention; RA – Re‐Admisssion
AC – All Complications; AMC – All Major Complications; AIC – All Intra‐operative complication; MIC – Major Intra‐operative complications; APC – All Post‐operative complications; MPC – Major Post‐operative Complications
PPS – Post‐operative Pain Score; D1PS – Day 1 Pain Score; D2PS – Dat 2 Pain Score; INU – Intra‐operative Narcotic Usage; PNU; Post‐operative Narcotic Usage
C‐ Charges; TC – Total Costs
Rec ‐ Recurrences
A summary of the outcomes is shown in Table 2. Across all studies, there was no statistically significant difference in the duration of surgery or operating room times (Table 2). However, the one randomized controlled study reported shorter operating times (Figure 2) and total operating room times for robotic surgery.32 This contrasted with the retrospective cohort studies that reported a longer operating time of 18.4 minutes (95%CI = 2.0–34.7 min) for the robotic arm (Figure 2) but no difference in the total operating theatre time (Table 2). One study reported a longer time from arrival in theatre to the surgical incision for robotic surgery (Table 2).39 The number of days stay in hospital was shorter in the robotic arm compared with standard laparoscopy (Figure 3).
Table 2.
Studies, participants and outcomes in a meta‐analysis comparing robotic to standard laparoscopy for endometrial cancer – Summary of 36 studies
| Outcome or subgroup | Studies | Participants | Statistical method | Effect estimate |
|---|---|---|---|---|
| Operation and hospital durations | ||||
| Operation time (m) | 27 | 4665 | Mean difference (IV, random, 95% CI) | 16.42 (−0.04, 32.88) |
| Operating room time (m) | 7 | 1647 | Mean difference (IV, random, 95% CI) | 17.76 (−15.09, 50.61) |
| In OR to incision time (m) | 1 | 181 | Mean difference (IV, random, 95% CI) | 6.00 (2.80, 9.20) |
| Hospital stay (days) | 25 | 4367 | Mean difference (IV, random, 95% CI) | −0.46 (−0.66, −0.26)* |
| Receiving full diet (days) | 1 | 236 | Mean difference (IV, random, 95% CI) | −0.20 (−0.35, −0.05)* |
| Days return to normal activity (days) | 1 | 70 | Mean difference (IV, random, 95% CI) | −7.50 (−12.04, −2.96)* |
| Lymph nodes | ||||
| Total lymph node count (n) | 14 | 2086 | Mean difference (IV, random, 95% CI) | −0.14 (−5.73, 5.46) |
| Pelvic lymph node count (n) | 18 | 2852 | Mean difference (IV, random, 95% CI) | 1.24 (−0.75, 3.22) |
| Para‐aortic lymph node count (n) | 13 | 1908 | Mean difference (IV, random, 95% CI) | 0.83 (−1.04, 2.71) |
| Bleeding | ||||
| Blood loss (ml) | 28 | 5115 | Mean difference (IV, random, 95% CI) | −57.74 (−77.20, −38.27)* |
| Blood transfusions | 21 | 4911 | Risk ratio (M‐H, random, 95% CI) | 0.77 (0.5, 1.07) |
| Postoperative Haemoglobin (g/L) | 1 | 99 | Mean difference (IV, random, 95% CI) | −5.00 (−10.77, 0.77) |
| Drop in Haemoglobin (g/L) | 5 | 457 | Mean difference (IV, random, 95% CI) | −3.93 (−8.72, 0.87) |
| Adverse events | ||||
| Conversion to laparotomy | 28 | 6558 | Risk ratio (M‐H, random, 95% CI) | 0.41 (0.29, 0.59)* |
| Re‐operation/re‐intervention | 3 | 594 | Risk ratio (M‐H, random, 95% CI) | 0.78 (0.02, 30.03) |
| Re‐admission | 9 | 1823 | Risk ratio (M‐H, random, 95% CI) | 1.55 (0.82, 2.92) |
| All complications | 25 | 5823 | Risk ratio (M‐H, random, 95% CI) | 0.82 (0.72, 0.93)* |
| All major complications | 16 | 3787 | Risk ratio (M‐H, random, 95% CI) | 1.06 (0.61, 1.90) |
| Intra‐operative complications | 22 | 4853 | Risk ratio (M‐H, random, 95% CI) | 0.81 (0.61, 1.06) |
| Major intra‐operative complication | 18 | 3957 | Risk ratio (M‐H, random, 95% CI) | 0.85 (0.58, 1.23) |
| Post‐operative complications | 18 | 4327 | Risk ratio (M‐H, random, 95% CI) | 0.85 (0.72, 1.02) |
| Major post‐operative complications | 9 | 2430 | Risk ratio (M‐H, random, 95% CI) | 1.18 (0.79, 1.76) |
| Pain and analgesia | ||||
| Postoperative visual analogue pain score (0–10) | 5 | 1070 | Mean difference (IV, random, 95% CI) | −0.08 (−0.36, 0.20) |
| Day 1 visual analogue pain score (0–10) | 3 | 788 | Mean difference (IV, random, 95% CI) | −0.48 (−1.07, 0.10) |
| Day 2 visual analogue pain score (0–10) | 1 | 27 | Mean difference (IV, random, 95% CI) | 0.00 (−1.31, 1.31) |
| Intra‐operative narcotic usage (mg m‐e) | 2 | 179 | Mean difference (IV, random, 95% CI) | −40.00 (−52.13, −27.87) |
| Post‐operative narcotic usage (mg m‐e) | 2 | 180 | Mean difference (IV, random, 95% CI) | −1.50 (−8.83, 5.82) |
| Finances | ||||
| Charges ($) | 1 | 70 | Mean difference (IV, random, 95% CI) | 1746.20 (63.37, 3429.03)* |
| Total costs ($) | 6 | 788 | Mean difference (IV, random, 95% CI) | 1869.42 (267.89, 3470.94)* |
| Oncological Ourtomes | ||||
| Recurrences | 2 | 453 | Risk ratio (M‐H, random, 95% CI) | 0.66 (0.33, 1.34) |
= Statistically Significant
IV = Inverse Variance
M‐H = Mantel–Haenzel
Figure 2.
