Abstract
The aim of this study was to analyse the effect of age at onset on the long‐term clinical, social and global outcomes of schizophrenia through a systematic review and a meta‐analysis. Original studies were searched from Web of Science, PsycINFO, Pubmed and Scopus, as well as manually. Naturalistic studies with at least a 2‐year follow‐up were included. Of the 3509 search results, 81 articles fulfilled the inclusion criteria. The meta‐analysis was performed in Stata as a random‐effect analysis with correlation coefficients between age at onset and the outcomes (categorized into remission, relapse, hospitalization, positive symptoms, negative symptoms, total symptoms, general clinical outcome, employment, social/occupational functioning and global outcome). There was a statistically significant (P < .05) correlation between younger age at onset and more hospitalizations (number of studies, n = 9; correlation, r = 0.17; 95% confidence interval, CI 0.09–0.25), more negative symptoms (n = 7; r = 0.14; 95% CI 0.01–0.27), more relapses (n = 3; r = 0.11; 95% CI 0.02–0.20), poorer social/occupational functioning (n = 12; r = 0.15; 95% CI 0.05–0.25) and poorer global outcome (n = 13; r = 0.14; 95% CI 0.07–0.22). Other relationships were not significant. This was the first systematic review of the effects of age at onset on the long‐term outcomes of schizophrenia. The results show that age at onset has a small, but significant impact on some of the outcomes of schizophrenia.
Keywords: age of onset, schizophrenia, patient outcome assessment, meta‐analysis
1. INTRODUCTION
Age at onset is one of the most commonly analysed predictor of outcome in schizophrenia but the results obtained in different original studies are far from conclusive: some have found a lower age at onset to have a positive effect on outcomes (Bland & Parker, 1978; Bland, Parker, & Orn, 1976; Stefanopoulou et al., 2011; Stephens, Richard, & McHugh, 1997), some a negative effect (Altamura, Bassetti, Sassella, Salvadori, & Mundo, 2001; Juola, Miettunen, Veijola, Isohanni, & Jääskeläinen, 2013) and others no effect at all (Üçok, Gorwood, & Karadayı, 2012). In their systematic review, Menezes and colleagues studied age at onset alongside other predictors of “good” and “bad” outcomes, functional recovery, intermediate outcome, relapse, readmission, Global Assessment of Functioning (GAF) Scale and employment/education in first‐episode psychosis. They concluded that there was no support for age at onset having an influence on any of the studied outcomes. (Menezes, Arenovich, & Zipursky, 2006) However, in original long‐term studies, earlier age at onset has been associated with lower probability of symptomatic remission (Juola et al., 2013) and having more hospital admissions after over 10 years since onset (Rabinowitz, Levine, & Häfner, 2006). Even with these inconsistent findings, it is not uncommon that in clinical textbooks onset early in life is regarded as a predictor for a poorer outcome (Cohen, 2003; Semple & Smyth, 2013).
There are several dimensions of outcomes in schizophrenia. These include clinical outcomes such as symptomatology and remission as well as broader outcomes including vocational functioning and global outcome (Færden, Nesvåg, & Marder, 2008; Liberman, Kopelowicz, Ventura, & Gutkind, 2002; Shrivastava, Johnston, Shah, & Bureau, 2010). The clinical and social/functional dimensions of outcome do not recover at the same rate (Harding, Brooks, Ashikaga, Strauss, & Breier, 1987; Strauss & Breier, 1987) and hence, they should be evaluated separately when reporting outcome. Inconsistency in the definitions of outcomes has made it difficult to draw conclusions and to make comparisons between different studies (Liberman et al., 2002).
The aim of this study was to analyse the effect of age at onset of psychosis on the long‐term outcomes of schizophrenia through a systematic review and meta‐analysis. The follow‐up period was set to a minimum of 2 years in order to focus on the long‐term outcome and the different outcome dimensions were evaluated separately. To our knowledge, this is the first systematic review on association between age at onset and long‐term outcomes of schizophrenia that includes both first‐episode patients and those with a longer history of illness.
2. MATERIALS AND METHODS
2.1. Data collection
We applied the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) guidelines for systematic reviews and meta‐analyses (Stroup et al., 2000). The search included 4 databases: Web of Science, PsycInfo, Pubmed and Scopus. The search was conducted in August 2015. The search query was the following: ("age at onset" OR "onset age" OR "age of onset") AND (schizoaffective OR schizophr* OR psychosis OR psychoses) AND (outcome OR symptoms OR hospitalization OR work OR occupation* OR employment OR prognosis OR remission OR relapse). The search was limited to title, abstract and key words. No language or time limit was applied and non‐English articles were translated when necessary. The search query was approved by an information scientist. In addition, a manual literature search was performed, utilizing material from a previous meta‐analysis carried out by our study group. This material consisted of 5009 articles that had been analysed to identify predictors for recovery. In the analysis, age at onset had been one of the searched predictors.(2013 Nov) The following information was collected from the included original studies: the used diagnostic system, setting of the study (inpatient/outpatient), duration of schizophrenia at baseline, sample size, definition and source of age at onset, onset age, follow‐up time, used outcome measures, and the main result of the study and any other especially noteworthy information. This is the first presentation of the review protocol of this meta‐analysis.
2.2. Study selection
The included articles were required to meet all the following criteria:
The follow‐up period or length of illness was a minimum of 2 years.
At least 80% of the study sample had a schizophrenia‐spectrum diagnosis (schizophrenia, schizophreniform disorder, schizoaffective disorder and delusional disorder) and individuals with schizophrenia had to be included (ie, the sample did not consist only of cases with schizophrenia spectrum disorders other than schizophrenia).
The study was not an intervention or clinical trial, as these limit the representativeness of the samples.
The used diagnosis system was reported (any version of Diagnostic and Statistical Manual of Mental Disorders [DSM], International Classification of Diseases [ICD] or Research Diagnostic Criteria [RDC]).
The study included an evaluation of the connection between age at onset and the outcome of schizophrenia: clinical outcome (positive and negative symptoms, total symptoms, remission and hospitalization), social/occupational outcome (frequency and quality of social connections, occupational functioning and employment) or global outcome (combined occupational or social and clinical course).
