Abstract
In the phase II IM103‐100 study, kidney transplant recipients were first randomized to belatacept more‐intensive‐based (n = 74), belatacept less‐intensive‐based (n = 71), or cyclosporine‐based (n = 73) immunosuppression. At 3‐6 months posttransplant, belatacept‐treated patients were re‐randomized to receive belatacept every 4 weeks (4‐weekly, n = 62) or every 8 weeks (8‐weekly, n = 60). Patients initially randomized to cyclosporine continued to receive cyclosporine‐based immunosuppression. Cumulative rates of biopsy‐proven acute rejection (BPAR) from first randomization to year 10 were 22.8%, 37.0%, and 25.8% for belatacept more‐intensive, belatacept less‐intensive, and cyclosporine, respectively (belatacept more‐intensive vs cyclosporine: hazard ratio [HR] = 0.95; 95% confidence interval [CI] 0.47‐1.92; P = .89; belatacept less‐intensive vs cyclosporine: HR = 1.61; 95% CI 0.85‐3.05; P = .15). Cumulative BPAR rates from second randomization to year 10 for belatacept 4‐weekly, belatacept 8‐weekly, and cyclosporine were 11.1%, 21.9%, and 13.9%, respectively (belatacept 4‐weekly vs cyclosporine: HR = 1.06, 95% CI 0.35‐3.17, P = .92; belatacept 8‐weekly vs cyclosporine: HR = 2.00, 95% CI 0.75‐5.35, P = .17). Renal function trends were estimated using a repeated‐measures model. Estimated mean GFR values at year 10 for belatacept 4‐weekly, belatacept 8‐weekly, and cyclosporine were 67.0, 68.7, and 42.7 mL/min per 1.73 m2, respectively (P<.001 for overall treatment effect). Although not statistically significant, rates of BPAR were 2‐fold higher in patients administered belatacept every 8 weeks vs every 4 weeks.
Keywords: clinical research/practice, clinical trial, glomerular filtration rate (GFR), immunosuppressant – fusion proteins and monoclonal antibodies: belatacept, kidney (allograft) function/dysfunction, kidney transplantation/nephrology, kidney transplantation: living donor
Short abstract
This post hoc analysis of a phase II study shows no difference at 10 years post–kidney transplant in cumulative rates for biopsy‐proven acute rejection between belatacept more‐intensive–, belatacept less‐intensive–, and cyclosporine‐based immunosuppression, and although the rate by subgroup analysis was two‐fold higher with belatacept administered every 8 weeks versus every 4 weeks, the difference was not significant, while the estimated mean glomerular filtration rate was significantly higher in both belatacept groups versus cyclosporine.
Abbreviations
- AE
adverse event
- BENEFIT
Belatacept Evaluation of Nephroprotection and Efficacy as First‐line Immunosuppression Trial
- BENEFIT‐EXT
BENEFIT‐Extended Criteria Donors Trial
- BPAR
biopsy‐proven acute rejection
- CI
confidence interval
- CsA
cyclosporine
- HR
hazard ratio
- LI
less intensive
- MI
more intensive
- PTLD
posttransplant lymphoproliferative disorder
- SD
standard deviation
1. INTRODUCTION
Short‐term outcomes in kidney transplant recipients have improved, with reductions in the incidence of early acute rejection, but there have been only modest improvements in long‐term survival.1 There are multiple causes for the lack of progress in long‐term outcomes, including the adverse effects of calcineurin inhibitors on cardiovascular risk factors, which can lead to premature death with a functioning graft;2, 3 calcineurin inhibitor–associated nephrotoxicity;4, 5, 6 and chronic antibody‐mediated rejection. A calcineurin inhibitor–free immunosuppressive regimen could potentially provide improved efficacy, safety, and preservation of renal function.
Belatacept is a selective T cell co‐stimulation blocker approved in the United States, European Union, and other countries for preventing organ rejection in adult kidney transplant recipients.7, 8 Belatacept was evaluated as part of a calcineurin inhibitor–free immunosuppressive regimen in the phase II IM103‐100 study, in which patients undergoing renal transplantation were randomized to receive 1 of 2 belatacept‐based dosing regimens or cyclosporine (CsA)‐based immunosuppression.9 While the efficacy of belatacept was comparable with CsA, renal function was significantly better in belatacept‐treated vs CsA‐treated patients at 12 months posttransplant.9 The present post hoc analysis compared outcomes at 10 years posttransplant in belatacept‐treated and CsA‐treated patients participating in IM103‐100.