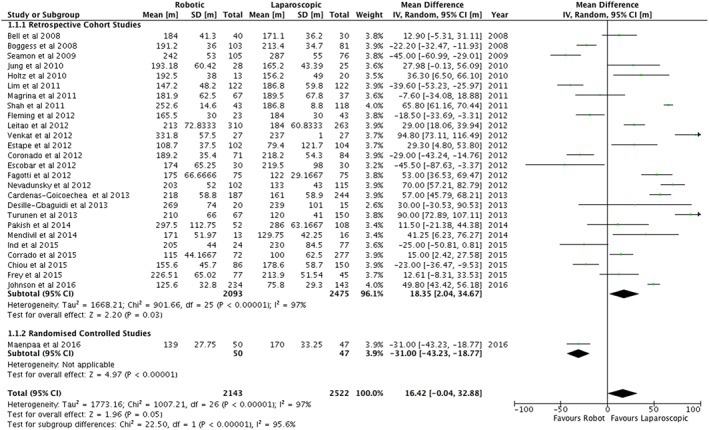
Duration of operations for endometrial cancer (mins)
Figure 3.
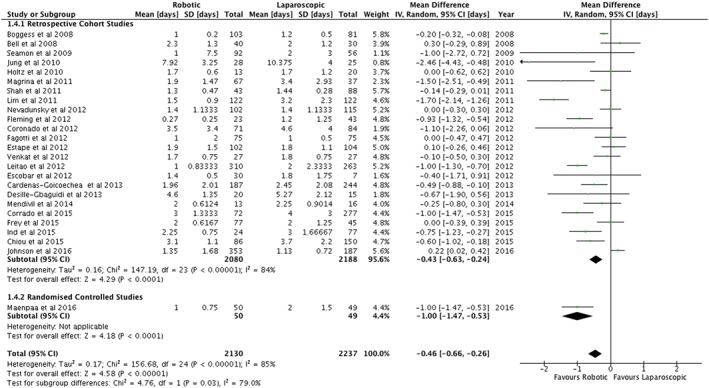
Days in hospital following surgery for endometrial cancer
There was no difference in the total number of lymph nodes removed in the two arms (Table 2). Furthermore, there were no differences between the pelvic and para‐aortic lymph node yields when analysed separately (Table 2).
The estimated blood loss was on average 57.7 mL less during robotic surgery (95%CI 38.3 to 77.2) (Figure 4). This difference was not reflected in the use of blood transfusions, which was significantly less for robotic surgery in the retrospective studies but not in the randomized controlled study nor in a meta‐analysis of all the papers (Figure 5). No differences were found in the post‐operative haemoglobin nor in the post‐operative drop in haemoglobin concentration (Table 2).
Figure 4.
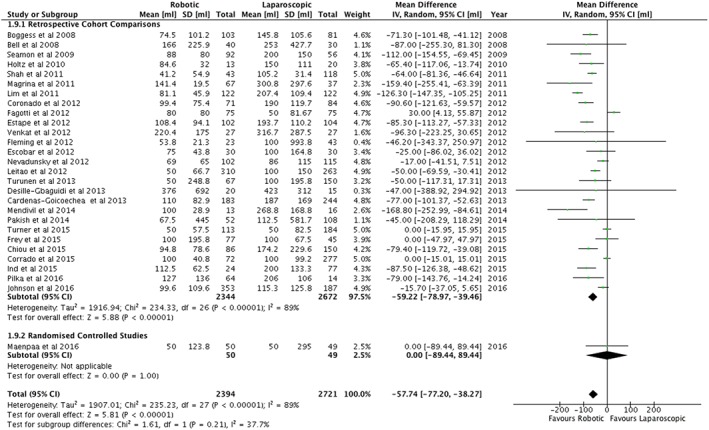
Mean estimated blood loss (mL) following surgery for endometrial cancer
Figure 5.