The sample was not biased based on age at onset (ie, it did not include only early or late onset patients). This was required as we wanted the effect sizes to be comparable between the studies.
The sample was not biased based on the severity or the outcome of the illness (eg, the sample did not include only chronic patients or patients with good/bad outcome). This was required as we wanted the effect sizes to be comparable between the studies.
2.3. Definitions of outcomes and age at onset
In line with previous research, the outcome categories were selected to cover the different dimensions of clinical, social, occupational and global outcomes of schizophrenia (Penttilä, Jääskeläinen, Hirvonen, Isohanni, & Miettunen, 2014).
Clinical outcomes included remission, relapse, hospitalization, different symptomatic outcomes and general clinical outcome. Remission was most often defined as absence of symptoms or at most mild symptoms. Most studies utilized the symptomatic severity criteria of Andreasen et al. (2005) and many studies also applied their duration criteria. Relapse was mostly defined as worsening of symptoms. Mere hospitalization was not viewed here as a relapse but was included in the hospitalization outcome. Other hospitalization outcomes included number and length of hospitalizations. The symptomatic outcomes were negative symptoms, positive symptoms and total symptoms, which were measured by, for example, Scale for the Assessment of Negative Symptoms (SANS), Scale for the Assessment of Positive Symptoms (SAPS) and Positive and Negative Syndrome Scale (PANSS). General clinical outcome included outcomes defined as good or bad clinical outcome and was measured by, for example, Clinical Global Impression (CGI) Scale.
Social/occupational outcomes included measures of social or occupational capacity including, for example, the ability to provide for oneself, work performance and length of work history, and the quality and measure of social contacts. Specific measures included, for example, Social and Occupational Functioning Assessment Scale (SOFAS) and subscales from Disability Assessment Scale (DAS) and the Strauss‐Carpenter Outcome Scale. Employment status was studied separately.
Global outcomes encompass outcomes that have clinical, social/occupational and functional aspects in a combined measure. These were measured by, for example, The Strauss‐Carpenter Outcome Scale and GAF Scale.
Individual symptoms, quality of life and suicidality were excluded from the outcomes because they were not widely used outcomes in the included studies. The connection between age at onset and cognition has previously been studied through a meta‐analysis and hence it was not included in this study (Rajji, Ismail, & Mulsant, 2009). Thus, there were altogether 10 different outcomes included in the analysis.
Age at onset can be defined in different ways. The different definitions used in original studies can be seen from Table S1, Supporting Information. They include definitions such as age at first admission, age at first (positive) psychotic symptoms and age at first contact with healthcare professionals. As these measures have been identified to correlate and to occur within 6 to 18 months of each other (DeLisi, 1992; DeLisi, Goldin, Maxwell, Kazuba, & Gershon, 1987; DeLisi et al., 1991), we accepted the different definitions used in individual studies.
2.4. Statistical methods
We expected heterogeneity in the relationship between age at onset and the different outcomes. Thus, a random‐effects model was used to pool estimates of effect sizes. In a random‐effects analysis, each study was weighted by the inverse of its variance and the between‐studies variance. (Sterne, 2009) Estimation of the relationship between age at onset and the outcome variables was made using correlation coefficients. If the results were reported from more than 1 follow‐up point, the results from the longer follow‐up time were used in the analysis. With classified variables, the average value of the classes was used. Unadjusted results were preferred if they were given in the original studies, as adjusted results were reported less often and with varying covariates. Effect measures other than correlations were transformed into correlations (r) using formulas by Rosenthal, Cooper, and Hedges (1994) and Rosenthal, Rosnow, and Rubin (2000). In this study, positive correlation indicates that patients with lower age at onset have poorer outcomes. Correlation coefficients can be interpreted as indicating a small 0.10, a moderate 0.30 or a large 0.50 effect (Cohen, 1992).
When several papers were available on the same or overlapping cohorts reporting results of the same outcome, we selected the results from the studies that had the longest follow‐up. If the follow‐up periods were equal, we chose the largest sample size. If 2 papers reported identical results in identical effect measures, we reported the paper published first as the reference. If they reported identical results in different effect size measures we chose the 1 that reported correlations over other measures. This selection was not necessary when the papers reported different outcomes using the same or overlapping cohorts. The reported sample sizes per outcome have been calculated by taking this into consideration.
Heterogeneity was studied using the I 2 statistic. The value of I 2 ranges from 0% to 100% and it reflects the proportion of the total variation between studies which is beyond chance. 25% is low, 50% is moderate and 75% high (Higgins, Thompson, Deeks, & Altman, 2003). The statistical significance of heterogeneity was tested with the χ 2 test. Age at onset, gender, length of illness at follow‐up, study design (retrospective/prospective) and sample type (first‐episode/not first‐episode sample) were studied as confounding factors through meta‐regression. Possible publication bias was examined using Egger's test for small‐study effects (Egger, Davey Smith, Schneider, & Minder, 1997). Stata version 11 was used in all analyses (StataCorp, 2009; Sterne, 2009). An alpha level of .05 was used in all statistical tests.
3. RESULTS
After removal of duplicates there were 3509 search results, of which 79 were found through manual searching. All the abstracts were analysed by 2 authors (J.I. and J.M.) and potentially relevant studies, altogether 274, were read in full by 1 author (J.I.). The excluded articles were reviewed by 2 authors (H.K. and J.I.). All problematic studies were discussed with the other authors. Ultimately, 81 papers fulfilled the inclusion criteria for the systematic review and 46 were included in the meta‐analysis. The flow chart of study inclusion is presented in Figure 1.
Figure 1.
