Abstract
Introduction
We present findings of a novel and ecologically relevant associative memory test, the Object Location Touchscreen Test (OLTT), which was posited as sensitive to early medial temporal lobe compromise associated with mild cognitive impairment (MCI).
Methods
A total of 114 participants, including healthy young and older controls and patients with MCI, completed the OLTT and standard neuropsychological testing. The OLTT required participants to recall the location of objects under free and cued recall conditions, with accuracy evaluated using distance measures (i.e., a continuous error score), and a standard recognition format. Correlations between performance and volumetric data were evaluated from a subset of 77 participants.
Results
Significant age effects were dwarfed by MCI effects across all test conditions. OLTT Cued Recall was strongly and specifically related to the volume of disease-relevant medial temporal lobe regions, generally more than traditional memory tests.
Discussion
The OLTT may be sensitive to early structural compromise in regions affected by Alzheimer's disease.
Keywords: MCI, Alzheimer's disease, Dementia, Ecological validity, Hippocampus, Entorhinal cortex, MRI, Volumetrics, Brain volume, Memory, Cognitive
Highlights
-
•
Evaluated age and mild cognitive impairment effects using ecologically relevant object location (OL) task.
-
•
Performance evaluated using both continuous and dichotomous measures of accuracy.
-
•
Greater decline in OL memory with mild cognitive impairment than with “healthy” aging.
-
•
Performance, especially continuous measure, reflected medial temporal integrity.
-
•
Novel OL memory task may be sensitive to early structural compromise.
1. Introduction
A substantial body of evidence indicates that Alzheimer's disease (AD) begins years before the onset of clinical symptoms [1], with neurofibrillary tau deposition in the transentorhinal region of the medial temporal lobes being among the earliest pathological changes [2], [3] and particularly associated with cognitive decline [4]. This anatomic location is significant because the entorhinal cortex serves as (1) the convergence zone for multimodal input from the amygdala, perirhinal, parahippocampal, auditory, and olfactory cortices and (2) the “gateway” to the hippocampus via the perforant and alvear pathways that directly innervate the subiculum, CA1, CA3, and dentate gyrus [5], [6]. Not surprisingly then, memory deficits are typically the first cognitive symptom of AD and form the basis for the diagnosis of (amnestic) mild cognitive impairment (MCI) [7], [8], generally considered a precursor to the dementia associated with AD. Such memory deficits are typically quantified using accepted neuropsychological measures such as memory for word lists, prose, and visuospatial designs, yet older adults [9] and early-stage patients frequently report trouble with associative tasks such as recalling the locations of objects [10], [11]. Thus, there may be particular merit in using paradigms that emulate such real-world complaints when evaluating memory in older adults.
A few previous studies have examined object location (OL) memory in patients with MCI, and all have reported significant impairment relative to cognitively intact older adults [12], [13], [14], [15]. Such findings are intriguing in light of the cognitive processes and associated neuroanatomic correlates of OL memories (see [15], [16], [17]). Specifically, to successfully form a new OL memory, an individual must engage ventral visual stream areas, including the perirhinal cortex [18], [19], to identify the object. Processing the object's location is known to engage the parahippocampal gyrus [20]. These different aspects of information about the object must be held in mind via the lateral frontoparietal working memory network until they are bound into a long-term memory, with associative binding generally believed to occur in the hippocampus [21]. The transfer of information from working- to long-term memory is consonant with Baddeley's [22] construct of an episodic buffer, which may be mediated by the entorhinal cortex in the case of OL memories based on the above noted projections into and out of this region as well as neurophysiological evidence of persistent neuronal activation during time delays (see [23]). Thus, early AD-related pathology in the entorhinal region may result in an incomplete integration of objects and their locations, thereby compromising the hippocampal binding process.
The present study extends our earlier work with OL memory [15], [17], [24], [25] and had two primary goals. First, we examined the effects of age and the cognitive phenotype of amnestic MCI on OL memory. While earlier studies required participants to remember an object's location within a standard [12], [14] or modified grid (street map) [13] using the traditional dichotomous view of memory (i.e., each item scored as correct vs. incorrect), our paradigm presents common objects within realistic environments [15], [17], [26] and quantifies the magnitude of impairment using a distance measurement. We posit that this continuous memory measure is especially sensitive to neuroanatomical compromise. Thus, our second goal was to evaluate the relationship between OL memory and the volume of the medial temporal lobes, as measured via magnetic resonance imaging (MRI) data. We predicted that performance would be related to the volume of the entorhinal and parahippocampal cortices and the hippocampus.
2. Methods
2.1. Participants
A total of 114 participants completed the Object Location Touchscreen Test (OLTT) and a brief neuropsychological protocol (see Table 1). Of these, 36 were healthy young controls (HYCs) and 31 were healthy older controls (HOCs). These participants were free of subjective complaints or objective evidence of memory impairment (i.e., they performed within 1 SD of the mean on the Immediate and Delayed Memory Indices of the Repeatable Battery for the Assessment of Neuropsychological Status [RBANS]) and were independent in all instrumental activities of daily living. The remaining 47 participants had been diagnosed with amnestic MCI according to Petersen's criteria [7] during a consensus conference before study referral. Specifically, there was a subjective decline in memory (reported by the patient or an informant) and objective evidence of memory impairment within the context of relatively preserved everyday functioning. The measures in Table 1 were independent of those used to determine the clinical diagnosis of MCI, thereby reinforcing the stability of the observed memory deficits.
