Abstract
Objective: Dental plaque accumulation and inadequate personal oral hygiene (OH) are known major risk factors of periodontitis. Nevertheless, the magnitude of their effects has not yet been the subject of a meta-analysis. Material and methods: The Medline and Scopus databases were searched up to May 2016. Observational studies were eligible if they assessed associations between OH and periodontitis in adult subjects. A multivariate random-effects meta-analysis was used to pool the effects of fair/poor OH versus good OH on periodontitis across studies. The associations between oral care habits and periodontitis were also assessed. Results: A total of 50 studies were eligible; 15 were used for pooling the effect of fair OH versus good OH and poor OH versus good OH on periodontitis, with pooled odds ratios (ORs) of 2.04 [95% confidence interval (CI): 1.65–2.53] and 5.01 (95% CI: 3.40–7.39), respectively. Eleven studies examined oral care habits measured according to toothbrushing regularity and dental visit frequency; pooled ORs of 0.66 (95% CI: 0.47–0.94) and 0.68 (95% CI: 0.47–0.98) were obtained, respectively. Conclusions: Fair to poor OH increases the risk of periodontitis by two- to five-fold. This risk can be reduced by regular toothbrushing and dental visits.
Key words: Meta-analysis, oral hygiene, periodontitis, risk factor, systematic review
Introduction
Periodontitis is the most common oral disease worldwide, with an age-standardised prevalence of 11.2%1. It is a multifactorial disease2, with risk factors such as diabetes mellitus (DM), smoking and, most commonly, inadequate oral hygiene (OH)3. The accumulation of dental plaque and calculus is usually caused by improper toothbrushing techniques, failure to carry out interdental cleaning and irregular dental visits. This accumulation predictably results in gingival inflammation. Persistent gingivitis is a key risk predictor for the breakdown of periodontal attachment. Although poor OH is a well-accepted and important risk factor for periodontitis, the magnitude of the association between OH and periodontitis has not yet been explored in a meta-analysis. Therefore, we conducted a systematic review and meta-analysis aiming to estimate the effects of OH on periodontitis, as measured by the Oral Hygiene Index (OHI), Plaque Index (PI) and plaque score (PSc). A second aim was to pool the magnitudes of association between oral care habits (regular toothbrushing, interdental cleaning and dental visits) and periodontitis.
Methods
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines for conducting a meta-analysis were followed4. The checklist is provided in Appendix S1 (PROSPERO registration number: CRD42015019036).
Search strategy
Relevant studies were identified from Medline and Scopus databases, searched up to May 2016 using standardised methodological filters. Search strategies were mainly constructed based on the primary objective with three domains (i.e. periodontitis, OH and general aspects for observational studies), as follows: (‘periodontitis’ OR ‘periodontal’) AND (‘poor oral hygiene’ OR ‘plaque index’ OR ‘oral hygiene index’ OR ‘plaque score’) AND (‘relation’ OR ‘association’ OR ‘risk factor’). The search terms and strategies are described in Table S1.
Inclusion criteria
Studies were screened based on titles and abstracts; if a decision could not be made based on this information, full papers were reviewed. Any type of observational study (e.g. cohort, case–control or cross-sectional) published in English was included if it met the following criteria: (i) assessed associations between OH and periodontitis in either general or specific types of adult populations; (ii) had at least two outcome groups, namely periodontitis versus non-periodontitis, or mild, moderate and severe periodontitis versus normal periodontium; (iii) assessed OH using standard tools, such as the OHI or Simplified Oral Hygiene Index (OHI-S)5, PI6, plaque control record/PSc7 or a questionnaire including the frequency of brushing, interdental cleaning and dental visits; (iv) reported/possibly calculated the mean and standard deviation (SD) of OH scores among periodontitis groups or a contingency table between non-periodontitis/periodontitis and OH groups. Studies were excluded if they had insufficient data for pooling after contacting the authors for additional data.
Two of three reviewers (A.L., S.R. and S.A.) independently evaluated the studies for eligibility, extracted the data and assessed the risk of bias. Any discrepancies between reviewers were discussed and resolved by consensus.
Study factors
The primary study factor was OH, objectively measured using the OHI, PI or PSc. Secondary study factors were oral care habits, which were subjectively assessed using questionnaires assessing the frequency of toothbrushing, interdental cleaning and dental visits.
Outcome
The outcome of interest was periodontitis, which was defined according to the original studies. The definition of periodontitis was based on periodontal probing depth, clinical attachment level or radiographs without a restricted periodontitis definition.
Data extraction
Study characteristics, including study design (cohort, case–control or cross-sectional), population type (general population or specific disease) and study location (community or hospital) were extracted. Subject characteristics (i.e. percentage of male subjects, smoking habits and the presence of DM) and clinical data (i.e. periodontitis definition and details of OH assessments) were also extracted.
Risk of bias assessment
The quality of the studies was assessed using the modified Newcastle–Ottawa Quality Assessment Scale8 (Appendix S2), which considers three domains: the representativeness of the studied subjects; the comparability between groups; and the ascertainment of outcome and exposure. Each domain was graded by assigning stars if there was a low risk of bias. Individual studies were categorised, according to these stars, as having a low, moderate or high risk of bias if the percentage of stars was ≥75%, 50–74% and <50%, respectively.
Statistical analysis
Data were pooled if there were at least two studies reporting the same outcomes and study factors. Data analysis was performed separately according to the type of OH data (i.e. categorical or continuous data), as described below.
For categorical data, the odds ratio (OR) of having periodontitis for fair OH versus good OH (OR1) and poor OH versus good OH (OR2), along with their 95% confidence intervals (95% CIs) were estimated for each study. For studies with two or more OH groups, a multivariate random-effects meta-analysis was applied for pooling ORs. This method considers within-study variation using Riley's method9., 10.. For studies in which OH was divided into more than two groups and ORs were reported without frequency data, the variance-covariance was assumed to be zero.
For continuous data, the mean difference in OH scores between periodontitis and non-periodontitis groups was estimated and pooled using a standardised mean difference (SMD). If logistic model correlation coefficients were reported instead of the mean and SD, the beta coefficients were then pooled using the pooling mean method.
Heterogeneity was assessed using Cochrane's Q test and the I2 statistic. If heterogeneity was present (Q test <0.1 or I2 ≥ 25%), a random-effects model (DerSimonian and Laird)11 was used. Otherwise, a fixed-effects model was applied using the inverse variance method.
Sources of heterogeneity were explored using a Galbraith plot to identify outlier studies. Covariables (i.e. population type, age, gender, smoking, DM, index use, periodontitis definition) were then fitted one-by-one into a meta-regression model. If there was a suggested association, a sensitivity analysis excluding the outlier studies and/or a subgroup analysis was performed.
Finally, potential publication bias was explored using the Egger test and a funnel plot. If either of these indicated asymmetry, a contour-enhanced funnel plot was constructed to identify the cause of asymmetry. All analyses were performed using STATA software version 14 (StataCorp, College Station, TX, USA). Two-sided P < 0.05 was considered statistically significant except for the heterogeneity test, in which P < 0.10 was used.
Grade of evidence
The system from the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group)12., 13. was used for grading the quality of evidence mainly based on the study design, risk of bias, indirectness of evidence, publication bias, heterogeneity and imprecision of results.