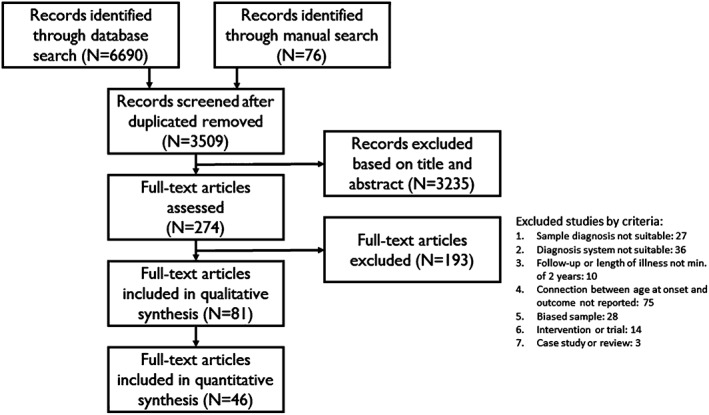
Flow diagram of the selection of studies (n, number of articles).
3.1. Study characteristics and quality
There was high variability between the studies in all measures. The included studies were published between 1974 and 2015. A minority were retrospective studies (n = 19) and of the remainder the follow‐up period ranged from 1 to 31 years. A minority of the studies (n = 37) included a sample of first‐onset, first‐admission or recent‐onset schizophrenia patients, whereas the rest included patients with a duration of schizophrenia at the baseline ranging from 4.5 to 22 years. A minority of the papers did not report a definition for onset age (n = 29). The sample size at the end of the follow‐up period varied considerably, ranging from 28 to 22 395, and was less than a 100 in 43 papers. The most frequently used diagnostic system was DSM in its different versions (DSM‐II, n = 2; DSM‐III, n = 6; DSM‐III‐R, n = 15; and DSM‐IV, n = 22). ICD was also widely used (ICD‐9, n = 12; ICD‐8, n = 2; and ICD‐10, n = 4), and a few studies used RDC (n = 3) or multiple different systems (n = 15). Details of the included papers are presented in Table S1. The outcomes reported in each included paper and their inclusion in the meta‐analysis can be seen in Table S2 and full references for the papers in Appendix S1.
3.2. Association between age at onset and clinical outcomes
3.2.1. Remission
There were 17 papers that studied remission in relation to age at onset, with a total population of 13 081 patients when excluding papers reporting from the same samples. In most studies, the effect of onset age on remission was not significant. The main portion of the study population, 11 078, came from The Worldwide‐Schizophrenia Outpatient Health Outcomes (W‐SOHO) study, in which there was no statistical difference between the age at first treatment of the patients achieving clinical remission and those who did not (Haro et al., 2011). Six studies reported at most a modest significant relationship between age at onset and achieving remission. There were no statistically significant relationships between age at onset and remission in meta‐analysis (n = 6; r = 0.05; 95% CI −0.02 to 0.12; P = .13).
3.2.2. Relapse
There were 6 papers that reported results for relapse, with a total population of 8329 patients. The largest portion of the patients was from the European part of the W‐SOHO study, in which there was no relationship between age at onset and relapsing (Haro et al., 2006). Three studies reported a significant relationship between age at onset and relapsing: Ascher‐Svanum et al. (2010) found that those with relapse during the 1‐year follow‐up period were over 2 years younger at onset than those not having relapse and (Altamura et al.’s (2001) results showed a 2.9‐year difference. Eaton's results in India, however, showed that age at onset above 25 increased the risk of relapsing (Eaton, Thara, Federman, & Tien, 1998). In meta‐analysis, there was a small but statistically significant relationship between age at onset and relapse (n = 3; r = 0.11; 95% CI 0.02–0.20; P = .01).
3.2.3. Hospitalization
There were altogether 19 papers that reported results for hospitalization outcomes. The total patient population was 78 817. There were 10 studies showing a statistically significant relationship between age at onset and hospitalization outcomes. The presented results, including some relatively strong correlations, suggested that earlier age at onset results in more frequent or longer hospitalizations. For example, Eaton et al. 1992a and 1992b conducted an extensive study in Australia, England, United States and Denmark and their results showed a significant relationship between earlier age at onset and increased risk of re‐hospitalization in all countries except the United States. In Denmark, individuals with first admission after age 60 had only 39% of the risk of re‐hospitalization compared with those with first admission before age 20. In meta‐analysis, age at onset had a small but statistically significant relationship with hospitalization (n = 9; r = 0.17; 95% CI 0.09–0.25; P < .001).
3.2.4. Symptomatic outcomes
Nine studies reported results for positive symptoms, with a total sample of 2359. Only 1 study reported a statistically significant relationship. Negative symptoms and age at onset were studied in 14 papers with 2595 patients. Four studies reported a significant relationship with effect sizes ranging from weak to modest. Fewer papers, altogether 7, investigated total symptoms with a sample of 892 patients. Only 2 studies with a combined sample of 160 individuals revealed a significant relationship between age at onset and total symptoms. There were no statistically significant relationships between age at onset and positive symptoms (n = 4; r = 0.05; 95% CI −0.05 to 0.15; P = .33) or total symptoms (n = 3; r = 0.25; 95% CI −0.05 to 0.56; P = .1). A lower age at onset had a small but significant relationship with more negative symptoms (n = 7; r = 0.14; 95% CI 0.01–0.27; P = .04).
3.2.5. General clinical outcome
There were 17 papers with a total sample of 1725 patients reporting results for general clinical outcome. Approximately half of the included studies reported a statistically significant relationship. In these studies, age at onset was almost equally reported as having a positive or a negative relationship with the outcome. According to this, there was no statistically significant relationship between age at onset and the outcome in meta‐analysis (n = 8; r = 0.06; 95% CI −0.07 to 0.19; P = .36).
3.3. Association between age at onset and occupational and social outcomes
3.3.1. Employment status
One of the 4 included studies reported significant results showing that an earlier onset decreased the percentage of time employed (Westermeyer & Harrow, 1984). The sample size for this outcome was rather small, including only 575 individuals. There was no statistically significant relationship between age at onset and employment status in meta‐analysis (n = 3; r = 0.15; 95% CI −0.11 to 0.41; P = .28).