Table 1.
Demographic and neuropsychological test performance data
| HYC (n = 36) |
HOC (n = 31) |
MCI (n = 47) |
HYC vs. HOC t1,65 = |
HOC vs. MCI t1,76 = |
|
|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |||
| Age (years) | 24.47 (5.1) | 70.35 (5.8) | 72.15 (7.4) | 34.47 (P < .001) | 1.14 (P = .26) |
| Education | 16.08 (2.1) | 16.77 (1.8) | 15.87 (2.7) | 1.46 (P = .15) | 1.62 (P = .11) |
| WTAR (scaled score) | 116.94 (5.9) | 115.32 (9.2) | 110.34 (13.8) | 0.86 (P = .39) | 1.77 (P = .08) |
| RBANS indices (standard score) | |||||
| Immediate memory | 107.28 (11.9) | 112.29 (10.8) | 86.45 (14.3) | 1.79 (P = .08) | 8.58 (P < .001) |
| Visuospatial/constructional | 104.19 (10.8) | 100.65 (14.6) | 95.15 (17.1) | 1.14 (P = .15) | 1.47 (P = .15) |
| Language | 104.75 (10.9) | 104.71 (11.1) | 93.89 (15.4) | 0.15 (P = .99) | 3.37 (P = .001) |
| Attention | 109.36 (14.8) | 107.03 (13.8) | 97.60 (12.9) | 0.66 (P = .5) | 3.08 (P = .003) |
| Delayed memory | 103.81 (7.4) | 107.55 (8.3) | 81.34 (17.6) | 1.96 (P = .06) | 7.74 (P < .001) |
| Total | 108.25 (11.7) | 108.97 (11.9) | 88.53 (10.5) | 0.25 (P = .8) | 7.97 (P < .001) |
| Trail Making Test A (in T-score) | 47.70 (12.5) | 49.81 (8.6) | 48.43 (11.7) | 0.73∗ (P = .47) | 0.56 (P = .58) |
| Trail Making Test B (in T-score) | 52.70 (9.7) | 50.87 (9.4) | 48.67 (12.1) | 0.70∗ (P = .49) | 0.85 (P = .40) |
| EWCST (raw scores) | |||||
| Sorts completed | - | 5.09 (1.1) | 3.19 (1.71) | n/a | 5.47 (P < .001) |
| Preservative errors | - | 1.77 (2.6) | 6.78 (6.83) | n/a | 3.90 (P < .001) |
| Set loss errors | - | 0.94 (1.4) | 1.62 (1.74) | n/a | 1.74 (P = .07) |
| OLTT | See Results | ||||
| Calibration | 0.36 (0.1) | 0.30 (0.15) | 0.39 (0.10) | ||
| Free recall | 5.80 (2.3) | 6.92 (2.0) | 10.96 (2.86) | ||
| Cued recall | 2.59 (1.9) | 4.24 (2.7) | 8.58 (2.88) | ||
| Recognition | 13.56 (1.8) | 12.29 (2.44) | 8.62 (2.21) | ||
Abbreviations: HYC, healthy young controls; HOC, healthy older controls; MCI, mild cognitive impairment; WTAR, Wechsler Test of Adult Reading; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status (standard scores provided); EWCST, Emory Short form of the Wisconsin Card Sorting Test; OLTT, Object Location Touchscreen Test.
Some of the healthy young did not complete the TMT, which resulted in t1,52.
General exclusion criteria included history of neurologic diseases other than MCI (e.g., stroke, moderate–severe traumatic brain injury), psychiatric disorders (e.g., severe depression, bipolar disorder), current or past substance dependence, and learning or attentional disorders.
Participants were recruited from the Atlanta, GA, area between December 2011 and January 2014. All testing was performed in a quiet office setting. The Emory University Institutional Review Board and Atlanta VAMC R&D committee approved the study methods. All participants provided written informed consent.
2.2. Object Location Touchscreen Test
We recently detailed the development and structure of the OLTT and, therefore, provide only a summary below (see [26]). It arose from our previous functional MRI research [15] and was designed as an ecologically relevant memory task that emulates everyday memory demands. In each of three versions of the OLTT, participants were instructed to learn the locations of 15 objects within 5 rooms (3 objects per room) that were presented on a computer screen. Memory was tested following a 15-minute delay (see below).
The OLTT was run using a Dell laptop computer and a 19” ELO touchscreen monitor (model 1915L) using a locally developed software program. The program automatically randomized the order of stimuli for each administration and phase, thereby eliminating any potential order effects.