Results
Identifying studies
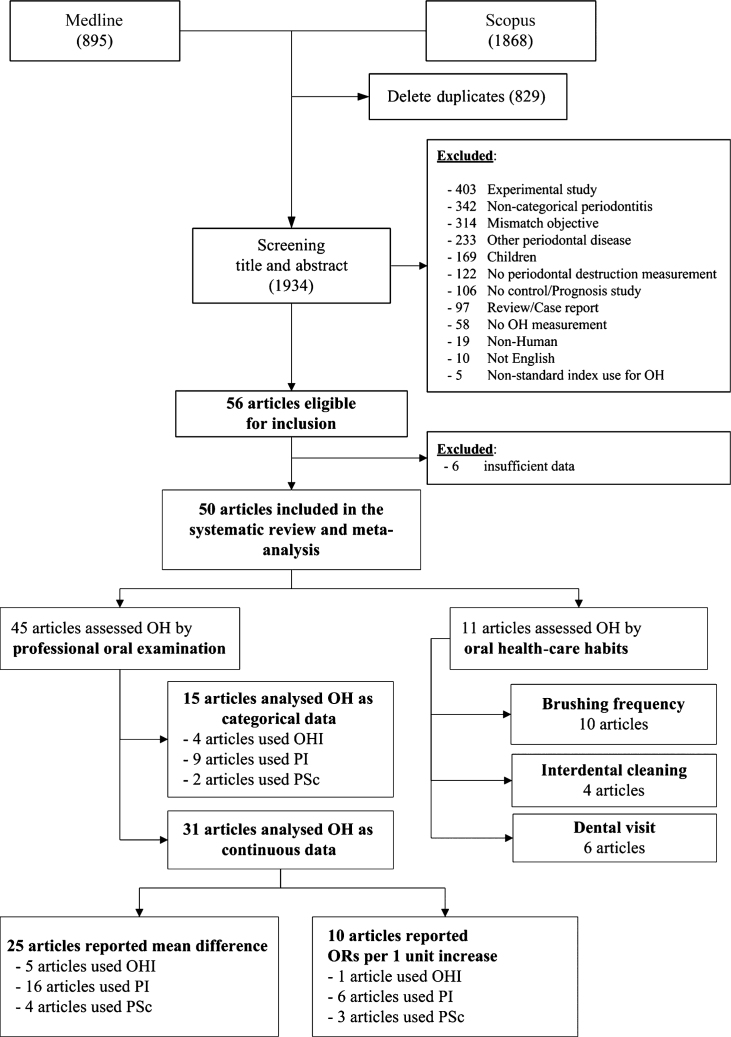
A total of 2,763 studies were identified from Medline and Scopus, and 1,934 studies remained after removing duplicates. Of these, 1,878 studies were ineligible for reasons described in Figure 1, leaving 5614., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42., 43., 44., 45., 46., 47., 48., 49., 50., 51., 52., 53., 54., 55., 56., 57., 58., 59., 60., 61., 62., 63., 64., 65., 66., 67., 68., 69. that were eligible for review. Six studies14., 47., 48., 51., 52., 57. were excluded because of insufficient data after contacting the authors. Of the remaining 50 studies, 4515., 16., 17., 18., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 33., 35., 36., 37., 38., 39., 42., 43., 44., 45., 46., 49., 50., 53., 54., 55., 56., 58., 59., 60., 61., 62., 63., 64., 65., 66., 67., 68., 69. objectively assessed OH using an oral examination. Of these 45 studies, 1515., 17., 22., 26., 27., 28., 29., 31., 35., 36., 37., 38., 39., 46., 65. analysed OH as categorical data, 3116., 18., 20., 21., 22., 23., 24., 25., 30., 33., 42., 43., 44., 45., 49., 50., 53., 54., 55., 56., 58., 59., 60., 61., 62., 63., 64., 66., 67., 68., 69. as continuous data and one22 as both. Eleven studies provided the association between periodontitis and oral care habits measured according to the frequency of brushing29., 32., 33., 34., 36., 37., 40., 41., 44., 56., interdental cleaning29., 41., 44., 56. and dental visits19., 33., 34., 36., 40., 56..
Figure 1.
Flow chart of identification and selection of studies. OH, oral hygiene; OHI, Oral Hygiene Index; PI, Plaque Index; PSc, plaque score.
Subject characteristics
The characteristics of the 50 included studies are described in Table 1. Most study designs were cross-sectional, most studies investigated a general population and 34 were based in hospitals. The mean subject age ranged from 15 to 65 years. The percentages of male subjects, smokers and people with diabetes are also shown in Table 1. While the definition of periodontitis varied across the studies, most (92%) used periodontal probing depth and/or clinical attachment level.
Table 1.
Characteristics of included studies
| Authors | Study type | Study base | Population | OH measurement | Age | Male (%) | Smoking (%) | DM (%) | Periodontitis definition |
|---|---|---|---|---|---|---|---|---|---|
| Imaki15 | Cross-sectional | Community | General | PI | 38.1 | 100 | 56.1 | N/A | CPITN: 3–4 |
| Norderyd16 | Cross-sectional | Community | General | PSc | 48 | 48.7 | 20 | 4 | Radiography: bone loss more than one-third of root length |
| Wakai17 | Cross-sectional | Hospital | General | PI | 51.1 | 82.1 | 34.4 | N/A | CPITN: 3–4 |
| Papapanou18 | Case–control | Hospital | General | PSc | 50.9 | 47.3 | 32.2 | N/A | One site or more with PPD ≥5 mm AND CAL ≥3 mm |
| Hashim19 | Cohort | Community | General | OHI, Dental visit | 15 | 54.2 | 33.3 | N/A | One site or more with ≥4 mm increase in CAL |
| Tezal20 | Cross-sectional | Community | General | PI | 48.7 | 48.2 | 61.8 | N/A | Mean CAL ≥2 mm |
| Hugoson21 | Cross-sectional | Community | General | PSc | 65.0 | 52.7 | 42.9 | N/A | Radiography: bone loss more than one-third of root length |
| Do22 | Cross-sectional | Community | General | PI | 40 | 40.3 | 28.9 | N/A | Two or more sites with CAL ≥5 mm AND one site or more with PPD ≥4 mm |
| Meisel23 | Cross-sectional | Community | General | PSc | 51.0 | 46.6 | 49.5 | 6 | 4th–5th quintiles of the percentage of sites with CAL >4 mm |
| Alpagot24 | Cohort | Hospital | Patients with HIV | PI | 34.1 | 57.9 | N/A | 0 | One site or more with PPD ≥4 mm OR CAL ≥2 mm |
| Solis25 | Cross-sectional | Hospital | General | PI | 37.4 | 35.3 | 23.5 | 0 | Two or more sites with CAL ≥6 mm AND one site or more with PPD ≥5 mm |
| Wickholm26 | Cross-sectional | Community | General | PI | 36.7 | 49.2 | 44.7 | N/A | Three or more teeth with PPD ≥5 mm |
| Natto27 | Cross-sectional | Community | General | PI | 36.4 | 64.9 | 70 | N/A | ≥10 sites with PPD ≥5 mm |
| Torrungruang28 | Cross-sectional | Community | General | PSc | 60 | 74.4 | 14.3 | 15.8 | Mean CAL >2.5 mm |
| de Macêdo29 | Cross-sectional | Community | General | PSc, Flossing, Brushing | N/A | 33.8 | 31.4 | N/A | Four or more teeth with PPD ≥4 mm AND CAL ≥3 mm at the same site |
| Khader30 | Cross-sectional | Hospital | General | PI | 39.4 | 44.8 | N/A | N/A | Khader's risk score |
| Vandana31 | Cross-sectional | Hospital | Dental fluorosis | OHI | 25.36 | 68.6 | N/A | 0 | CPITN: 3–4 |
| Wang32 | Cross-sectional | Community | General | Brushing | N/A | 45.7 | 27.7 | 0 | Mean CAL ≥3 mm |