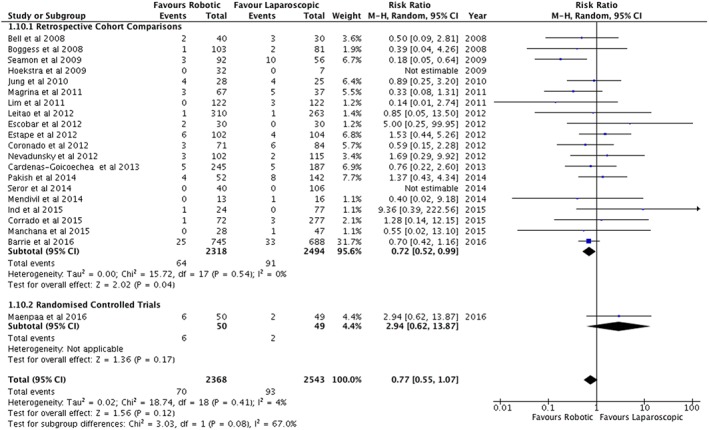
Blood transfusions following surgery for endometrial cancer
For adverse outcomes, significant differences were not found for re‐interventions, re‐admissions, major complications, intra‐operative complications, major intra‐operative complications, post‐operative complications or major post‐operative complications (Table 2). However, there were less total complications in the robotic arm (RR = 0.82, 95%CI = 0.72 to 0.93) (Figure 6). Furthermore, there were significantly less conversions to laparotomy for robotic surgery compared with standard laparoscopy (RR = 0.41, 95%CI = 0.29 to 0.59) (Figure 7).
Figure 6.
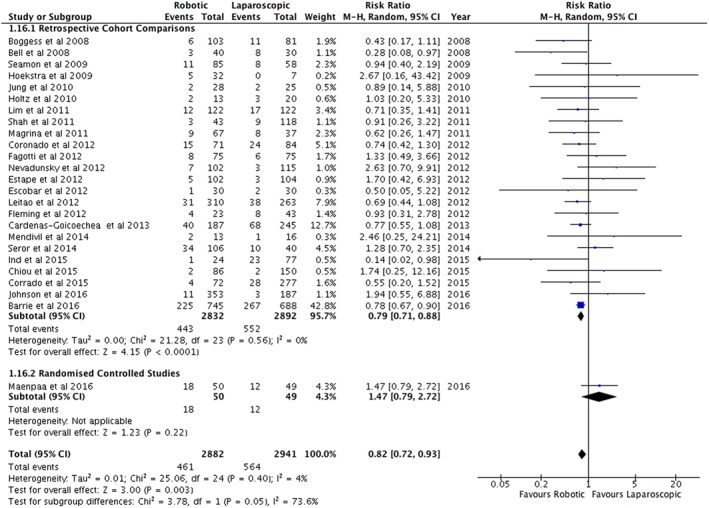
All complications related to surgery for endometrial cancer
Figure 7.
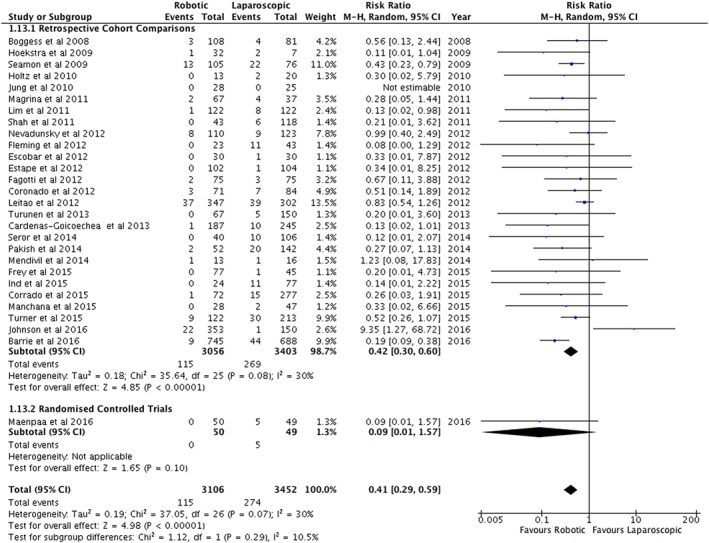
Conversions to laparotomy following surgery for endometrial cancer
No differences could be demonstrated between the two groups for pain scores or post‐operative analgesia usage (Table 2). However, data from two studies showed significantly less intra‐operative narcotic analgesia usage in the robotic group (−40 mg morphine equivalents, 95%CI = −52.11 to −27.85 mg) although this was heavily weighted by one study.22 No differences were demonstrated in the risk of recurrence (Table 2).