3.3.2. Social or occupational capacity
There were a total of 25 papers reporting social or occupational capacity outcomes, of which 15 reported a statistically significant relationship indicating that lower age at onset predicts a poorer outcome in this outcome category. The total sample of patients was 14 192, including over half of the patients from the W‐SOHO study that reported a small, but significant relationship between age at onset and the outcome (Haro et al., 2011). One study reported in 2 papers indicated an opposite significant relationship between the variables (Bland & Parker, 1978; Bland et al., 1976). Stronger (r ≥ 0.30) correlations were also reported (Boato, Caputo, Comazzi, & Ferrari, 1995; Bora, Eryavuz, Kayahan, Sungu, & Veznedaroglu, 2006; Greig, Bell, Kaplan, & Bryson, 2000). In meta‐analysis, a lower age at onset predicted poorer social/occupational functioning (n = 12; r = 0.15; 95% CI 0.05–0.25; P = .002).
3.4. Association between age at onset and global outcomes
There were 23 papers in this category, of which 10 reported a significant relationship between age at onset and the measured outcome. The reported correlations ranged from 0.004 to 0.3, showing variability between the included studies. The total patient population, 1999, was smaller than for most of the measured outcomes. In meta‐analysis, lower age at onset predicted poorer global outcome (n = 13; r = 0.14; 95% CI 0.07–0.22; P < 0.001).
Overall correlations for each outcome are summarized in a forest plot in Figure 2. The forest plots for each individual outcome can be found in Figures [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link].
Figure 2.

Correlations between age at onset and outcome categories. Positive correlation indicates that a younger age at onset results in poorer outcome (CI, conference interval; n, number of studies).
3.5. Heterogeneity in meta‐analysis
Heterogeneity was statistically significant for hospitalization (I 2 = 95.1%, P < 0.001), negative symptoms (I 2 = 63.5%, P = .01), total symptoms (I 2 = 88.8%, P < 0.001), general clinical outcome (I 2 = 69.7%, P = .002), social/occupation functioning (I 2 = 80.8%, P < 0.001) and global outcome (I 2 = 47.7%, P = .03). It was not significant for relapse (I 2 = 11.4%, P = .32), remission (I 2 = 39.6%, P = .14) or positive symptoms (I 2 = 0.0%, P = .69).
3.6. Covariates in meta‐regression and publication bias
Gender, length of illness at follow‐up, study design (retrospective/prospective) and sample type (first‐episode/not first‐episode sample) were studied as confounding factors through meta‐regression. Neither length of illness at follow‐up nor study design affected the correlations between age at onset and the studied outcomes. Male gender had a confounding effect (t = 2.69, P = .036): the correlation between lower age at onset and poorer general clinical outcome became significant in samples with a higher proportion of males. First‐episode sample type in turn weakened the relationship between hospitalization and age at onset (t = −3.56, P < 0.001). No statistically significant bias was found through the Egger's test for small study effects for the identified significant predictors with an alpha level of .05 or .10.
4. DISCUSSION
Based on this study, there is a statistically significant association between age at onset and the outcome of schizophrenia, though the association is small and less important than presented in some clinical textbooks (Cohen, 2003; Semple & Smyth, 2013). There was no significant correlation between age at onset and clinical outcomes of remission, general clinical outcome, positive symptoms or total symptoms. We found a small but statistically significant association between lower age at onset and more hospitalizations, more negative symptoms, more relapses, poorer social/occupational functioning and poorer global outcome. Male gender had a confounding effect, according to which with a higher proportion of males in the sample the correlation between earlier age at onset and poorer general clinical outcome became significant. Thus, the effect of age at onset is mostly independent from gender but it is possible that males with an early onset have a poorer clinical outcome. First‐episode sample type in turn weakened the relationship between hospitalization and age at onset, which may be because non‐first‐episode samples may include more chronic patients who are in more need of inpatient care.
4.1. Comparison with earlier studies on predictors of outcomes in schizophrenia
The effect sizes in systematic reviews focusing on other predictors of outcomes in schizophrenia have been similar to those identified here. For example, longer duration of untreated psychosis (DUP) (Penttilä et al., 2014), family history of psychosis (Käkelä et al., 2014) and current substance use (Large, Mullin, Gupta, Harris, & Nielssen, 2014) all had small but significant association with poorer outcomes. These factors may be in interplay with one another. Patients with an earlier onset have a longer DUP (Ballageer, Malla, Manchanda, Takhar, & Haricharan, 2005) and a higher family loading or family history of psychosis (Esterberg, Trotman, Holtzman, Compton, & Walker, 2010; Suvisaari, Haukka, Tanskanen, & Lonnqvist, 1998) and more substance or drug abuse (Cantwell & Group, 2003). It is also possible that duration of illness partly explains the connection between age at onset and poorer outcomes. Our study included both first‐episode and cross‐sectional studies, and hence in the latter the role of age at onset could be masked by the length of illness. There was only 1 original study in which this had been taken into account, and in this study age at onset remained a significant predictor of the measured outcome even when length of illness was controlled for (Kao & Liu, 2010). Thus, further research on the topic is needed. Nevertheless, an earlier age at onset results in longer length of illness considering the patient's life span, and thus results in higher costs for society due to loss in productive employment and health care costs.
Even though the identified associations between earlier age of onset and poorer outcomes are weak, the results are nevertheless scientifically and clinically important.
4.2. Strengths and limitations of the study and risk of bias
To our knowledge, this is the first systematic review and meta‐analysis of age at onset and the outcome of schizophrenia. The performed literature search was extensive, as multiple databases were used in addition to a manual search. However, it is possible that some studies reporting results for the relationship between age at onset and outcomes for schizophrenia were not included, if for example this association was not the main focus of the study.
There are a few other limitations in this study: the low number of included studies in some outcome categories, controlling of confounding factors, and heterogeneity and sampling in the included original studies.
The most important limitation is the small number of studies in some outcome categories making the meta‐analysis regarding these outcomes less reliable. For example, only 3 studies were included in clinical outcome categories of total symptoms, relapse and employment. A larger number of studies would have improved the reliability of the results. Publication bias was studied through Egger's test for the small study effect. It should be noted that the power of the test is low when the number of studies is small. Hence, a publication bias remains possible.