2. METHODS
2.1. Study design
The design of IM103‐100 (NCT00035555) has been described.9 Briefly, IM103‐100 was a 12‐month, open‐label, phase II study of kidney transplant recipients aged ≥18 years. Study participants were recipients of a primary or repeat transplant from a living or deceased donor. Patients were first randomized to receive belatacept more‐intensive (MI)‐based, belatacept less‐intensive (LI)‐based, or CsA‐based immunosuppression, hereafter referred to as the population at first randomization (see Figure 1 for dose schedule). The belatacept LI regimen used in this study differed from the subsequently approved regimen.7, 8 Patients randomized to belatacept MI or belatacept LI were re‐randomized at 6 months or 3 months posttransplant, respectively, to receive belatacept 5 mg/kg either every 4 weeks or every 8 weeks, hereafter referred to as the population at second randomization (Figure 1); the belatacept 4‐weekly and 8‐weekly dosing schedules were unique to this study. Patients first randomized to CsA received CsA‐based immunosuppression throughout the study. All patients received basiliximab induction, mycophenolate mofetil, and corticosteroids. If approved by the treating physician, patients were eligible to continue the treatment to which they had been assigned at the second randomization beyond 12 months.10
Figure 1.
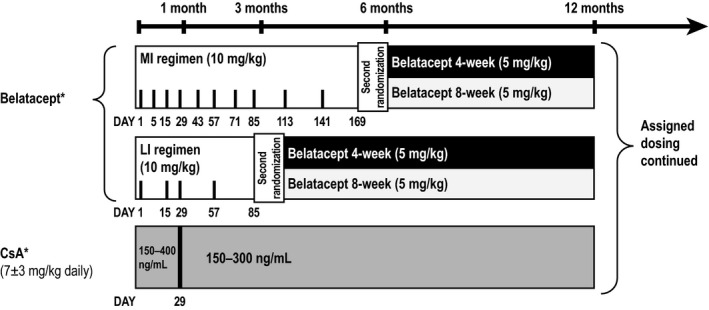
Study design. CsA, cyclosporine; LI, less intensive; MI, more intensive. *All patients received basiliximab induction, mycophenolate mofetil, and corticosteroid taper
This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. The institutional review board/ethics committee at each site approved the study protocol. All patients provided written informed consent.
2.2. Outcomes and statistics
This intent‐to‐treat post hoc analysis examined efficacy and safety in all evaluable patients at 10 years posttransplant. The evaluable population was composed of patients who were alive and observable at 10 years postrandomization or who had died or experienced graft loss by year 10.
Biopsy‐proven acute rejection (BPAR) was defined as histologically confirmed acute rejection by the central pathologist, regardless of the reason for biopsy. The cumulative event rates for BPAR were calculated for each regimen using the Kaplan‐Meier method and compared using a log‐rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were derived using Cox regression. Time to death or graft loss was determined using the Kaplan‐Meier method and compared between regimens using a log‐rank test.
Renal function was estimated using the 6‐variable Modification of Diet in Renal Disease equation.11 Estimated mean GFR and 95% CIs were determined from month 1 to month 120 (year 10) using a repeated‐measures model with an unstructured covariance matrix. This model included time, treatment, and a time‐by‐treatment interaction. No further adjustment was made for other potentially confounding covariates. Time was regarded as a categorical variable (intervals of 3 months up to month 12 and every 6 months thereafter). Missing data were assumed to be missing at random. A slope‐based model was also used to determine whether there was a difference between the slope for each belatacept regimen and the slope for the CsA regimen. The slope‐based model assumed that the relationship between GFR values over time was linear. The difference between slopes was tested using a contrast statement within the SAS model (SAS software, version 9.2; SAS Institute, Cary, NC). Time was regarded as a continuous variable, treatment as a fixed effect, and the intercept and time as random effects; no further adjustment was made for other potentially confounding covariates. Sensitivity analyses were performed in which GFR values that were missing due to death or graft loss were imputed as zero; the same models were used as for the analyses without imputation.
Adverse events (AEs) were mapped to terms from the Medical Dictionary for Regulatory Activities version 15.0 (MedDRA MSSO, McLean, VA) and expressed as incidence rates adjusted per 100 person‐years of exposure to assigned treatment.