Six studies reported the total costs of surgery and could be used in a meta‐analysis. All but one showed an increased cost with the robotic arm with a mean additional cost of $1869.42 (95%CI = $267.89 to $3470.94).
4. DISCUSSION
These data are favourable towards the robotic arm for hospital stay, return to normal activity, return to a normal diet, conversion to laparotomy, operative complications and blood loss. The total cost is in favour of standard laparoscopy. All but three studies assessed are retrospective cohort reviews, two are matched retrospective reviews and one a randomized controlled study (Table 2). Therefore, the quality of the evidence is low although it is bolstered by large numbers of papers and patients. One criticism is that in many of the papers, the robotic arm consists of an early series for the surgical teams. Outcomes with robotic surgery improve with numbers performed29, 52 so this would potentially be biasing the results in favour of the more established standard laparoscopy arm. Furthermore, some authors have acknowledged worse co‐morbidity in the robotic arms5, 45 of their studies with obesity in particular associated with worse outcomes.53 Therefore the data in favour of robotic laparoscopy is in spite of adverse confounders.
Other recent reviews and meta‐analyses of the subject exist.54, 55 They do not include all the citations that are in this study nor the randomized controlled study. Some of these meta‐analyses include registry studies even though some analyse the same databases and include patients reported in the institutional cohorts. However, the findings of less operative conversions, lower blood loss, and a shorter hospital stay are consistent findings within meta‐analyses but this study also demonstrates less overall complications in the robotic arm as well as higher costs.54, 55
This study found significantly longer operating times for robotic surgery in the retrospective cohort studies. However, the one randomized controlled study showed shorter operating times for robotic surgery.32 This may be due to the ‘early series’ effect described when a teams first few operations took longer than the later procedures in their series but in one study where the surgeon and team was already experienced in robotic surgery, longer operating times were still demonstrated.5 It is possible that this is a power effect and a larger study with even more numbers would have demonstrated a longer duration of surgery. From studies reporting outcomes from registries and databases, one study reported a non‐significant shorter operative time in the robotic arm and no studies report longer operative times.46 The mean difference of 18 minutes has to be put in perspective as most people accept the benefits of laparoscopic compared to open surgery for endometrial cancer.1, 56 A meta‐analysis has shown that a standard laparoscopic approach has an additional operative duration of 33 minutes over laparotomy.56
This study demonstrates a shorter hospital stay for robotic cases. This is supported by one registry study that showed a significantly lower proportion of women staying three nights or more in hospital.51 One other registry study reports a non‐significant shorter stay in the robotic group.46 Return to normal activity is shorter for robotics in the one study that reports this outcome in the meta‐analysis.12 One registry study reports on this.46 That study46 reports on a 6.7 days quicker return to normal activity for the robotic arm but reports this as being non‐significant. However, using the Inverse Variance method this would have 95% confidence intervals of 2.05 to 11.35 days shorter return to normal activity which supports the data we report. The reduction in conversion to laparotomies and less complications might explain these findings as one would expect a patient who had a laparotomy or one who suffered complications to spend longer in hospital and take longer to return to normal activity.
In this analysis we demonstrated less blood loss in the robotic arm. However, this could be perceived as a surrogate outcome as 50 mL less blood loss might not be reflected in a drop in haemoglobin concentration or the use of blood transfusions. Although blood transfusion usage was much lower in the robotic arm (RR = 0.76, 95%CI 0.57 to 1.01) this failed to reach statistical significance. Furthermore, no difference in the drop in haemoglobin could be demonstrated either. Blood loss was reported in one registry study and was not significantly different.46 Blood transfusion usage was not shown to be different in any of the registry studies but was lower in all four papers that reported this outcome.46, 49, 50, 51 Therefore, the importance or not in the finding of 50 mL less blood loss remains to be defined.
The finding of less conversions to laparotomy is an important one as the relative risk is 0.42 with tight confidence intervals (0.30 to 0.59). This is likely to be related to the increased ergonomics of robotic surgery over standard laparoscopy.57 However, the outcome is not supported in a registry study.51 Re‐operation and re‐admission rates are also reported in registry studies without any demonstrable significant difference.