We studied the effects of multiple covariates, but there might be other confounding factors in addition to those included in our meta‐regression analysis. For example, social and occupational functioning may be dependent on pre‐morbid adjustment, which may be dependent on the age at onset. Other possible confounders are DUP and substance abuse. Therefore, the possible bias due to confounding factors should be considered when interpreting the results of this meta‐analysis.
There are also limitations within the included original studies themselves. Heterogeneity between the studies varied in different outcome categories. There is rather low variability in how age at onset can be defined, and so the heterogeneity is probably due to variation in the studied outcomes. Differences in defining and measuring the outcomes as well as in the used methodologies may have affected our results and conclusions. Especially outcomes including social aspects showed some variation. As the results were presented with various effect size measures, we were forced to convert them into correlations. These conversions may result in inaccuracy, as the used equations assume that the variables are normally distributed.
We aimed to reduce the possibility of selection bias by excluding studies with biased samples. Previously, studies focusing on early onset schizophrenia have reported poorer long‐term clinical outcomes compared with adult onset (Biswas, Malhotra, Malhotra, & Gupta, 2006; Schmidt, Blanz, Dippe, Koppe, & Lay, 1995; Vourdas, Pipe, Corrigall, & Frangou, 2003). These studies are often based on samples from different populations, as early onset patients and adult onset patients are often treated in different facilities. Hence, there is a possible selection bias (Amminger et al., 2011) and thus these studies were excluded from this study. Neither did we include studies focusing only on childhood nor adolescence schizophrenia, in order to ensure comparability of effect sizes between studies. Extreme onset ages are rare and might not be present in the samples of the original studies that we included especially when the sample sizes were small. The results might have been different if the sample sizes had been larger and more childhood onset and late onset schizophrenia cases had been included in the samples of the original studies. Thus, our results concern mostly adult and late adolescence onset schizophrenia.
4.3. Implications
Social and occupational functioning in schizophrenia is unfortunately poor (Perälä et al., 2008). There is a suggestion that especially employment outcomes have not improved and on the contrary might have slightly weakened during recent years. For example, in Finland, when studying employment rates of schizophrenia patients discharged in the years 1986, 1990 and 1994, the younger cohorts had consistently lower employment rates compared to the older ones (Honkonen, Stengård, Virtanen, & Salokangas, 2007). It has been suggested that an earlier onset disrupts the social and cognitive development of a person and hence results in weaker social and occupational skills (Häfner, Nowotny, Löffler, an der Heiden, & Maurer, 1995; Hafner et al., 2003). In a recent meta‐analysis, it was indeed discovered that an earlier onset age is associated with poorer cognition in schizophrenia (Rajji et al., 2009).
Our results confirm the importance of early intervention services, including occupational rehabilitation. Interventions supporting social recovery and employment should be offered to patients with schizophrenia, as stated in different national clinical guidelines (Gaebel, Riesbeck, & Wobrock, 2011). Especially early intervention services, focusing usually on younger patients, are becoming more widely offered and there has been an upsurge in research on such services (Behan, Masterson, & Clarke, 2016). In studies focusing on interventions, a younger age of onset has been associated with poorer pre‐morbid functioning and more severe baseline illness in schizophrenia: a lower onset age is related to having more negative symptoms (Ballageer et al., 2005; Pencer, Addington, & Addington, 2005), impaired social functioning (Hui et al., 2014; Vourdas et al., 2003) and poorer work history (Hui et al., 2014; Mueser, Salyers, & Mueser, 2001) at baseline as well as poorer pre‐morbid functioning in the form of, for example, education (Ballageer et al., 2005; Hui et al., 2014).
Overall, a lower age at onset may result in poorer baseline functioning especially regarding work and social adjustment, although this connection may dilute over time as a result of the intervention programmes. In intervention studies that report later outcomes, there are fewer differences between early and late onset participants. For example, age at onset was not relevant for risk of relapse in a 3‐year follow‐up (Caseiro et al., 2012) or for achieving symptomatic remission in a 2‐year follow‐up, (Addington & Addington, 2008) and the earlier described baseline differences in negative symptoms, employment and social functioning for early onset participants disappeared during a 2‐year follow‐up (Mueser et al., 2001; Pencer et al., 2005). Early onset participants even showed better global, social and vocational functional outcomes after 7.4 years since onset compared to participants with an adult onset (Amminger et al., 2011). Fewer results indicate that a deteriorating illness course is related to an earlier disease onset even in interventional settings (Addington & Addington, 2005). Based on this and on our results, the importance of vocational rehabilitation and supported employment may be especially important among individuals with early onset of schizophrenia, as they possibly benefit most from these kinds of interventions.
5. CONCLUSIONS
This was the first systematic review of the effects of age at onset on a wide variety of long‐term outcomes of schizophrenia. The results show that age at onset has a small, although significant impact on some of the outcomes of schizophrenia.
Supporting information
Table S1. Table of the characteristics and quality of the included studies. For full references, see Appendix S1.
Table S2. References and reported outcomes. x indicates that the paper includes outcome in this category, (m) indicates that the presented results are included in the meta‐analysis and bold indicates that the presented results are statistically significant in the original study. For full references, see Appendix S1.
Appendix S1. Full references of included studies.
Figure S1. Relapse.
Figure S2. Remission.
Figure S3. Hospitalization.
Figure S4. Positive symptoms.
Figure S5. Negative symptoms.
Figure S6. Total symptoms.
Figure S7. General clinical outcome.
Figure S8. Employment.
Figure S9. Social/occupational functioning.
Figure S10. Global outcome.
ACKNOWLEDGMENTS
This work was supported by Academy of Finland (grant numbers 268336 and 278286), NARSAD: The Brain and Behavior Research Foundation, the Sigrid Jusélius Foundation, the Northern Finland Health Care Support Foundation and the European Union's Horizon 2020 research and innovation programme (grant agreement number 643552). We thank information scientist, Dr. Noora Hirvonen for her advice in performing the literature search.