| Akhter33 | Case–control | Hospital | General | PI, Brushing, Dental visit | 38.5 | 50 | 45.7 | N/A | Two or more sites with CAL ≥6 AND one site or more with PPD ≥5 mm |
| Kumar34 | Cross-sectional | Community | General | Brushing, Dental visit | 33.9 | 100 | N/A | N/A | CPITN: 3–4 |
| Benguigui35 | Cross-sectional | Community | General | PI | 58 | 54.9 | 19.2 | 6.7 | CDC/AAP |
| Saxlin36 | Cohort | Community | General | PI, Brushing, Dental visit | 41.86 | 27 | 0 | 0 | New teeth with PPD ≥4 mm |
| Bawadi37 | Cross-sectional | Hospital | General | PI, Brushing | 36.4 | 49.4 | 20.3 | 17.9 | Four or more teeth with PPD ≥4 mm AND CAL ≥3 mm at the same site |
| Carrilho Neto38 | Cross-sectional | Hospital | Inpatients | OHI | 45.7 | 59.7 | 42.7 | N/A | One site or more with PPD >4 mm |
| Mathur39 | Cross-sectional | Hospital | General | OHI | N/A | 57.3 | N/A | N/A | N/A |
| Teng40 | Cross-sectional | Hospital | Psychiatric inpatients | Brushing, Dental visit | 41 | 62.5 | 42.5 | N/A | CPITN: 3–4 |
| Crocombe41 | Cross-sectional | Community | General | Brushing, Flossing | N/A | 50 | 15 | 4.3 | One site or more with CAL ≥4 mm |
| Mannem42 | Cross-sectional | Hospital | General | PI | 52.5 | 44.1 | 34.2 | N/A | Four or more teeth with PPD ≥4 mm AND CAL ≥3 mm at the same site |
| Raja43 | Cross-sectional | Hospital | General | PI | 36.5 | 53.3 | 96.7 | 0 | Four or more sites with CAL ≥4 mm |
| Vogt44 | Cross-sectional | Hospital | Pregnancy | PSc, Flossing, Brushing | 27.2 | 0 | 15.87 | 0 | Four or more teeth with PPD ≥4 mm AND CAL ≥4 mm at the same site |
| Fiyaz45 | Case–control | Hospital | General | OHI | N/A | N/A | 0 | 0 | One site or more with PPD >4 mm OR CAL >1.5 mm |
| Palle46 | Cross-sectional | Hospital | CVD | OHI | 57.2 | 84.1 | 32.3 | 52.2 | Five or more sites with CAL ≥5 mm |
| Cakmak49 | Case–control | Hospital | General | PI | 38.3 | 49.1 | 0 | 0 | One site or more with PPD ≥5 mm AND CAL ≥4 mm |
| Develioglu50 | Case–control | Hospital | General | PI | 46.7 | N/A | 0 | 33.3 | ≥30% sites with PPD ≥5 mm AND CAL ≥3 mm |
| Jacob53 | Case–control | Hospital | General | PI | 37.3 | 75.6 | 33.3 | 0 | CDC/AAP |
| Kaur54 | Case–control | Hospital | General | PI | N/A | 66.7 | 25 | 0 | N/A |
| Koseoglu55 | Case–control | Hospital | General | PI | 34.0 | 50 | 0 | 0 | Four or more teeth with PPD ≥5 mm AND CAL ≥4 mm in each jaw |
| Kovačević56 | Cross-sectional | Hospital | General | OHI, Brushing, Flossing, Dental visit | 38.9 | 77.2 | 31.7 | N/A | CPITN: 3–4 |
| Lavu58 | Case–control | Hospital | General | OHI | 33.6 | 50.4 | 0 | 0 | CAL >1 mm at least 30% sites |
| Lutfioglu59 | Case–control | Hospital | General | PI | 33.1 | 53.3 | 51.1 | 0 | One site or more with PPD ≥5 mm with radiographic evidence of bone loss |
| Meenawat60 | Case–control | Hospital | General | PI | 43.2 | 100 | 41.4 | 0 | Four or more teeth with PPD >4 mm AND CAL >2 mm |
| Mesa61 | Case–control | Hospital | General | PSc | 46.3 | 40.3 | 46.8 | N/A | Four or more teeth with PPD ≥4 mm AND CAL ≥3 mm at the same sites |
| Perayil62 | Case–control | Hospital | General | OHI | 43.1 | 43.3 | 0 | 0 | Five or more teeth with PPD ≥5 mm AND CAL ≥3 mm |
| Pereira63 | Case–control | Hospital | General | PSc | 38.4 | 33.7 | 0 | 0 | Four or more teeth with PPD ≥4 mm AND CAL ≥3 mm |
| Petrović64 | Case–control | Hospital | General | PI | 36.1 | 38.8 | 22.4 | 0 | Thre or more quadrants with three or more sites with PPD ≥3 mm AND CAL ≥2 mm |
| Pranckeviciene65 | Cross-sectional | Hospital | Type I and type II DM | PI | 43.86 | N/A | 25.9 | 100 | One site or more with CAL >5 mm |
| Puri66 | Case–control | Hospital | General | OHI | 39.78 | N/A | 0 | 0 | AAP 1999 |
| Singh67 | Case–control | Hospital | General | PI | 43.5 | 52.5 | 0 | 0 | One site or more with PPD ≥5 mm AND CAL ≥2 mm |
| Toyman68 | Case–control | Hospital | General | PI | 34.6 | 51.2 | 0 | 0 | Six teeth or more with PPD ≥5 mm with radiographic evidence of bone loss |
| Varghese69 | Case–control | Hospital | General | PI | N/A | 65.3 | 0 | 0 | ≥30% sites with PPD ≥6 mm AND CAL ≥5 mm |
CAL, clinical attachment level; CDC/AAP, periodontitis definition of the Centers for Disease Control and Prevention in collaboration with the American Academy of Periodontology; CPITN, the Community Periodontal Index of Treatment Needs; CVD, cardiovascular disease; DM, diabetes mellitus; HIV, human immunodeficiency virus; N/A, not available; OH, oral hygiene; OHI, oral hygiene index; PI, plaque index; PPD, periodontal pocket depth; PSc, plaque score.