The findings of less overall complications may also be related to ergonomic reasons although it will be interesting to see with time how further studies not influenced by the ‘early series’ effect will alter the analysis of intra‐operative, post‐operative, and major complications. The registry studies have conflicting results for this outcome. Total complication rates are very heterogeneous as they are dependent on the definition of a complication and the systematic way in which complications are collected. One registry study reported ‘similar morbidity’ yet the analysis in a table showed significantly less medical complications, significantly less bladder injuries, and significantly less re‐operations for robotic surgery compared with standard laparoscopy.50 Another study by the same group showed a 4% increase in all complications and medical complications in the robotic arm.49
The cost analysis is in favour of the standard laparoscopy arm of the study being $1869.42 less expensive. This is consistent with outcomes from a large registry study where standard laparoscopy was $1291.00 cheaper than a robotic approach to endometrial cancer.50 This figure reduces to $688.00 for individual surgeons who perform more than 50 cases a year48 and that caseload could be considered as an absolute minimum for endometrial cancer surgeons. Other studies that report on hospital charges rather than costs show greater differences.51, 58 However, some might argue that such an increased cost compares favourably compared with other interventions in the field of gynecological oncology such as some chemotherapy agents. What a straight comparison between robotic and standard approaches does not reveal is the additional cost from those patients who have open surgery in institutions not using robotics. To date, two studies have demonstrated greater utilisation of laparoscopic approaches with the use of the robot with less laparotomies, less complications and less overall costs when including the expense of open surgery into the cohorts.5, 6 One problem with analysing cost data in such a way is that different countries have variable healthcare reimbursement systems and wage costs. For example in some countries where there is social healthcare, surgeons are salaried by institutions and in other countries they charge separately. Therefore, a cost–benefit may exist in one healthcare system and not in another and it is difficult to interpret how this data would apply to a single institution although it is clearly of interest.
One matter to consider when assessing these outcomes is the innovation in new platforms over time. In early series, the Da‐Vinci Standard® system will have been used, whereas in latter series the fourth generation of platform (DaVinci Xi®) may have been available. To date there is no published data on the value of the updated systems on outcomes and it would be interesting to analyse this. Furthermore, different institutions have different protocols for para‐aortic and pelvic lymph node dissections resulting in a heterogeneity of operations performed across institutions. If a consensus ever occurs on the role of lymphadenectomy in endometrial cancer then it would be wise to assess separate subgroups but this is not possible currently.
In summary, this study demonstrates that the current evidence is in favour of robotic assisted laparoscopy for endometrial cancer over standard laparoscopy for clinic outcomes but costs are probably greater. To date there are only 99 patients recruited to randomized controlled trials32 and an increase in this number will undoubtedly provide stronger evidence.
CONFLICT OF INTERESTS
Marielle Nobbenhuis and Thomas Ind have proctored for Intuitive Surgical.
ETHICS
As this is a review no ethics was required.
Ind T, Laios A, Hacking M, Nobbenhuis M. A comparison of operative outcomes between standard and robotic laparoscopic surgery for endometrial cancer: A systematic review and meta‐analysis. Int J Med Robotics Comput Assist Surg. 2017;13:e1851 https://doi.org/10.1002/rcs.1851
REFERENCES
- 1. Galaal K, Bryant A, Fisher AD, Al‐Khaduri M, Kew F, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database of Systematic Reviews. 2012;(9): https://doi.org/10.1002/14651858.CD006655.pub2 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 2. Willis SF, Barton D, Ind TE. Laparoscopic hysterectomy with or without pelvic lymphadenectomy or sampling in a high‐risk series of patients with endometrial cancer. Int Seminars Surg Oncol: ISSO. 2006;3:28 https://doi.org/10.1186/1477–7800–3‐28 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rashid TG, Kini M, Ind TE. Comparing the learning curve for robotically assisted and straight stick laparoscopic procedures in surgical novices. Int J Med Robot + Comput Assist Surg: MRCAS. 2010;6(3):306‐310. https://doi.org/10.1002/rcs.333 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 4. Haider JN, Ind TE. Comparison of knot tying in robotic, laparoscopic, and open surgery: Robotic knots as tight as but more secure than open knots. J Gynecol Surg. 2013;29(6):287‐291. [Google Scholar]
- 5. Ind TE, Marshall C, Hacking M, et al. Introducing robotic surgery into an endometrial cancer service ‐‐ a prospective evaluation of clinical and economic outcomes in a UK institution. Int J Med Robot + Comput Assist Surg: MRCAS. 2016;12(1):137‐144. https://doi.org/10.1002/rcs.1651 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lau S, Vaknin Z, Ramana‐Kumar AV, Halliday D, Franco EL, Gotlieb WH. Outcomes and cost comparisons after introducing a robotics program for endometrial cancer surgery. Obstet Gynecol. 2012;119:717‐724. [DOI] [PubMed] [Google Scholar]
- 7. Pilka R, Marek R, Adam T, et al. Systemic inflammatory response after open, laparoscopic and robotic surgery in endometrial cancer patients. Anticancer Res. 2016;36(6):2909‐2922. [PubMed] [Google Scholar]
- 8. Review Manager (RevMan) (program) . 5.3 version. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.: The Nordic Cochrane Centre, 2014.