3. RESULTS
3.1. Population at first randomization
3.1.1. Efficacy
Of the 218 patients enrolled, 74 were initially randomized to belatacept MI, 71 to belatacept LI, and 73 to CsA (Figure 2). The cumulative event rate for BPAR from first randomization to year 10 was 22.8% for belatacept MI, 37.0% for belatacept LI, and 25.8% for CsA (Figure 3A). The HR for the comparison of belatacept MI with CsA was 0.95 (95% CI 0.47−1.92; P = .89). The HR for the comparison of belatacept LI with CsA was 1.61 (95% CI 0.85−3.05; P = .15). Grades of BPAR are summarized in Table S1.
Figure 2.
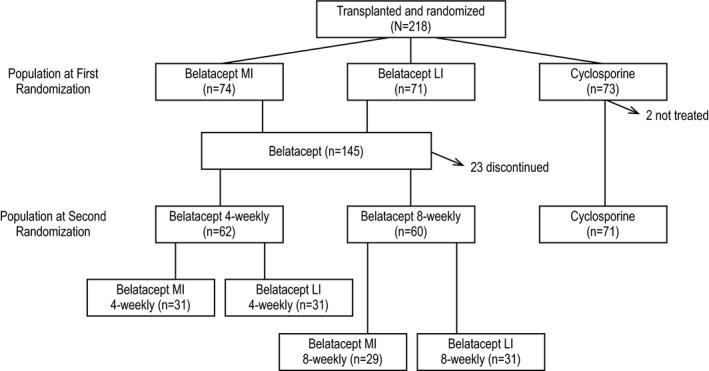
Patient disposition. LI, less intensive; MI, more intensive
Figure 3.
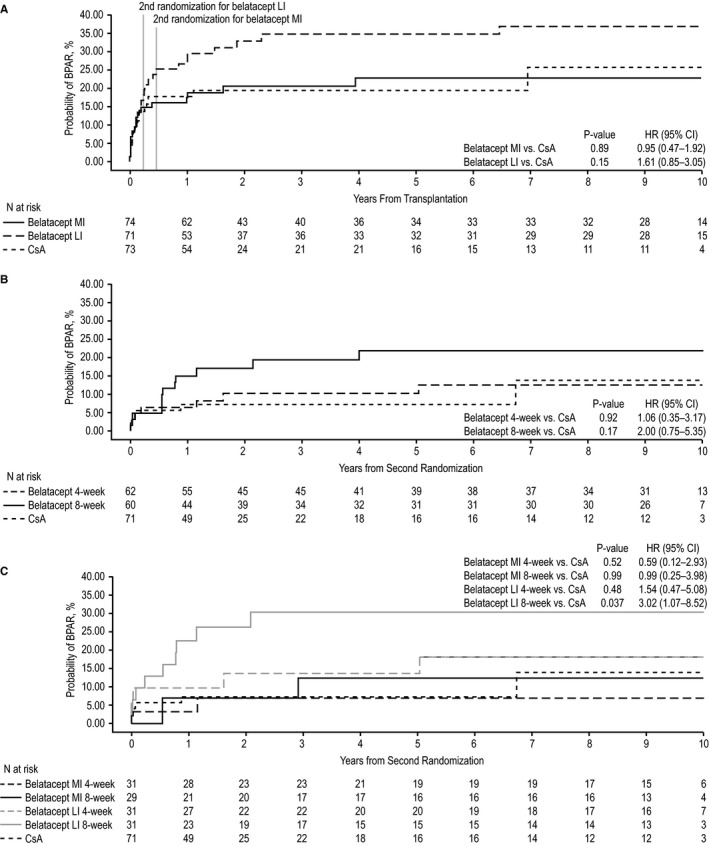
Biopsy‐proven acute rejection from randomization to year 10 in (A) the population at first randomization, (B) the population at second randomization, and (C) the population at second randomization stratified by belatacept dosing frequency. BPAR, biopsy‐proven acute rejection; CI, confidence interval; CsA, cyclosporine; HR, hazard ratio; LI, less intensive; MI, more intensive
Death or graft loss status at 10 years posttransplant was assessed in 37.8% (28 of 74) of belatacept MI‐treated patients, 25.4% (18 of 71) of belatacept LI‐treated, and 15.1% (11 of 73) of CsA‐treated patients. The Kaplan‐Meier estimated rate of death or graft loss at year 10 was 24.0% for belatacept MI, 6.1% for belatacept LI, and 16.8% for CsA (Figure 4A). The HR for the comparison of belatacept MI with CsA was 0.95 (95% CI 0.38‐2.36; P = .91); the HR for the comparison of belatacept LI with CsA was 0.24 (95% CI 0.06‐0.91; P = .037). Adjudicated causes of death and graft loss are summarized in Tables S2 and S3, respectively.