Risk of bias assessment
The results of the risk of bias assessments are described in Table S2. Most (72%) studies provided inadequate details for sample selection; hence, representativeness was unclear. For example, some authors did not mention their sampling methods or clearly describe their process for selecting cases and controls. Twenty-seven (46%) studies were potentially biased because of improper statistical adjustments for confounding factors. Almost all studies measured periodontitis via an oral examination, which was objective and valid. However, 16 (32%) studies used partial-mouth examination protocols, 16 (32%) studies diagnosed periodontitis without data regarding clinical attachment level and 25 (50%) studies did not provide details about intra/interexaminer agreement. The numbers of studies with low, moderate and high risks of bias were 23, 19 and 8, respectively.
Oral hygiene
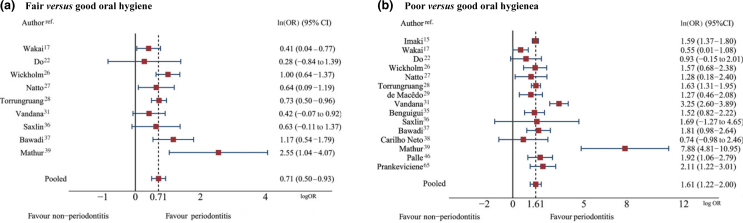
Of the 15 studies which reported OH as categorical data, six15., 29., 35., 38., 46., 65. categorised OH as good or poor, whereas nine17., 22., 26., 27., 28., 31., 36., 37., 39. categorised OH as good, fair or poor. The criteria for classifying OH are presented in Table S3. Pooled ln(ORs) determined using a multivariate meta-analysis (Figure 2) were 0.71 (95% CI: 0.50–0.93) and 1.61 (95% CI: 1.22–2.00), which yielded pooled ORs of 2.04 (95% CI: 1.65–2.53) and 5.01 (95% CI: 3.40–7.39), respectively, for fair OH and poor OH. These results indicate that fair OH and poor OH increase the risk of periodontitis by approximately two- and five-fold compared with good OH with an I2 of 40% and 78%, respectively. The details of each individual study are shown in Table S4.
Figure 2.
Pooling effects of fair oral hygiene (OH) versus good OH (a) and poor OH versus good OH (b) on periodontitis. 95% CI, 95% confidence interval; OR, odds ratio.
Population type appeared to be a large source of heterogeneity. Subgroup analyses in community-based studies yielded lower heterogeneity levels [i.e. the I2 values were 4% and 0% for fair and poor versus good OH, respectively, with corresponding pooled ORs of 2.23 (95% CI: 1.85–2.69) and 4.78 (95% CI: 4.10–5.58)]. In addition, a sensitivity analysis focussing on 11 studies15., 17., 22., 26., 27., 28., 29., 35., 36., 37., 39. of general populations decreased the degree of heterogeneity to 22% and 49% for fair OH versus good OH and poor OH versus good OH, with pooled ORs of 2.10 (95% CI: 1.76–2.49) and 4.21 (95% CI: 3.21–5.51), respectively. Moreover, the periodontitis definitions and index types used, as well as smoking behaviour, also contributed to heterogeneity (Table S5).
Among 31 studies that measured OH on a continuous scale, 2518., 24., 25., 33., 42., 43., 44., 45., 49., 50., 53., 54., 55., 56., 58., 59., 60., 61., 62., 63., 64., 66., 67., 68., 69. compared OH between periodontitis and non-periodontitis groups using the mean scores. The SMDs were highly heterogeneous (I2 = 95.6%), with a pooled SMD of 2.04 (95% CI: 1.59–2.50) (Table S6). From these findings, it could be interpreted that periodontitis subjects had a significantly higher OH score of 2.04 standardised units than did non-periodontitis subjects.
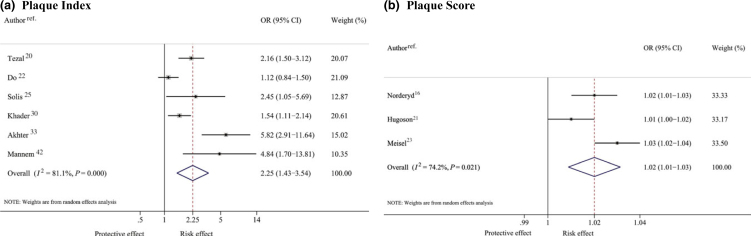
Six20., 22., 25., 30., 33., 42. and three16., 21., 23. studies reported the effects of PI and PSc on periodontitis as coefficients [i.e. ln(OR)] of logistic regression models. Pooling these corresponding effects yielded pooled ORs of 2.25 (95% CI: 1.43–3.54) and 1.02 (95% CI: 1.01–1.03), and high heterogeneity was found for both (Figure 3). These findings could be interpreted to indicate that each one-unit increase in the measures of PI and PSc would increase the odds of having periodontitis by 2.25 and 1.02, respectively.
Figure 3.
Pooling odds ratios (ORs) of plaque index (a) and plaque score (b) on periodontitis.
Oral health-care habits
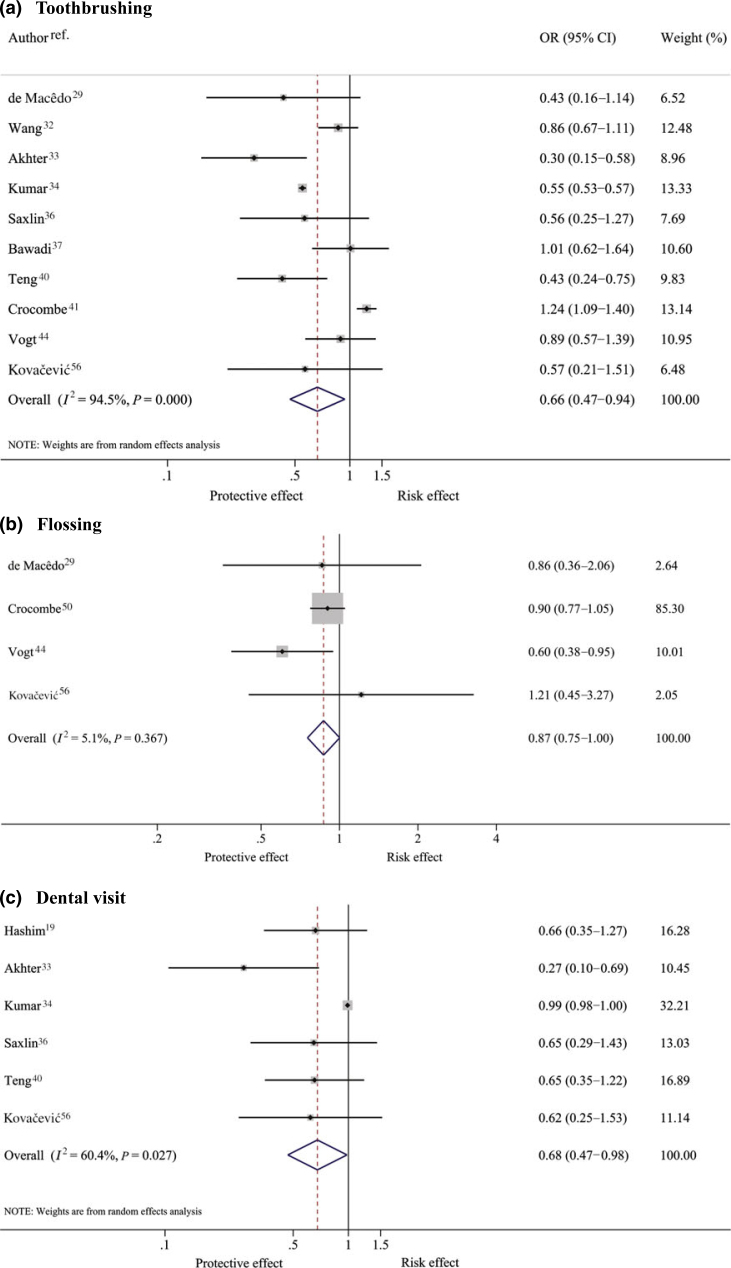
Ten29., 32., 33., 34., 36., 37., 40., 41., 44., 56., four29., 41., 44., 56. and six19., 33., 34., 36., 40., 56. studies assessed the effects of brushing, dental floss and dental visits on periodontitis (Table S7). The pooled ORs (Figure 4) suggested that toothbrushing and dental visits were significantly associated with periodontitis, although the I2 values showed high heterogeneity, at 94.5% and 60.4%, respectively. Subjects who brushed their teeth regularly had approximately 34% significantly lower odds of having periodontitis (pooled OR = 0.66; 95% CI: 0.47–0.94). Smoking, the definition of regular brushing and periodontitis were potential sources of heterogeneity (Table S8).