- 9. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Nat Cancer Instit. 1959;22:719‐748. [PubMed] [Google Scholar]
- 10. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135(11):1301‐1309. [DOI] [PubMed] [Google Scholar]
- 11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13 https://doi.org/10.1186/1471–2288–5‐13 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bell MC, Torgerson J, Seshadri‐Kreaden U, Suttle AW, Hunt S. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol. 2008;111(3):407‐411. https://doi.org/10.1016/j.ygyno.2008.08.022 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 13. Boggess JFG, Cantrell PA, Shafer L, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: Robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199:360. [DOI] [PubMed] [Google Scholar]
- 14. Cardenas‐Goicoechea J, Soto E, Chuang L, et al. Integration of robotics into two established programs of minimally invasive surgery for endometrial cancer appears to decrease surgical complications. J Gynecol Oncol. 2013;24(1):21‐28. https://doi.org/10.3802/jgo.2013.24.1.21 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiou HY, Chiu LH, Chen CH, Yen YK, Chang CW, Liu WM. Comparing robotic surgery with laparoscopy and laparotomy for endometrial cancer management: a cohort study. Int J Surg (London, England). 2015;13:17‐22. https://doi.org/10.1016/j.ijsu.2014.11.015 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 16. Coronado PJ, Herraiz MA, Magrina JF, Fasero M, Vidart JA. Comparison of perioperative outcomes and cost of robotic‐assisted laparoscopy, laparoscopy and laparotomy for endometrial cancer. Europ J Obstet Gynecol Reproduct Biol. 2012;165(2):289‐294. https://doi.org/10.1016/j.ejogrb.2012.07.006 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 17. Corrado G, Cutillo G, Pomati G, et al. Surgical and oncological outcome of robotic surgery compared to laparoscopic and abdominal surgery in the management of endometrial cancer. Euro J Surg Oncol: J Europ Soc Surg Oncol British Assoc Surg Oncol. 2015;41(8):1074‐1081. https://doi.org/10.1016/j.ejso.2015.04.020 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 18. Desille‐Gbaguidi H, Hebert T, Paternotte‐Villemagne J, Gaborit C, Rush E, Body G. Overall care cost comparison between robotic and laparoscopic surgery for endometrial and cervical cancer. Europ J Obstet Gynecol Reproduct Biol. 2013;171(2):348‐352. https://doi.org/10.1016/j.ejogrb.2013.09.025 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 19. Escobar PF, Frumovitz M, Soliman PT, et al. Comparison of single‐port laparoscopy, standard laparoscopy, and robotic surgery in patients with endometrial cancer. Annals Surg Oncol. 2012;19(5):1583‐1588. https://doi.org/10.1245/s10434‐011‐2136‐y (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 20. Estape R, Lambrou N, Estape E, Vega O, Ojea T. Robotic‐assisted total laparoscopic hysterectomy and staging for the treatment of endometrial cancer: A comparison with conventional laparoscopy and abdominal approaches. J Robot Surg. 2012;6(3):199‐205. https://doi.org/10.1007/s11701‐011‐0290‐7 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 21. Fagotti A, Gagliardi ML, Fanfani F, et al. Perioperative outcomes of total laparoendoscopic single‐site hysterectomy versus total robotic hysterectomy in endometrial cancer patients: A multicentre study. Gynecol Oncol. 2012;125(3):552‐555. https://doi.org/10.1016/j.ygyno.2012.02.035 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 22. Fleming ND, Axtell AE, Lentz SE. Operative and anesthetic outcomes in endometrial cancer staging via three minimally invasive methods. J Robot Surg. 2012;6(4):337‐344. https://doi.org/10.1007/s11701‐011‐0319‐y (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 23. Frey MK, Lin JF, Stewart LE, Makaroun L, Panico VJ, Holcomb K. Comparison of two minimally invasive approaches to endometrial cancer staging: A single‐surgeon experience. J Reproduct Med. 2015;60(3–4):127‐134. [PubMed] [Google Scholar]
- 24. Hoekstra AV, Jairam‐Thodla A, Rademaker A, et al. The impact of robotics on practice management of endometrial cancer: Transitioning from traditional surgery. Int J Med Robot. 2009;5(4):392‐401. https://doi.org/10.1002/rcs.268 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 25. Holtz DO, Miroshnichenko G, Finnegan MO, Chernick M, Dunton CJ. Endometrial cancer surgery costs: Robot vs laparoscopy. J Minimal Invas Gynecol. 2010;17(4):500‐503. https://doi.org/10.1016/j.jmig.2010.03.012 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 26. Johnson L, Bunn WD, Nguyen L, Rice J, Raj M, Cunningham MJ. Clinical comparison of robotic, laparoscopic, and open hysterectomy procedures for endometrial cancer patients. J Robot Surg. 2016; https://doi.org/10.1007/s11701‐016‐0651‐3 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 27. Jung YW, Lee DW, Kim SW, et al. Robot‐assisted staging using three robotic arms for endometrial cancer: Comparison to laparoscopy and laparotomy at a single institution. J Surg Oncol. 