Figure 4.
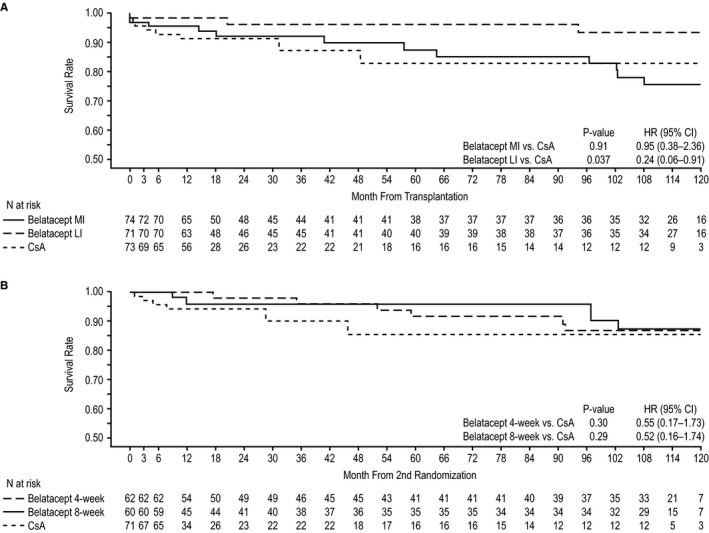
Time to death or graft loss from randomization to year 10 in (A) the population at first randomization and (B) the population at second randomization. CI, confidence interval; CsA, cyclosporine; HR, hazard ratio; LI, less intensive; MI, more intensive
3.1.2. Renal function
Estimated mean GFR was stable over 10 years for both belatacept‐based regimens, but declined for the CsA‐based regimen. Estimated mean GFR values at year 10 for belatacept MI‐treated, belatacept LI‐treated, and CsA‐treated patients were 66.3, 66.6, and 42.4 mL/min per 1.73 m2, respectively. The estimated differences in GFR significantly favored each belatacept‐based regimen vs the CsA‐based regimen (P < .001 for overall treatment effect) (Figure 5A). Per the slope‐based model (and relative to month 1), belatacept MI‐treated and belatacept LI‐treated patients experienced estimated mean GFR gains of +0.25 (95% CI −0.38 to 0.87) and +0.38 (95% CI −0.25 to 1.00) mL/min per 1.73 m2 per year, respectively. Patients randomized to CsA had an estimated mean decline in GFR equivalent to −1.14 (95% CI −2.06 to −0.22) mL/min per 1.73 m2 per year. Compared with CsA, the GFR slopes diverged over time for belatacept MI (P = .015) and belatacept LI (P = .008). The sensitivity analysis yielded similar results (Figure S1).
Figure 5.
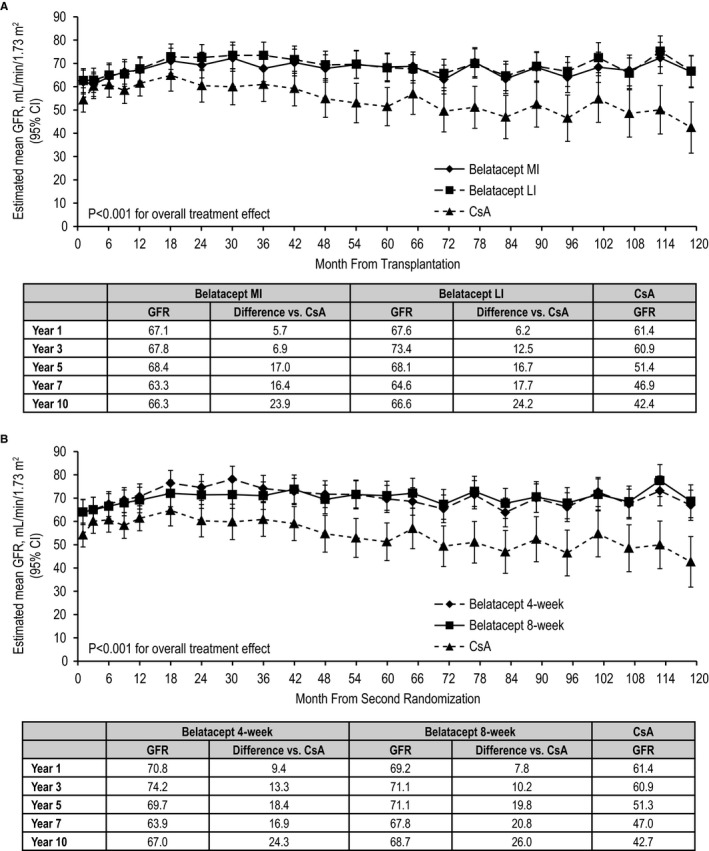
Estimated mean GFR over 10 years in (A) the population at first randomization and (B) the population at second randomization (repeated‐measures modeling without imputation). CsA, cyclosporine; LI, less intensive; MI, more intensive
3.1.3. Safety
Serious AEs occurred in 83.8% (62 of 74) of belatacept MI‐treated, 87.3% (62 of 71) of belatacept LI‐treated, and 69.9% (51 of 73) of CsA‐treated patients. The incidence rates of serious infections, any‐grade viral infections, any‐grade fungal infections, and malignancies per 100 person‐years of treatment exposure are summarized in Table 1. Four patients experienced posttransplant lymphoproliferative disorder (PTLD) by year 10 (belatacept MI, n = 3; belatacept LI, n = 0; CsA, n = 1). The 3 cases of PTLD in belatacept MI‐treated patients had their onset on days 112, 262, and 396 posttransplantation.
Table 1.
Cumulative incidence rates of selected safety events adjusted per 100 person‐years of treatment exposure in the population at first randomization