Figure 4.
Pooling effect of oral care habits – toothbrushing (a), flossing (b) and dental visits (c) –on periodontitis.
For dental visits, the sensitivity analysis was performed by considering four of six studies that had clearly defined a regular dental visit as at least one visit per year19., 33., 36., 56.. This yielded a significant effect size of 0.56 (95% CI: 0.37–0.83) with an I2 of 0%, indicating that subjects who regularly visited dentists at least once a year had a 44% lower risk of periodontitis than those who did not. The effects of interdental cleaning with dental floss on periodontitis showed little heterogeneity (I2 = 5.1%), but the pooled OR was borderline significant (OR = 0.87; 95% CI: 0.75–1.00).
Publication bias
Publication bias was assessed for all pooled estimates using funnel plots (Figure S1) and Egger tests (Table S9). The results suggested symmetry except for the mean differences in OH score, PSc and dental visits. Contour-enhanced funnel plots were further constructed (Figure S2), and these indicated that the asymmetry of the funnels might be caused by both heterogeneity and publication bias.
Quality of evidence
The scoring using the GRADE framework is shown in Table 2 and Appendix S3. Based on observational studies, all pooled estimates were graded as low quality13. For the effects of fair OH and poor OH on periodontitis, this was upgraded to moderate quality because of large effect sizes and strong dose–response relationships. The effects of brushing and dental visits were downgraded to very low quality caused by heterogeneity and publication bias, respectively.
Table 2.
Overview of the meta-analysis
| Risk factor | No. of studies | Pooled OR (95% CI) | I2 (%) | Quality of evidence* |
|---|---|---|---|---|
| OH | ||||
| Categorical data | ||||
| Fair OH versus Good OH | 9 | 2.04 (1.65–2.53) | 40 | |
| Poor OH versus Good OH | 15 | 5.01 (3.40–7.39) | 78 | |
| Continuous data | Moderate | |||
| PI: 1-unit increase | 6 | 2.25 (1.43–3.54) | 81.1 | |
| PSc: 1-unit increase | 3 | 1.02 (1.01–1.03) | 74.2 | |
| OH score | 25 | 2.04 (1.59–2.50)† | 95.6 | |
| Oral health-care habits | ||||
| Toothbrushing | 10 | 0.66 (0.47–0.94) | 94.5 | Very low |
| Interdental cleaning | 4 | 0.87 (0.75–1.00) | 5.1 | Low |
| Dental visits | 6 | 0.68 (0.47–0.98) | 60.4 | Very low |
OH, oral hygiene; PI, plaque index; PSc, plaque score.
Quality of evidence: The Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group).
Pooled standard mean difference (SMD).
Discussion
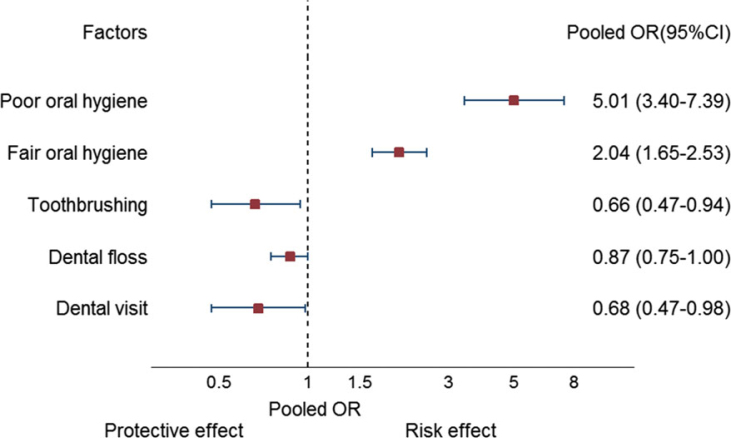
We conducted a systematic review and meta-analysis of the effects of OH on periodontitis. The results suggest a dose–response relationship between OH and periodontitis, with fair and poor OH significantly increasing the risk of having periodontitis by two- and five-fold, respectively, compared with good OH. In contrast, regular toothbrushing and dentist visits could reduce periodontitis by 34% and 32%, respectively. These pooled OH effects and oral care habits are summarised in Table 2 and Figure 5.
Figure 5.
Summary of pooled effect of oral hygiene (OH) and oral care habits on periodontitis. OR, odds ratio.
The effect of OH on periodontitis was stronger than those of other risk factors, such as DM70 (OR = 2.6; 95% CI: 1.0–6.6), smoking71 (OR = 2.82; 95% CI: 2.36–3.39) or obesity72 (OR = 2.13; 95% CI: 1.40–3.26). Our results also showed protective effects of regular toothbrushing, which were consistent with the findings of a previous meta-analysis73 that reported a significant risk for severe periodontitis caused by infrequent brushing (OR = 1.44; 95% CI: 1.21–1.71). However, our study could only identify a small effect of interdental cleaning with dental floss (i.e. a non-significant reduction of 13% in the risk of periodontitis). This result was also consistent with a previous meta-analysis74, which found little benefit from self-performed flossing on plaque or periodontal parameters.
Although the use of OH assessments varied between the included studies, approximately half commonly used the PI with similar cut-off points. OH was defined as poor for a PI of >2 or if the patient had a moderate accumulation of soft deposits visible by the naked eye; and OH was defined as fair for PI values ranging from 1 to 2 or if the patient had a film of plaque adhering to the tooth as detected by disclosing solution or probe.
To address concerns about the varying quality of the individual studies, a sensitivity analysis was also performed, including only studies with a low risk of bias22., 26., 27., 28., 29., 35., 36., 37., 46., 65.. The results showed little difference compared with those of the main analysis, but heterogeneity was much lower.