2010;101(2):116‐121. https://doi.org/10.1002/jso.21436 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 28. Leitao MM Jr, Bartashnik A, Wagner I, et al. Cost‐effectiveness analysis of robotically assisted laparoscopy for newly diagnosed uterine cancers. Obstet Gynecol. 2014;123(5):1031‐1037. https://doi.org/10.1097/aog.0000000000000223 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leitao MM Jr, Briscoe G, Santos K, et al. Introduction of a computer‐based surgical platform in the surgical care of patients with newly diagnosed uterine cancer: Outcomes and impact on approach. Gynecol Oncol. 2012;125(2):394‐399. https://doi.org/10.1016/j.ygyno.2012.01.046 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 30. Leitao MM Jr, Malhotra V, Briscoe G, et al. Postoperative pain medication requirements in patients undergoing computer‐assisted ('Robotic') and standard laparoscopic procedures for newly diagnosed endometrial cancer. Annals Surg Oncol. 2013;20(11):3561‐3567. https://doi.org/10.1245/s10434‐013‐3064‐9 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 31. Lim PC, Kang E, Park DH. A comparative detail analysis of the learning curve and surgical outcome for robotic hysterectomy with lymphadenectomy versus laparoscopic hysterectomy with lymphadenectomy in treatment of endometrial cancer: A case‐matched controlled study of the first one hundred twenty two patients. Gynecol Oncol. 2011;120(3):413‐418. https://doi.org/10.1016/j.ygyno.2010.11.034 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 32. Maenpaa MM, Nieminen K, Tomas EI, Laurila M, Luukkaala TH, Maenpaa JU. Robotic‐assisted vs traditional laparoscopic surgery for endometrial cancer: A randomized controlled trial. Am Jo Obstet Gynecol. 2016;215(5):588 e1‐588 e7. https://doi.org/10.1016/j.ajog.2016.06.005 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 33. Magrina JF, Zanagnolo V, Giles G, Noble BN, Kho RMC, Magtibay PM. Robotic suregery for endometrial cancer: Comparison of peri‐operative outcomes and recurrence with laparoscopy, vaginal/laparoscopy, and laparotomy. Europ J Gynaecol Oncol. 2011;32(5):476‐480. [PubMed] [Google Scholar]
- 34. Manchana T, Puangsricharoen P, Sirisabya N, et al. Comparison of perioperative and oncologic outcomes with laparotomy, and laparoscopic or robotic surgery for women with endometrial cancer. Asian Pacific J Cancer Prevent: APJCP. 2015;16(13):5483‐5488. [DOI] [PubMed] [Google Scholar]
- 35. Martino MA, Shubella J, Thomas MB, et al. A cost analysis of postoperative management in endometrial cancer patients treated by robotics versus laparoscopic approach. Gynecol Oncol. 2011;123(3):528‐531. https://doi.org/10.1016/j.ygyno.2011.08.021 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 36. Mendivil AA, Rettenmaier MA, Abaid LN, et al. A comparison of open surgery, robotic‐assisted surgery and conventional laparoscopic surgery in the treatment of morbidly obese endometrial cancer patients. J Soc Laparoendosc Surg. 2015;19(1):e2014 00001. https://doi.org/10.4293/jsls.2014.00001): (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nevandunsky N, Clark R, Muto M, et al. Robotic assisted, total laparoscopic, and total abdominal hysterectomy for management of uterine cancer. J Cancer Therapy. 2012;3(2):162‐166. https://doi.org/10.4236/jct.2012.32022 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pakish J, Soliman PT, Frumovitz M, et al. A comparison of extraperitoneal versus transperitoneal laparoscopic or robotic para‐aortic lymphadenectomy for staging of endometrial carcinoma. Gynecol Oncol. 2014;132(2):366‐371. https://doi.org/10.1016/j.ygyno.2013.12.019 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seamon LG, Cohn DE, Henretta MS, et al. Minimally invasive comprehensive surgical staging for endometrial cancer: Robotics or laparoscopy? Gynecol Oncol. 2009;113(1):36‐41. https://doi.org/10.1016/j.ygyno.2008.12.005 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 40. Seror J, Bats AS, Huchon C, Bensaid C, Douay‐Hauser N, Lecuru F. Laparoscopy vs robotics in surgical management of endometrial cancer: comparison of intraoperative and postoperative complications. J Minimal Invas Gynecol. 2014;21(1):120‐125. https://doi.org/10.1016/j.jmig.2013.07.015 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 41. Shah NT, Wright KN, Jonsdottir GM, Jorgensen S, Einarsson JI, Muto MG. The feasibility of societal cost equivalence between robotic hysterectomy and alternate hysterectomy methods for endometrial cancer. Obstet Gynecol Int. 2011;2011:570464. https://doi.org/10.1155/2011/570464: (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turner TB, Habib AS, Broadwater G, et al. Postoperative pain scores and narcotic use in robotic‐assisted versus laparoscopic hysterectomy for endometrial cancer staging. J Minimal Invas Gynecol. 