| Belatacept MI (n = 74) | Belatacept LI (n = 71) | CsA (n = 73) | |
|---|---|---|---|
| Serious infectionsa | 10.36 | 6.71 | 14.99 |
| Any‐grade fungal infectionb | 7.89 | 4.23 | 3.74 |
| Any‐grade viral infectionb | 17.53 | 16.89 | 14.92 |
| Any malignancya | 3.14 | 2.54 | 3.01 |
CsA, cyclosporine; LI, less intensive; MI, more intensive.
The exposure (patient‐years) of a patient was calculated from the randomization date to the event date, to the date of last follow‐up, or to year 10, whichever was earliest.
The exposure (patient‐years) of a patient was calculated from the randomization date to the event date, to the date of last dose of study medication plus 56 d, or to year 10, whichever was earliest.
3.2. Subgroup analysis of the population at second randomization
3.2.1. Efficacy
Between the first and second randomizations, 23 belatacept‐treated patients discontinued the study, and 2 patients randomized to CsA did not receive treatment. Consequently, 62 belatacept‐treated patients were subsequently randomized to receive belatacept every 4 weeks and 60 were subsequently randomized to receive belatacept every 8 weeks. Seventy‐one CsA‐treated patients continued with CsA‐based immunosuppression (Figure 2). The cumulative event rate for BPAR from second randomization to year 10 was 11.1% for belatacept 4‐weekly, 21.9% for belatacept 8‐weekly, and 13.9% for CsA (Figure 3B). Although not statistically significant, the HR was 2.00 (95% CI 0.75‐5.35; P = .17) for the comparison of belatacept 8‐weekly with CsA. The HR for the comparison of belatacept 4‐weekly with CsA was 1.06 (95% CI 0.35‐3.17; P = .92). Grades of BPAR are presented in Table S4.
In total, 21.0% (13 of 62) of patients assigned to belatacept 4‐weekly, 20.0% (12 of 60) of patients assigned to belatacept 8‐weekly, and 14.1% (10 of 71) of CsA‐treated patients were assessed for death or graft loss at 10 years posttransplant. The Kaplan‐Meier estimated rate of death or graft loss at year 10 was 13.1% for belatacept 4‐weekly, 12.5% for belatacept 8‐weekly, and 14.4% for CsA (Figure 4B). The HR for the comparison of belatacept 4‐weekly with CsA was 0.55 (95% CI 0.17‐1.73; P = .30); the HR for the comparison of belatacept 8‐weekly with CsA was 0.52 (95% CI 0.16‐1.74; P = .29).