Good OH and oral care habits should be encouraged and promoted in public health campaigns. Dentists and dental hygienists should regularly educate, motivate and assess patients’ perceptions for improving oral health behaviours. Additionally, dental nurses or assistants should encourage and provide general, useful information. Repeated and individually tailored OH instructions are key elements in achieving gingival health. Goal setting, self-monitoring and planning are effective interventions for improving OH-related behaviours in patients with periodontitis. Recognising the benefits of behaviour changes, their own susceptibility and the deleterious effects of periodontitis are important messages in periodontitis prevention75.
Patients should be able to access dental care regularly for professional cleaning together with tailoring and monitoring their OH75. They should also be taught how to perform plaque removal efficiently. Generally, mechanical plaque controlled by twice-daily toothbrushing with a fluoride-containing dentifrice is an accepted recommendation. The proper duration of toothbrushing is also mentioned as an important determinant of plaque removal; therefore, it should be stressed during toothbrushing instruction76. The current scientific data show that dental floss is not effective as a tool for removal of interdental plaque. It requires the user to be instructed about specific skills in order to be more effective. Interdental brushes have been shown to be the most effective method for removal of interdental plaque77; however, the selection of interdental aids must be at the clinician's discretion based on a patient's needs and dexterity and the characteristics of a patient's interdental spaces.
This study has some strengths. It includes studies of the effects of OH using both objective and subjective assessments. The magnitudes of the effects were pooled and reported. The results of subgroup analyses (i.e. population type, study base, periodontitis definition and smoking) were also explored. We used rigorous pooling methods (multivariate random-effects meta-analysis), which considered the variance-covariance between the studies.
However, this study also has some limitations. Our pooled ORs were based on summary data of observational studies. Some data were reported without adjusting for potential confounders; thus, the pooled results might be prone to bias. Moreover, the definition of periodontitis varied among studies, which resulted in high heterogeneity, although the subgroup analyses did reduce this effect. Furthermore, the assessments of publication bias using funnel plots and Egger tests with the low numbers of included studies in some meta-analyses may not be valid. Failure to detect asymmetry cannot rule out a reporting bias or vice versa.
In conclusion, poor OH increases the risk of periodontitis by approximately two- to five-fold compared with good OH. Oral care habits, including regular brushing and dental visits, can decrease the risk of periodontitis and should thus be promoted as a public health intervention.
Acknowledgements
This manuscript is a part of Attawood Lertpimonchai's PhD thesis in Clinical Epidemiology, the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand.
Conflicts of interest
The authors declare no conflicts of interest. This study received no external funding, apart from the support of the authors’ institution.
Supporting Information
Figure S1. Funnel plots of publication bias assessment.
Figure S2. Contour-enhanced funnel plots.
Table S1. Search terms and search strategy.
Table S2. Risk of bias assessment.
Table S3. Categorisation of OH level.
Table S4. Pooling effects of fair and poor versus good OH on periodontitis.
Table S5. Subgroup and sensitivity analysis according to sources of heterogeneity of fair and poor versus good OH.
Table S6. Pooling SMD of OH scores between periodontitis and non-periodontitis.
Table S7. Pooled effect size of oral care habits on periodontitis.
Table S8. Sources of heterogeneity of tooth brushing meta-analysis.
Table S9. Publication bias assessment by Egger test.
Appendix S1. PRISMA checklist.
Appendix S2. Modified Newcastle-Ottawa Quality Assessment Scale.
Appendix S3. GRADE approach.
References
- 1.Kassebaum NJ, Bernabe E, Dahiya M, et al. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93:1045–1053. doi: 10.1177/0022034514552491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Bakdash B. Oral hygiene and compliance as risk factors in periodontitis. J Periodontol. 1994;65(5 Suppl):539–544. doi: 10.1902/jop.1994.65.5s.539. [DOI] [PubMed] [Google Scholar]
- 4.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 6.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 7.O'Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 8.Wells GA, Shea B, O'Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 22 August 2016.
- 9.White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J. 2011;11:255–270. [Google Scholar]
- 10.Riley RD, Thompson JR, Abrams KR. An alternative model for bivariate random-effects meta-analysis when the within-study correlations are unknown. Biostatistics. 2008;9:172–186. doi: 10.1093/biostatistics/kxm023. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control CliniTrials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Bagramian RA, Farghaly MM, Lopatin D, et al. A comparison of periodontal disease among rural Amish and non-Amish adults. J Clin Periodontol. 1994;21:386–390. doi: 10.1111/j.1600-051x.1994.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 15.Imaki M, Yoshida Y, Tanada S. Relation between smoking and periodontal disease by oral hygiene status in Japanese factory workers. Appl Human Sci. 1997;16:77–81. doi: 10.2114/jpa.16.77. [DOI] [PubMed] [Google Scholar]
- 16.Norderyd O, Hugoson A. Risk of severe periodontal disease in a Swedish adult population. A cross-sectional study. J Clin Periodontol. 1998;25:1022–1028. doi: 10.1111/j.1600-051x.1998.tb02408.x. [DOI] [PubMed] [Google Scholar]
- 17.Wakai K, Kawamura T, Umemura O, et al. Associations of medical status and physical fitness with periodontal disease. J Clin Periodontol. 1999;26:664–672. doi: 10.1034/j.1600-051x.1999.261006.x. [DOI] [PubMed] [Google Scholar]
- 18.Papapanou PN, Neiderud AM, Papadimitriou A, et al. “Checkerboard” assessments of periodontal microbiota and serum antibody responses: a case-control study. J Periodontol. 2000;71:885–897. doi: 10.1902/jop.2000.71.6.885. [DOI] [PubMed] [Google Scholar]
- 19.Hashim R, Thomson WM, Pack ARC. Smoking in adolescence as a predictor of early loss of periodontal attachment. Community Dent Oral Epidemiol. 2001;29:130–135. doi: 10.1034/j.1600-0528.2001.290207.x. [DOI] [PubMed] [Google Scholar]
- 20.Tezal M, Grossi SG, Ho AW, et al. The effect of alcohol consumption on periodontal disease. J Periodontol. 2001;72:183–189. doi: 10.1902/jop.2001.72.2.183. [DOI] [PubMed] [Google Scholar]