Conflict of interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Immonen J, Jääskeläinen E, Korpela H and Miettunen J. Age at onset and the outcomes of schizophrenia: A systematic review and meta‐analysis. Early Intervention in Psychiatry. 2017;11:453–460. https://doi.org/10.1111/eip.12412
Funding information Academy of Finland, Grant/Award number: 268336, 278286; NARSAD: The Brain and Behavior Research Foundation; Sigrid Jusélius Foundation; Northern Finland Health Care Support Foundation; European Union's Horizon 2020 Research and Innovation Programme, Grant/Award number: 643552.
REFERENCES
- Addington, J. , & Addington, D. (2005). Patterns of premorbid functioning in first episode psychosis: Relationship to 2‐year outcome. Acta Psychiatrica Scandinavica, 112(1), 40–46. [DOI] [PubMed] [Google Scholar]
- Addington, J. , & Addington, D. (2008). Symptom remission in first episode patients. Schizophrenia Research, 106(2), 281–285. [DOI] [PubMed] [Google Scholar]
- Altamura, A. , Bassetti, R. , Sassella, F. , Salvadori, D. , & Mundo, E. (2001). Duration of untreated psychosis as a predictor of outcome in first‐episode schizophrenia: A retrospective study. Schizophrenia Research, 52(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Amminger, G. P. , Henry, L. P. , Harrigan, S. M. , Harris, M. G. , Alvarez‐Jimenez, M. , Herrman, H. , et al. (2011). Outcome in early‐onset schizophrenia revisited: findings from the Early Psychosis Prevention and Intervention Centre long‐term follow‐up study. Schizophrenia Research, 131(1), 112–119. [DOI] [PubMed] [Google Scholar]
- Andreasen, N. C. , Carpenter, W. T. , Kane, J. M. , Lasser, R. A. , Marder, S. R. , & Weinberger, D. R. (2005). Remission in schizophrenia: Proposed criteria and rationale for consensus. The American Journal of Psychiatry, 162(3), 441–449. [DOI] [PubMed] [Google Scholar]
- Ascher‐Svanum, H. , Zhu, B. , Faries, D. E. , Salkever, D. , Slade, E. P. , Peng, X. , et al. (2010). The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry, 10, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballageer, T. , Malla, A. , Manchanda, R. , Takhar, J. , & Haricharan, R. (2005). Is adolescent‐onset first‐episode psychosis different from adult onset? Journal of the American Academy of Child and Adolescent Psychiatry, 44(8), 782–789. [DOI] [PubMed] [Google Scholar]
- Behan, C. , Masterson, S. , & Clarke, M. (2016). Systematic review of the evidence for service models delivering early intervention in psychosis outside the stand‐alone centre. Early Intervention in Psychiatry, doi: 10.1111/eip.12334. [DOI] [PubMed] [Google Scholar]
- Biswas, P. , Malhotra, S. , Malhotra, A. , & Gupta, N. (2006). A comparative study of clinical correlates in schizophrenia with onset in childhood, adolescence and adulthood. Journal of Indian Association for Child and Adolescent Mental Health, 2(1), 18–30. [DOI] [PubMed] [Google Scholar]
- Bland, R. C. , & Parker, J. H. (1978). Prognosis in schizophrenia: Prognostic predictors and outcome. Archives of General Psychiatry, 35(1), 72–77. [DOI] [PubMed] [Google Scholar]
- Bland, R. C. , Parker, J. H. , & Orn, H. (1976). Prognosis in schizophrenia: A ten‐year follow‐up of first admissions. Archives of General Psychiatry, 33(8), 949–954. [DOI] [PubMed] [Google Scholar]
- Boato, P. , Caputo, A. , Comazzi, M. , & Ferrari, L. (1995). Premorbid functioning and prognosis of chronic schizophrenia. New Trends in Experimental and Clinical Psychiatry, 11, 75–78. [Google Scholar]
- Bora, E. , Eryavuz, A. , Kayahan, B. , Sungu, G. , & Veznedaroglu, B. (2006). Social functioning, theory of mind and neurocognition in outpatients with schizophrenia: Mental state decoding may be a better predictor of social functioning than mental state reasoning. Psychiatry Research, 145(2), 95–103. [DOI] [PubMed] [Google Scholar]
- Cantwell, R. , & Group, S. C. S. (2003). Substance use and schizophrenia: Effects on symptoms, social functioning and service use. British Journal of Psychiatry, 182, 324–329. [DOI] [PubMed] [Google Scholar]
- Caseiro, O. , Pérez‐Iglesias, R. , Mata, I. , Martínez‐Garcia, O. , Pelayo‐Terán, J. M. , Tabares‐Seisdedos, R. , et al. (2012). Predicting relapse after a first episode of non‐affective psychosis: A three‐year follow‐up study. Journal of Psychiatric Research, 46(8), 1099–1105. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1992). A power primer. Psychological Bulletin, 112(1), 155. [DOI] [PubMed] [Google Scholar]
- Cohen, B. J. (2003). Theory and practice of psychiatry. New York: Oxford University Press. [Google Scholar]
- DeLisi, L. E. (1992). The significance of age of onset for schizophrenia. Schizophrenia Bulletin, 18(2), 209–215. [DOI] [PubMed] [Google Scholar]
- DeLisi, L. E. , Goldin, L. R. , Maxwell, M. E. , Kazuba, D. M. , & Gershon, E. S. (1987). Clinical features of illness in siblings with schizophrenia or schizoaffective disorder. Archives of General Psychiatry, 44(10), 891–896. [DOI] [PubMed] [Google Scholar]
- DeLisi, L. E. , Hoff, A. L. , Schwartz, J. E. , Shields, G. W. , Halthore, S. N. , Gupta, S. M. , et al. (1991). Brain morphology in first‐episode schizophrenic‐like psychotic patients: A quantitative magnetic resonance imaging study. Biological Psychiatry, 29(2), 159–175. [DOI] [PubMed] [Google Scholar]