3.2.2. Renal function
Estimated mean GFR was stable over 10 years for both belatacept‐based regimens (4‐weekly or 8‐weekly), but declined for CsA‐based treatment. Estimated mean GFR values at year 10 for the belatacept 4‐weekly, belatacept 8‐weekly, and CsA groups were 67.0, 68.7, and 42.7 mL/min per 1.73 m2, respectively. The estimated differences in GFR significantly favored each belatacept‐based regimen vs the CsA‐based regimen (P < .001 for overall treatment effect) (Figure 5B). Per the slope‐based model (and relative to month 1), patients administered belatacept every 4 weeks had an estimated mean GFR change of −0.08 (95% CI −0.68 to 0.52) mL/min per 1.73 m2 per year. Patients randomized to belatacept every 8 weeks had an estimated mean GFR gain of +0.50 (95% CI −0.16 to 1.15) mL/min per 1.73 m2 per year, respectively. Patients randomized to CsA had an estimated mean GFR decline equivalent to −1.15 (95% CI −2.07 to −0.23) mL/min per 1.73 m2 per year. The GFR slopes diverged over time between belatacept 8‐weekly and CsA (P = .004), but not between belatacept 4‐weekly and CsA (P = .06). The sensitivity analysis yielded similar results (Figure S2).
3.2.3. Safety
Serious AEs occurred in 67.7% (42 of 62) of patients receiving belatacept 4‐weekly, 73.3% (44 of 60) of patients receiving belatacept 8‐weekly, and 60.6% (43 of 71) of CsA‐treated patients. The incidence rates of serious infections, any‐grade viral infections, any‐grade fungal infections, and malignancies per 100 person‐years of treatment exposure are presented in Table S5.
3.3. Biopsy‐proven acute rejection from the time of second randomization by treatment arm and belatacept dosing frequency
BPAR rates from the time of second randomization to year 10 were stratified by treatment arm and belatacept dosing frequency. Of those patients initially randomized to belatacept MI, 31 were subsequently randomized to receive belatacept MI every 4 weeks and 29 were subsequently randomized to receive belatacept MI every 8 weeks. The corresponding patient numbers in the original belatacept LI treatment group were 31 and 31, respectively. The cumulative event rate for BPAR from second randomization to year 10 was 6.9% for belatacept MI 4‐weekly, 12.4% for belatacept MI 8‐weekly, 15.2% for belatacept LI 4‐weekly, 30.4% for belatacept LI 8‐weekly, and 13.9% for CsA (Figure 3C). The HR for the comparison of belatacept LI 8‐weekly with CsA was statistically significant (HR 3.02; 95% CI 1.07‐8.52; P = .037).
4. DISCUSSION
In this post hoc analysis of the IM103‐100 study, no statistically significant differences in the Kaplan‐Meier cumulative event rates for BPAR were observed at 10 years posttransplant for the belatacept MI, belatacept LI, or CsA regimens overall. The comparable rates of acute rejection for belatacept‐based vs CsA‐based immunosuppression were preserved over 10 years: acute rejection rates in the belatacept treatment arms (6%‐7%) were noninferior to that observed in the CsA treatment arm (8%) at 6 months posttransplant in IM103‐100.10 Most acute rejection events in belatacept‐treated patients occur within 6 months of treatment initiation.10, 12, 13 In IM103‐100, most BPAR events in the population at first randomization were reported by month 6 (28 of 41, 68.3%).
The efficacy and safety of belatacept have also been examined in 2 phase III studies of de novo kidney transplant recipients: Belatacept Evaluation of Nephroprotection and Efficacy as First‐line Immunosuppression Trial (BENEFIT) and BENEFIT‐Extended Criteria Donors (BENEFIT‐EXT). Like the present phase II study, patients in BENEFIT and BENEFIT‐EXT were randomized to 1 of 2 belatacept dosing regimens (MI and LI) or CsA. However, it is difficult to compare the results from these phase III studies to IM103‐100 because enrollment to this phase II study was substantially lower and both the definition of acute rejection and the belatacept LI regimen administered were different. With regard to the latter, patients allocated to belatacept LI‐based immunosuppression in IM103‐100 received 5 doses of belatacept 10 mg/kg during the induction phase, while patients randomized to belatacept LI in BENEFIT12 and BENEFIT‐EXT13 received 6 induction doses of belatacept 10 mg/kg. The additional dose in BENEFIT and BENEFIT‐EXT was administered on day 4 posttransplant to optimize saturation of CD80/CD86 ligands and blockade of CD28 activation. Despite these caveats, in analyses performed at 1 and 7 years posttransplant, rates of acute rejection were higher for belatacept‐treated vs CsA‐treated patients participating in BENEFIT12, 14, 15 and similar for belatacept‐treated vs CsA‐treated patients participating in BENEFIT‐EXT.13, 16
At 10 years posttransplant in IM103‐100, the risk of death or graft loss was similar for belatacept MI‐based and CsA‐based immunosuppression, but was lower by 76% in belatacept LI‐treated vs CsA‐treated patients. The improved patient and graft survival seen with belatacept LI vs belatacept MI at 10 years posttransplant in IM103‐100 further supports the United States Food and Drug Administration approval of the reduced‐dose regimen.