- 21.Hugoson A, Ljungquist B, Breivik T. The relationship of some negative events and psychological factors to periodontal disease in an adult Swedish population 50 to 80 years of age. J Clin Periodontol. 2002;29:247–253. doi: 10.1034/j.1600-051x.2002.290311.x. [DOI] [PubMed] [Google Scholar]
- 22.Do GL, Spencer AJ, Roberts-Thomson K, et al. Smoking as a risk indicator for periodontal disease in the middle-aged Vietnamese population. Community Dent Oral Epidemiol. 2003;31:437–446. doi: 10.1046/j.1600-0528.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 23.Meisel P, Siegemund A, Grimm R, et al. The interleukin-1 polymorphism, smoking, and the risk of periodontal disease in the population-based SHIP study. J Dent Res. 2003;82:189–193. doi: 10.1177/154405910308200308. [DOI] [PubMed] [Google Scholar]
- 24.Alpagot T, Duzgunes N, Wolff LF, et al. Risk factors for periodontitis in HIV+ patients. J Periodontal Res. 2004;39:149–157. doi: 10.1111/j.1600-0765.2004.00718.x. [DOI] [PubMed] [Google Scholar]
- 25.Solis ACO, Lotufo RFM, Pannuti CM, et al. Association of periodontal disease to anxiety and depression symptoms, and psychosocial stress factors. J Clin Periodontol. 2004;31:633–638. doi: 10.1111/j.1600-051X.2004.00538.x. [DOI] [PubMed] [Google Scholar]
- 26.Wickholm S, Söder PÖ, Galanti MR, et al. Periodontal disease in a group of Swedish adult snuff and cigarette users. Acta Odontol Scand. 2004;62:333–338. doi: 10.1080/00016350410001801. [DOI] [PubMed] [Google Scholar]
- 27.Natto S, Baljoon M, Bergstrom J. Tobacco smoking and periodontal health in a Saudi Arabian population. J Periodontol. 2005;76:1919–1926. doi: 10.1902/jop.2005.76.11.1919. [DOI] [PubMed] [Google Scholar]
- 28.Torrungruang K, Tamsailom S, Rojanasomsith K, et al. Risk indicators of periodontal disease in older Thai adults. J Periodontol. 2005;76:558–565. doi: 10.1902/jop.2005.76.4.558. [DOI] [PubMed] [Google Scholar]
- 29.de Macêdo TCN, Costa MdCN, Gomes-Filho IS, et al. Factors related to periodontal disease in a rural population. Braz Oral Res. 2006;20:257–262. doi: 10.1590/s1806-83242006000300014. [DOI] [PubMed] [Google Scholar]
- 30.Khader YS. Factors associated with periodontal diseases in Jordan: principal component and factor analysis approach. J Oral Sci. 2006;48:77–84. doi: 10.2334/josnusd.48.77. [DOI] [PubMed] [Google Scholar]
- 31.Vandana KL, Sesha Reddy M. Assessment of periodontal status in dental fluorosis subjects using community periodontal index of treatment needs. Indian J Dent Res. 2007;18:67–71. doi: 10.4103/0970-9290.32423. [DOI] [PubMed] [Google Scholar]
- 32.Wang QT, Wu ZF, Wu YF, et al. Epidemiology and preventive direction of periodontology in China. J Clin Periodontol. 2007;34:946–951. doi: 10.1111/j.1600-051X.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 33.Akhter R, Hassan NMM, Aida J, et al. Relationship between betel quid additives and established periodontitis among Bangladeshi subjects. J Clin Periodontol. 2008;35:9–15. doi: 10.1111/j.1600-051X.2007.01164.x. [DOI] [PubMed] [Google Scholar]
- 34.Kumar TS, Dagli RJ, Mathur A, et al. Oral health status and practices of dentate Bhil adult tribes of southern Rajasthan, India. Int Dent J. 2009;59:133–140. [PubMed] [Google Scholar]
- 35.Benguigui C, Bongard V, Ruidavets JB, et al. Metabolic syndrome, insulin resistance, and periodontitis: a cross-sectional study in a middle-aged French population. J Clin Periodontol. 2010;37:601–608. doi: 10.1111/j.1600-051X.2010.01571.x. [DOI] [PubMed] [Google Scholar]
- 36.Saxlin T, Ylostalo P, Suominen-Taipale L, et al. Overweight and obesity weakly predict the development of periodontal infection. J Clin Periodontol. 2010;37:1059–1067. doi: 10.1111/j.1600-051X.2010.01633.x. [DOI] [PubMed] [Google Scholar]
- 37.Bawadi HA, Khader YS, Haroun TF, et al. The association between periodontal disease, physical activity and healthy diet among adults in Jordan. J Periodontal Res. 2011;46:74–81. doi: 10.1111/j.1600-0765.2010.01314.x. [DOI] [PubMed] [Google Scholar]
- 38.Carrilho Neto A, De Paula Ramos S, Sant'ana AC, et al. Oral health status among hospitalized patients. Int J Dent Hyg. 2011;9:21–29. doi: 10.1111/j.1601-5037.2009.00423.x. [DOI] [PubMed] [Google Scholar]
- 39.Mathur LK, Manohar B, Shankarapillai R, et al. Obesity and periodontitis: a clinical study. J Indian Soc Periodontol. 2011;15:240–244. doi: 10.4103/0972-124X.85667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teng PR, Su JM, Chang WH, et al. Oral health of psychiatric inpatients: a survey of central Taiwan hospitals. Gen Hosp Psychiatry. 2011;33:253–259. doi: 10.1016/j.genhosppsych.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Crocombe LA, Brennan DS, Slade GD, et al. Is self interdental cleaning associated with dental plaque levels, dental calculus, gingivitis and periodontal disease? J Periodontal Res. 2012;47:188–197. doi: 10.1111/j.1600-0765.2011.01420.x. [DOI] [PubMed] [Google Scholar]
- 42.Mannem S, Chava VK. The effect of stress on periodontitis: a clinicobiochemical study. J Indian Soc Periodontol. 2012;16:365–369. doi: 10.4103/0972-124X.100912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raja S, Joshi V, Shirahatti R, et al. “Is the periodontium an index of your mind?” A crossectional study to evaluate the relationship between life events and periodontitis. Int J Clin Dent. 2012;5:39–48. [Google Scholar]
- 44.Vogt M, Sallum AW, Cecatti JG, et al. Factors associated with the prevalence of periodontal disease in low-risk pregnant women. Reprod Health. 2012;9:3. doi: 10.1186/1742-4755-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiyaz M, Ramesh A, Ramalingam K, et al. Association of salivary calcium, phosphate, pH and flow rate on oral health: a study on 90 subjects. J Indian Soc Periodontol. 2013;17:454–460. doi: 10.4103/0972-124X.118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palle AR, Reddy CMSK, Shankar BS, et al. Association between obesity and chronic periodontitis: a cross-sectional study. J Contemp Dent Pract. 2013;14:168–173. doi: 10.5005/jp-journals-10024-1294. [DOI] [PubMed] [Google Scholar]
- 47.Alhabashneh R, Khader Y, Herra Z, et al. The association between periodontal disease and metabolic syndrome among outpatients with diabetes in Jordan. J Diabetes Metab Disord. 2015;14:67–73. doi: 10.1186/s40200-015-0192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhat M, Roberts-Thomson K, Do LG. Clustering of risk indicators for periodontal disease: a population-based study. Community Dent Health. 2015;32:158–162. [PubMed] [Google Scholar]
- 49.Cakmak O, Alkan BA, Ozsoy S, et al. Association of gingival crevicular fluid cortisol/dehydroepiandrosterone levels with periodontal status. J Periodontol. 2014;85:e287–e294. doi: 10.1902/jop.2014.130787. [DOI] [PubMed] [Google Scholar]