- Eaton, W. W. , Bilker, W. , Haro, J. M. , Herrman, H. , Mortensen, P. B. , Freeman, H. , et al. (1992b). Long‐term course of hospitalization for schizophrenia. Part II: Change with passage of time. Schizophrenia Bulletin, 18(2), 229. [DOI] [PubMed] [Google Scholar]
- Eaton, W. W. , Mortensen, P. B. , Herrman, H. , Freeman, H. , Bilker, W. , Burgess, P. , et al. (1992a). Long‐term course of hospitalization for schizophrenia: Part I: Risk for rehospitalization. Schizophrenia Bulletin, 18(2), 217. [DOI] [PubMed] [Google Scholar]
- Eaton, W. W. , Thara, R. , Federman, E. , & Tien, A. (1998). Remission and relapse in schizophrenia: The Madras Longitudinal Study. Journal of Nervous and Mental Disease, 186(6), 357–363. [DOI] [PubMed] [Google Scholar]
- Egger, M. , Davey Smith, G. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg, M. L. , Trotman, H. D. , Holtzman, C. , Compton, M. T. , & Walker, E. F. (2010). The impact of a family history of psychosis on age‐at‐onset and positive and negative symptoms of schizophrenia: A meta‐analysis. Schizophrenia Research, 120(1), 121–130. [DOI] [PubMed] [Google Scholar]
- Færden, A. , Nesvåg, R. , & Marder, S. R. (2008). Definitions of the term ‘recovered’ in schizophrenia and other disorders. Psychopathology, 41(5), 271–278. [DOI] [PubMed] [Google Scholar]
- Gaebel, W. , Riesbeck, M. , & Wobrock, T. (2011). Schizophrenia guidelines across the world: A selective review and comparison. International Review of Psychiatry, 23(4), 379–387. [DOI] [PubMed] [Google Scholar]
- Greig, T. C. , Bell, M. D. , Kaplan, E. , & Bryson, G. (2000). Object relations and reality testing in early‐and late‐onset schizophrenia. Journal of Clinical Psychology, 56(4), 505–517. [DOI] [PubMed] [Google Scholar]
- Hafner, H. , Maurer, K. , Loffler, W. , an der Heiden, W. , Hambrecht, M. , & Schultze‐Lutter, F. (2003). Modeling the early course of schizophrenia. Schizophrenia Bulletin, 29(2), 325–340. [DOI] [PubMed] [Google Scholar]
- Häfner, H. , Nowotny, B. , Löffler, W. , an der Heiden, W. , & Maurer, K. (1995). When and how does schizophrenia produce social deficits? European Archives of Psychiatry and Clinical Neuroscience, 246(1), 17–28. [DOI] [PubMed] [Google Scholar]
- Harding, C. M. , Brooks, G. W. , Ashikaga, T. , Strauss, J. S. , & Breier, A. (1987). The Vermont longitudinal study of persons with severe mental illness, II: Long‐term outcome of subjects who retrospectively met DSM‐III criteria for schizophrenia. The American Journal of Psychiatry, 144(6), 727–735. [DOI] [PubMed] [Google Scholar]
- Haro, J. M. , Novick, D. , Bertsch, J. , Karagianis, J. , Dossenbach, M. , & Jones, P. B. (2011). Cross‐national clinical and functional remission rates: Worldwide Schizophrenia Outpatient Health Outcomes (W‐SOHO) study. British Journal of Psychiatry, 199(3), 194–201. [DOI] [PubMed] [Google Scholar]
- Haro, J. M. , Novick, D. , Suarez, D. , Alonso, J. , Lepine, J. P. , Ratcliffe, M. , et al. (2006). Remission and relapse in the outpatient care of schizophrenia: Three‐year results from the Schizophrenia Outpatient Health Outcomes study. Journal of Clinical Psychopharmacology, 26(6), 571–578. [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkonen, T. , Stengård, E. , Virtanen, M. , & Salokangas, R. K. (2007). Employment predictors for discharged schizophrenia patients. Social Psychiatry and Psychiatric Epidemiology, 42(5), 372–380. [DOI] [PubMed] [Google Scholar]
- Hui, C. L. , Li, A. W. , Leung, C. , Chang, W. , Chan, S. K. , Lee, E. H. , et al. (2014). Comparing illness presentation, treatment and functioning between patients with adolescent‐and adult‐onset psychosis. Psychiatry Research, 220(3), 797–802. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen, E. , Juola, P. , Hirvonen, N. , McGrath, J. J. , Saha, S. , Isohanni, M. , et al. (2013 Nov). A systematic review and meta‐analysis of recovery in schizophrenia. Schizophrenia Bulletin, 39(6), 1296–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juola, P. , Miettunen, J. , Veijola, J. , Isohanni, M. , & Jääskeläinen, E. (2013). Predictors of short–and long‐term clinical outcome in schizophrenic psychosis—The Northern Finland 1966 Birth Cohort study. European Psychiatry, 28(5), 263–268. [DOI] [PubMed] [Google Scholar]
- Käkelä, J. , Panula, J. , Oinas, E. , Hirvonen, N. , Jääskeläinen, E. , & Miettunen, J. (2014). Family history of psychosis and social, occupational and global outcome in schizophrenia: A meta‐analysis. Acta Psychiatrica Scandinavica, 130(4), 269–278. [DOI] [PubMed] [Google Scholar]
- Kao, Y. C. , & Liu, Y. P. (2010). Effects of age of onset on clinical characteristics in schizophrenia spectrum disorders. BMC Psychiatry, 10, 63. doi:10.1186/1471‐244X‐10‐63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large, M. , Mullin, K. , Gupta, P. , Harris, A. , & Nielssen, O. (2014). Systematic meta‐analysis of outcomes associated with psychosis and co‐morbid substance use. The Australian and New Zealand Journal of Psychiatry, 48(5), 418–432. [DOI] [PubMed] [Google Scholar]
- Liberman, R. P. , Kopelowicz, A. , Ventura, J. , & Gutkind, D. (2002). Operational criteria and factors related to recovery from schizophrenia. International Review of Psychiatry, 14(4), 256–272. [Google Scholar]
- Menezes, N. , Arenovich, T. , & Zipursky, R. (2006). A systematic review of longitudinal outcome studies of first‐episode psychosis. Psychological Medicine, 36(10), 1349–1362. [DOI] [PubMed] [Google Scholar]