Long‐term use of belatacept was not associated with discernible nephrotoxicity in IM103‐100; estimated mean GFR was significantly higher for belatacept MI‐based and belatacept LI‐based vs CsA‐based immunosuppression at 10 years posttransplant. Further, renal function was stable in belatacept‐treated patients, with marginal GFR gains of +0.25−0.38 mL/min per 1.73 m2 per year, while GFR in CsA‐treated patients declined by −1.14 mL/min per 1.73 m2 per year. Estimated yearly gains in GFR were greater in belatacept‐treated patients participating in BENEFIT (1.30‐1.39 mL/min per 1.73 m2 per year) and BENEFIT‐EXT (1.45‐1.51 mL/min per 1.73 m2 per year).14, 16
Unlike BENEFIT and BENEFIT‐EXT wherein maintenance doses of belatacept were only administered every 4 weeks, the dose‐finding IM103‐100 study analyzed outcomes following a second randomization of belatacept‐treated patients to either every 4‐week or every 8‐week belatacept dosing. As observed in the population at first randomization, cumulative rates of BPAR in the population at second randomization did not differ statistically across treatment regimens at 10 years posttransplant, although rates of BPAR were 2‐fold higher in patients administered belatacept every 8 weeks vs every 4 weeks. Among belatacept‐treated patients in the population at second randomization, most BPAR events occurred by month 6 (13 of 18, 72.2%). In a further subgroup analysis in which study participants were stratified by both belatacept dosing regimen and frequency, the cumulative BPAR rate was greatest in the subset of patients who received belatacept LI every 8 weeks. From the available data, it is not possible to determine whether the increased rate of BPAR in the belatacept LI every 8‐week treatment arm was attributable to differences in the timing of belatacept conversion and/or dosing frequency. However, conversion of some patients to 8‐weekly administration could offer both logistical and practical advantages. A precision medicine study, Precision Medicine Offers Belatacept Monotherapy (PROBE; NCT02939365), will seek to convert patients to belatacept monotherapy. Following conversion to belatacept monotherapy, the dosing regimen in eligible patients—as determined via biomarker assessment—will be extended from every 4 weeks to every 8 weeks.
Time to death or graft loss was similar for belatacept 4‐weekly, belatacept 8‐weekly, and CsA. Renal function was significantly greater for both belatacept groups (4‐weekly and 8‐weekly) vs CsA. Notably, estimated mean GFR at year 10 was similar for the belatacept 4‐weekly (67.0 mL/min per 1.73 m2) and 8‐weekly (68.7 mL/min per 1.73 m2) regimens. Although extended‐interval belatacept dosing during the maintenance phase has the potential to reduce healthcare costs and ease administrative burdens, 8‐weekly dosing may lead to trough levels of belatacept that result in reduced CD86‐receptor occupancy and, consequently, less efficacy in some patients.17 Collectively, the data suggest that belatacept 8‐weekly dosing may not be sufficient for all patients, if initiated 3−6 months posttransplant. Additional studies may be warranted to determine the optimal timing of and to identify patients most likely to benefit from less frequent administration of belatacept.
With up to 10 years of follow‐up, IM103‐100 represents the longest randomized prospective clinical trial evaluating a non‐calcineurin inhibitor–based immunosuppressive regimen conducted in kidney transplant recipients. However, these exploratory analyses should be interpreted with caution as they were conducted post hoc with no adjustment for multiplicity testing, and patient numbers were small (both because of the original sample size and the rate of attrition over time). In addition, we assumed that confounders were randomly dispersed, which may not have been the case with such a long duration of follow‐up.