- 50.Develioglu H, Ozdemir H, Bostanci V. Comparative analysis of the blood flow values of patients with type 2 diabetes mellitus presenting with chronic periodontitis, patients with chronic periodontitis only and healthy individuals. West Indian Med J. 2014;63:359–363. doi: 10.7727/wimj.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsiao CN, Ko EC, Shieh TY, et al. Relationship between areca nut chewing and periodontal status of people in a typical aboriginal community in Southern Taiwan. J Dent Sci. 2015;10:300–308. [Google Scholar]
- 52.Ismail FB, Ismail G, Dumitriu AS et al. Identification of subgingival periodontal pathogens and association with the severity of periodontitis in patients with chronic kidney diseases: a cross-sectional study. Biomed Res Int 2015 2015. [DOI] [PMC free article] [PubMed]
- 53.Jacob PS, Nath S, Patel RP. Evaluation of interleukin-1β and 8 in gutka chewers with periodontitis among a rural Indian population. J Periodontal Implant Sci. 2014;44:126–133. doi: 10.5051/jpis.2014.44.3.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaur S, Narayanswamy S, Ramesh AV. Comparative evaluation of salivary soluble CD44 levels in periodontal health and disease. J Indian Soc Periodontol. 2014;18:734–738. doi: 10.4103/0972-124X.147409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Köseoʇlu S, Saʇlam M, Pekbaʇriyanik T, et al. Level of interleukin-35 in gingival crevicular fluid, saliva, and plasma in periodontal disease and health. J Periodontol. 2015;86:964–971. doi: 10.1902/jop.2015.140666. [DOI] [PubMed] [Google Scholar]
- 56.Kovačević V, Milosavljević M, Rančić N, et al. Assessment of the periodontal health and community periodontal index in the Army of Serbia. Vojnosanit Pregl. 2015;72:953–960. doi: 10.2298/vsp140812105k. [DOI] [PubMed] [Google Scholar]
- 57.Kvarnvik C, Soljegard E, Charalampakis G, et al. Periodontal disease in a remote Asian population: association between clinical and microbiological parameters. J Investig Clin Dent. 2016;7:246–253. doi: 10.1111/jicd.12156. [DOI] [PubMed] [Google Scholar]
- 58.Lavu V, Venkatesan V, Bhaskar LV, et al. Polymorphic regions in Fcgr and Tnf alpha genes and susceptibility to chronic periodontitis in a cohort from South India. J Periodontol. 2016;87:914–922. doi: 10.1902/jop.2016.150743. [DOI] [PubMed] [Google Scholar]
- 59.Lütfioğlu M, Aydoğdu A, Sakallioğlu EE, et al. Gingival crevicular fluid interleukin-8 and lipoxin A4 levels of smokers and nonsmokers with different periodontal status: a cross-sectional study. J Periodontal Res. 2015;51:471–480. doi: 10.1111/jre.12324. [DOI] [PubMed] [Google Scholar]
- 60.Meenawat A, Govila V, Goel S, et al. Evaluation of the effect of nicotine and metabolites on the periodontal status and the mRNA expression of interleukin-1β in smokers with chronic periodontitis. J Indian Soc Periodontol. 2015;19:381–387. doi: 10.4103/0972-124X.157879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mesa F, Magán-Fernández A, Muñoz R, et al. Catecholamine metabolites in Urine, as chronic stress biomarkers, are associated with higher risk of chronic periodontitis in adults. J Periodontol. 2014;85:1755–1762. doi: 10.1902/jop.2014.140209. [DOI] [PubMed] [Google Scholar]
- 62.Perayil J, Suresh N, Fenol A, et al. Comparison of glycated hemoglobin levels in individuals without diabetes and with and without periodontitis before and after non-surgical periodontal therapy. J Periodontol. 2014;85:1658–1666. doi: 10.1902/jop.2014.130661. [DOI] [PubMed] [Google Scholar]
- 63.Pereira AL, Franco GC, Cortelli SC, et al. Influence of periodontal status and periodontopathogens on levels of oral human β-defensin-2 in saliva. J Periodontol. 2013;84:1445–1453. doi: 10.1902/jop.2012.120321. [DOI] [PubMed] [Google Scholar]
- 64.Petrović SM, Zelić K, Milašin J, et al. Detection of herpes simplex virus type 1 in gingival crevicular fluid of gingival sulcus/periodontal pocket using polymerase chain reaction. Srp Arh Celok Lek. 2014;142:296–300. [PubMed] [Google Scholar]
- 65.Pranckeviciene A, Siudikiene J, Ostrauskas R, et al. Severity of periodontal disease in adult patients with diabetes mellitus in relation to the type of diabetes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:117–123. doi: 10.5507/bp.2013.098. [DOI] [PubMed] [Google Scholar]
- 66.Puri K, Chhokra M, Dodwad V, et al. Association of interleukin-1 α (-889) gene polymorphism in patients with generalized aggressive and chronic periodontitis. Dent Res J (Isfahan) 2015;12:76–82. doi: 10.4103/1735-3327.150338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh P, Gupta ND, Bey A, et al. Salivary TNF-alpha: a potential marker of periodontal destruction. J Indian Soc Periodontol. 2014;18:306–310. doi: 10.4103/0972-124X.134566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toyman U, Tüter G, Kurtiş B, et al. Evaluation of gingival crevicular fluid levels of tissue plasminogen activator, plasminogen activator inhibitor 2, matrix metalloproteinase-3 and interleukin 1-β in patients with different periodontal diseases. J Periodontal Res. 2015;50:44–51. doi: 10.1111/jre.12179. [DOI] [PubMed] [Google Scholar]
- 69.Varghese SS, Thomas H, Jayakumar ND, et al. Estimation of salivary tumor necrosis factor-alpha in chronic and aggressive periodontitis patients. Contemp Clin Dent. 2015;6:S152–S156. doi: 10.4103/0976-237X.166816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nelson RG, Shlossman M, Budding LM, et al. Periodontal disease and NIDDM in Pima Indians. Diabetes Care. 1990;13:836–840. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- 71.Papapanou PN. Periodontal diseases: epidemiology. Ann Periodontol. 1996;1:1–36. doi: 10.1902/annals.1996.1.1.1. [DOI] [PubMed] [Google Scholar]
- 72.Suvan J, D'Aiuto F, Moles DR, et al. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes Rev. 2011;12:e381–e404. doi: 10.1111/j.1467-789X.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- 73.Zimmermann H, Zimmermann N, Hagenfeld D, et al. Is frequency of tooth brushing a risk factor for periodontitis? A systematic review and meta-analysis. Community Dent Oral Epidemiol. 2015;43:116–127. doi: 10.1111/cdoe.12126. [DOI] [PubMed] [Google Scholar]
- 74.Berchier CE, Slot DE, Haps S, et al. The efficacy of dental floss in addition to a toothbrush on plaque and parameters of gingival inflammation: a systematic review. Int J Dent Hyg. 2008;6:265–279. doi: 10.1111/j.1601-5037.2008.00336.x. [DOI] [PubMed] [Google Scholar]
- 75.Tonetti MS, Eickholz P, Loos BG, et al. Principles in prevention of periodontal diseases: consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J Clin Periodontol. 2015;42(Suppl 16):S5–S11. doi: 10.1111/jcpe.12368. [DOI] [PubMed] [Google Scholar]
- 76.Slot DE, Van der Weijden FA. Group A. Initiator paper. Plaque control: home remedies practiced in developing countries. J Int Acad Periodontol. 2015;1(Suppl):4–15. [PubMed] [Google Scholar]
- 77.Salzer S, Slot DE, Van der Weijden FA, et al. Efficacy of inter-dental mechanical plaque control in managing gingivitis–a meta-review. J Clin Periodontol. 2015;42(Suppl 16):S92–S105. doi: 10.1111/jcpe.12363. [DOI] [PubMed] [Google Scholar]