2015;22(6):1004‐1010. https://doi.org/10.1016/j.jmig.2015.05.003 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 43. Turunen H, Pakarinen P, Sjoberg J, Loukovaara M. Laparoscopic vs robotic‐assisted surgery for endometrial carcinoma in a centre with long laparoscopic experience. J Obstet Gynaecol: J Instit Obstet Gynaecol. 2013;33(7):720‐724. https://doi.org/10.3109/01443615.2013.812623 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 44. Venkat P, Chen LM, Young‐Lin N, et al. An economic analysis of robotic versus laparoscopic surgery for endometrial cancer: Costs, charges and reimbursements to hospitals and professionals. Gynecol Oncol. 2012;125(1):237‐240. https://doi.org/10.1016/j.ygyno.2011.11.036 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 45. Barrie A, Freeman AH, Lyon L, et al. Classification of postoperative complications in robotic‐assisted compared with laparoscopic hysterectomy for endometrial cancer. J Minimal Invas Gynecol. 2016;23(7):1181‐1188. https://doi.org/10.1016/j.jmig.2016.08.832 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 46. Borgfeldt C, Kalapotharakos G, Asciutto KC, Lofgren M, Hogberg T. A population‐based registry study evaluating surgery in newly diagnosed uterine cancer. Acta Obstetricia et Gynecologica Scandinavica. 2016;95(8):901‐911. https://doi.org/10.1111/aogs.12918 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 47. Chan JK, Gardner AB, Taylor K, et al. Robotic versus laparoscopic versus open surgery in morbidly obese endometrial cancer patients ‐ a comparative analysis of total charges and complication rates. Gynecol Oncol. 2015;139(2):300‐305. https://doi.org/10.1016/j.ygyno.2015.09.006 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 48. Wright JD, Ananth CV, Tergas AI, et al. An economic analysis of robotically assisted hysterectomy. Obstet Gynecol. 2014;123(5):1038‐1048. https://doi.org/10.1097/aog.0000000000000244 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wright JD, Burke WM, Tergas AI, et al. Comparative effectiveness of minimally invasive hysterectomy for endometrial cancer. J Clinic Oncol: Official J Am Soc Clinic Oncol. 2016;34(10):1087‐1096. https://doi.org/10.1200/jco.2015.65.3212 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wright JD, Burke WM, Wilde ET, et al. Comparative effectiveness of robotic versus laparoscopic hysterectomy for endometrial cancer. J Clinic Oncol: Official J Am Soc Clinic Oncol. 2012;30(8):783‐791. https://doi.org/10.1200/jco.2011.36.7508 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zakhari A, Czuzoj‐Shulman N, Spence AR, Gotlieb WH, Abenhaim HA. Laparoscopic and robot‐assisted hysterectomy for uterine cancer: A comparison of costs and complications. Am J Obstet Gynecol. 2015;213(5):665 e1‐665 e7. https://doi.org/10.1016/j.ajog.2015.07.004 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 52. Lim PC, Kang E, Park DH. Learning curve and surgical outcome for robotic‐assisted hysterectomy with lymphadenectomy: Case‐matched controlled comparison with laparoscopy and laparotomy for treatment of endometrial cancer. J Minimal Invas Gynecol. 2010;17(6):739‐748. https://doi.org/10.1016/j.jmig.2010.07.008 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 53. Ind TEJ, Marshall C, Hacking M, Chiu S, Harris M, Nobbenhuis M. The effect of obesity on clinical and economic outcomes in robotic endometrial cancer surgery. Robot Surg: Res Rev. 2017;2017(4):33‐37. https://doi.org/10.2147/RSRR.S123108 (published Online First: Epub Date) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nevis IF, Vali B, Higgins C, Dhalla I, Urbach D, Bernardini MQ. Robot‐assisted hysterectomy for endometrial and cervical cancers: A systematic review. J Robot Surg. 2017;11(1):1‐16. https://doi.org/10.1007/s11701‐016‐0621‐9 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 55. Xie W, Cao D, Yang J, Shen K, Zhao L. Robot‐assisted surgery versus conventional laparoscopic surgery for endometrial cancer: A systematic review and meta‐analysis. J Cancer Res Clinic Oncol. 2016;142(10):2173‐2183. https://doi.org/10.1007/s00432‐016‐2180‐x. (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 56. He H, Zeng D, Ou H, Tang Y, Li J, Zhong H. Laparoscopic treatment of endometrial cancer: Systematic review. J Minimal Invas Gynecol. 2013;20(4):413‐423. https://doi.org/10.1016/j.jmig.2013.01.005 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]
- 57. Balasubramani LM, D. A.; Shepherd JH, Ind TEJ. Differences in hand movements and task completion times between laparoscopic, robotically assisted, and open surgery: An in vitro study. J Robot Surg. 2011;5:137‐140. [DOI] [PubMed] [Google Scholar]
- 58. Yu X, Lum D, Kiet TK, et al. Utilization of and charges for robotic versus laparoscopic versus open surgery for endometrial cancer. J Surg Oncol. 2013;107(6):653‐658. https://doi.org/10.1002/jso.23275 (published Online First: Epub Date) [DOI] [PubMed] [Google Scholar]