In conclusion, in both the population at first randomization and the population at second randomization, there were no statistically significant differences in the cumulative rates of BPAR between belatacept‐treated and CsA‐treated patients, although BPAR rates were 2‐fold higher for the belatacept 8‐weekly vs 4‐weekly regimen. In addition, belatacept‐based immunosuppression was associated with significantly higher levels of renal function, which were sustained over 10 years. These data support the long‐term trends observed at 7 years posttransplant in the phase III BENEFIT and BENEFIT‐EXT studies.14, 16 Importantly, no new safety signals emerged for belatacept with up to 10 years of exposure. These post hoc analyses suggest that extending belatacept dosing from every 4 weeks to every 8 weeks during the maintenance phase is not associated with an increased risk of death or graft loss. However, further study is needed.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. UMK, MP, and LY are full time employees of BMS. AD has received research support from Bristol‐Myers Squibb, and his spouse was once an employee of Bristol‐Myers Squibb. GB: research grant and expert board with Novartis, expert board with Astellas and BMS, participation in 1 Sandoz symposium. FV reports grant/research support with Astellas, Alexicon, Immucor, BMS, and Genentech. CL received grant funding for studies conducted at Emory from Bristol‐Myers Squibb. GG and JG report that they have nothing to disclose.
Supporting information
ACKNOWLEDGMENTS
This study was sponsored by Bristol‐Myers Squibb. Medical writing and editorial assistance for this manuscript were provided by Tiffany DeSimone, PhD, of CodonMedical, an Ashfield Company, and were funded by Bristol‐Myers Squibb.
Vincenti F, Blancho G, Durrbach A, et al. Ten‐year outcomes in a randomized phase II study of kidney transplant recipients administered belatacept 4‐weekly or 8‐weekly. Am J Transplant. 2017;00:1–9. https://doi.org/10.1111/ajt.14452
REFERENCES
- 1. Lamb KE, Lodhi S, Meier‐Kriesche HU. Long‐term renal allograft survival in the United States: A critical reappraisal. Am J Transplant. 2011;11:450‐462. [DOI] [PubMed] [Google Scholar]
- 2. Krämer BK, Zülke C, Kammerl MC, et al. Cardiovascular risk factors and estimated risk for CAD in a randomized trial comparing calcineurin inhibitors in renal transplantation. Am J Transplant. 2003;3:982‐987. [DOI] [PubMed] [Google Scholar]
- 3. Ligtenberg G, Hené RJ, Blankestijn PJ, Koomans HA. Cardiovascular risk factors in renal transplant patients: Cyclosporin A versus tacrolimus. J Am Soc Nephrol. 2001;12:368‐373. [DOI] [PubMed] [Google Scholar]
- 4. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326‐2333. [DOI] [PubMed] [Google Scholar]
- 5. Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931‐940. [DOI] [PubMed] [Google Scholar]
- 6. Gaston RS. Chronic calcineurin inhibitor nephrotoxicity: Reflections on an evolving paradigm. Clin J Am Soc Nephrol. 2009;4:2029‐2034. [DOI] [PubMed] [Google Scholar]
- 7. Squibb Bristol‐Myers. Belatacept (NULOJIX) Prescribing Information. Princeton, NJ: Bristol‐Myers Squibb Company; 2014. [Google Scholar]
- 8. Squibb Bristol‐Myers. Belatacept (NULOJIX) Summary of Product Characteristics. Uxbridge, UK: Bristol‐Myers Squibb Pharma; 2011. [Google Scholar]
- 9. Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770‐781. [DOI] [PubMed] [Google Scholar]
- 10. Vincenti F, Blancho G, Durrbach A, et al. Five‐year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol. 2010;21:1587‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461‐470. [DOI] [PubMed] [Google Scholar]
- 12. Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept‐based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535‐546. [DOI] [PubMed] [Google Scholar]
- 13. Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT‐EXT study). Am J Transplant. 2010;10:547‐557. [DOI] [PubMed] [Google Scholar]
- 14. Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long‐term outcomes in kidney transplantation. N Engl J Med. 2016;374:333‐343. [DOI] [PubMed] [Google Scholar]
- 15. Vincenti F, Grinyo J, Rostaing L, et al. Belatacept patients had superior graft survival compared with cyclosporine patients: Final results from BENEFIT [abstract O215]. Transplant Int. 2015;28(Suppl 4):1‐133. [Google Scholar]
- 16. Durrbach A, Pestana JM, Florman S, et al. Long‐term outcomes in belatacept‐ versus cyclosporine‐treated recipients of extended criteria donor kidneys: Final results from BENEFIT‐EXT, a phase III randomized study. Am J Transplant. 2016;16:3192‐3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Latek R, Fleener C, Lamian V, et al. Assessment of belatacept‐mediated costimulation blockade through evaluation of CD80/86‐receptor saturation. Transplantation. 2009;87:926‐933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