- Mueser, K. T. , Salyers, M. P. , & Mueser, P. R. (2001). A prospective analysis of work in schizophrenia. Schizophrenia Bulletin, 27(2), 281–296. [DOI] [PubMed] [Google Scholar]
- Pencer, A. , Addington, J. , & Addington, D. (2005). Outcome of a first episode of psychosis in adolescence: A 2‐year follow‐up. Psychiatry Research, 133(1), 35–43. [DOI] [PubMed] [Google Scholar]
- Penttilä, M. , Jääskeläinen, E. , Hirvonen, N. , Isohanni, M. , & Miettunen, J. (2014). Duration of untreated psychosis as predictor of long‐term outcome in schizophrenia: Systematic review and meta‐analysis. British Journal of Psychiatry, 205(2), 88–94. [DOI] [PubMed] [Google Scholar]
- Perälä, J. , Saarni, S. I. , Ostamo, A. , Pirkola, S. , Haukka, J. , Härkänen, T. , et al. (2008). Geographic variation and sociodemographic characteristics of psychotic disorders in Finland. Schizophrenia Research, 106(2), 337–347. [DOI] [PubMed] [Google Scholar]
- Rabinowitz, J. , Levine, S. Z. , & Häfner, H. (2006). A population based elaboration of the role of age of onset on the course of schizophrenia. Schizophrenia Research, 88(1), 96–101. [DOI] [PubMed] [Google Scholar]
- Rajji, T. K. , Ismail, Z. , & Mulsant, B. H. (2009). Age at onset and cognition in schizophrenia: meta‐analysis. British Journal of Psychiatry, 195(4), 286–293. [DOI] [PubMed] [Google Scholar]
- Rosenthal, R. , Cooper, H. , & Hedges, L. (1994). Parametric measures of effect size In Cooper H. & Hedges L. (Eds.), The handbook of research synthesis (pp. 231–244). New York: Russell Sage Foundation. [Google Scholar]
- Rosenthal, R. , Rosnow, R. L. , & Rubin, D. B. (2000). Contrasts and effect sizes in behavioral research: A correlational approach. New York: Cambridge University Press. [Google Scholar]
- Schmidt, M. , Blanz, B. , Dippe, A. , Koppe, T. , & Lay, B. (1995). Course of patients diagnosed as having schizophrenia during first episode occurring under age 18 years. European Archives of Psychiatry and Clinical Neuroscience, 245(2), 93–100. [DOI] [PubMed] [Google Scholar]
- Semple, D. , & Smyth, R. (2013). Oxford handbook of psychiatry (3rd ed.). Oxford, England: Oxford University Press. [Google Scholar]
- Shrivastava, A. , Johnston, M. , Shah, N. , & Bureau, Y. (2010). Redefining outcome measures in schizophrenia: Integrating social and clinical parameters. Current Opinion in Psychiatry, 23(2), 120–126. [DOI] [PubMed] [Google Scholar]
- StataCorp, L. (2009). Stata version 11.0. College Station, TX: StataCorp LP. [Google Scholar]
- Stefanopoulou, E. , Lafuente, A. R. , Fonseca, A. S. , Keegan, S. , Vishnick, C. , & Huxley, A. (2011). Global assessment of psychosocial functioning and predictors of outcome in schizophrenia. International Journal of Psychiatry in Clinical Practice, 15(1), 62–68. [DOI] [PubMed] [Google Scholar]
- Stephens, J. H. , Richard, P. , & McHugh, P. R. (1997). Long‐term follow‐up of patients hospitalized for schizophrenia, 1913 to 1940. Journal of Nervous and Mental Disease, 185(12), 715–721. [DOI] [PubMed] [Google Scholar]
- Sterne J. A. C. (Ed.). (2009). Meta‐analysis: An updated collection from the Stata journal (1st ed.). College Station, TX: Stata Press. [Google Scholar]
- Strauss, J. S. , & Breier, A. (1987). The Vermont longitudinal study of persons with severe mental illness. I: Methodology, study sample, and overall status 32 years later. The American Journal of Psychiatry, 144(6), 718–726. [DOI] [PubMed] [Google Scholar]
- Stroup, D. F. , Berlin, J. A. , Morton, S. C. , Olkin, I. , Williamson, G. D. , Rennie, D. , et al. (2000). Meta‐analysis of observational studies in epidemiology: A proposal for reporting. JAMA, 283(15), 2008–2012. [DOI] [PubMed] [Google Scholar]
- Suvisaari, J. M. , Haukka, J. , Tanskanen, A. , & Lonnqvist, J. K. (1998). Age at onset and outcome in schizophrenia are related to the degree of familial loading. British Journal of Psychiatry, 173, 494–500. [DOI] [PubMed] [Google Scholar]
- Üçok, A. , Gorwood, P. , & Karadayı, G. (2012). Employment and its relationship with functionality and quality of life in patients with schizophrenia: EGOFORS Study. European Psychiatry, 27(6), 422–425. [DOI] [PubMed] [Google Scholar]
- Vourdas, A. , Pipe, R. , Corrigall, R. , & Frangou, S. (2003). Increased developmental deviance and premorbid dysfunction in early onset schizophrenia. Schizophrenia Research, 62(1), 13–22. [DOI] [PubMed] [Google Scholar]
- Westermeyer, J. F. , & Harrow, M. (1984). Prognosis and outcome using broad (DSM‐II) and narrow (DSM‐III) concepts of schizophrenia. Schizophrenia Bulletin, 10(4), 624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Table of the characteristics and quality of the included studies. For full references, see Appendix S1.
Table S2. References and reported outcomes. x indicates that the paper includes outcome in this category, (m) indicates that the presented results are included in the meta‐analysis and bold indicates that the presented results are statistically significant in the original study. For full references, see Appendix S1.
Appendix S1. Full references of included studies.
Figure S1. Relapse.
Figure S2. Remission.
Figure S3. Hospitalization.
Figure S4. Positive symptoms.
Figure S5. Negative symptoms.
Figure S6. Total symptoms.
Figure S7. General clinical outcome.
Figure S8. Employment.
Figure S9. Social/occupational functioning.
Figure S10. Global outcome.