2.2.1. Stimuli
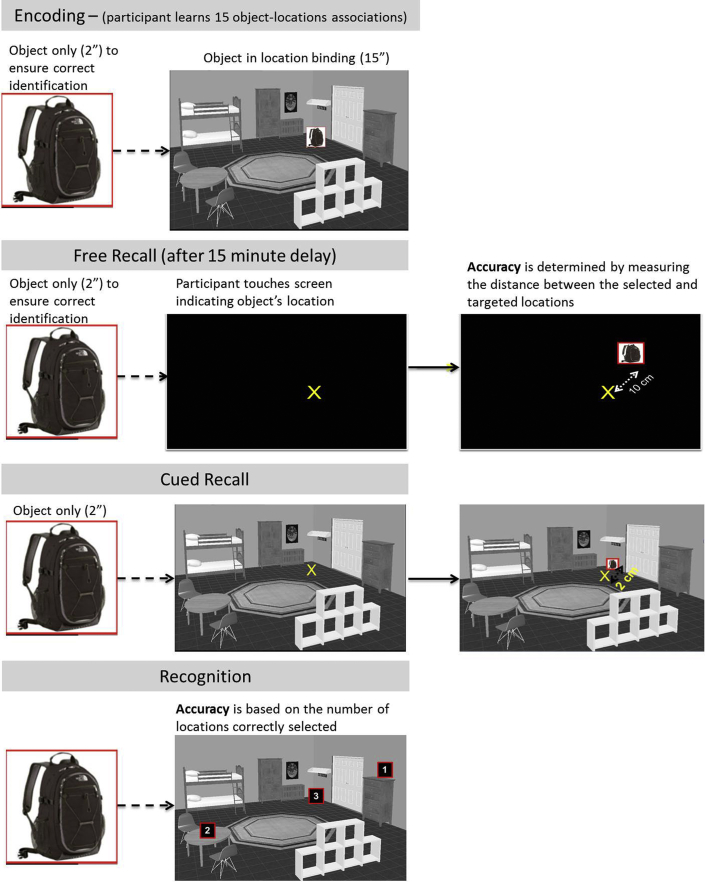
Following our earlier procedures [15], 45 common household objects were selected (15 stimuli per version), and we created 15 rooms using a computerized design program (www.Plan3d.com) that were used to create three OLTT versions. Within each room, we selected three locations and pseudorandomly assigned objects to each location. These OL pairs were inspected to ensure if there were no inherent (or implicit) relationships and to ensure that each object could reasonably be found in at least two of the room types (see example in Fig. 1).
Fig. 1.
Example from the Object Location Touchscreen Task.
2.2.2. Calibration phase
Participants began by touching a series of dots that appeared across the width and height of the screen, which provided an opportunity to practice using the touchscreen and ensured that they were accurately processing and touching the targeted location (e.g., ruling out dysmetria). The dependent variable of interest was the distance (in cm) between the “touched” point and the center of the target dot.
2.2.3. Encoding phase
Each of the 15 OL associations was presented for a total of 17 seconds; the object was first presented alone for 2 seconds (participants were required to name the object to ensure if it was accurately identified), immediately after which the object was shown in its location for 15 seconds. We allowed participants to take notes on a sheet of paper but removed this at the end of this phase. A 15-minute delay followed this phase and was filled with questionnaires (see Table 1).
2.2.4. Memory test phase (after 15-minute delay)
Memory was assessed for all 15 stimuli under three unique conditions that occurred in consecutive order. Free recall: First, participants saw a target object (2 seconds), presented in the center of the screen, followed by a blank screen and were instructed to touch the remembered location of the object as if it were in the room in which it appeared during encoding. This condition was designed to assess the spatial aspects of the memories. Cued recall: Participants saw the object (2 seconds) and then its associated room (without the object present) and were instructed to touch the remembered location of the object. This condition was designed to assess the associational aspects of the memories (i.e., the object to location binding). The primary dependent variable for both of these conditions was the distance (in cm) between the selected and target location (lower scores indicate more accurate performance). This approach allowed us to quantify the severity of memory failure on each trial, as opposed to relying on the traditional dichotomous assessment of memory as correct or incorrect. Recognition: Finally, participants completed a recognition trial in which they selected the location of the object from three potential locations. Including this trial allowed us to place the results within the context of traditional dichotomous views of memory. The dependent variable was the number of correctly selected locations.
2.3. Magnetic resonance imaging
A subset of 77 right-handed participants underwent MRI as part of other studies in our laboratory (26 HYC, 20 HOC, 31 MCI). All scans were performed using a Siemens Trio 3T MRI Scanner with a 12-channel head coil. High-resolution anatomic images were acquired using a 3D Magnetization Prepared Rapid Acquisition Gradient Echo (MPRAGE) sequence (TR 2300 ms, TE 3.9 ms, FA 8°) with 176, 1-mm-thick, sagittal slices (FOV 256 mm, in-plane resolution 1 × 1 mm, in-plane matrix 256 × 256). A senior MR technologist visually inspected the data and suboptimal scans were repeated (and replaced) at the time of acquisition to ensure usable data.
2.3.1. Volumetric analyses
Anatomic data sets were processed and analyzed with FreeSurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu/) following the automated procedure for volumetric measures described in detail by Fischl et al. [27], [28]. Hippocampal subfields were segmented using models as described by Van Leemput et al. [29]. All images were manually inspected and corrected (by S.T.) as necessary including using FSL [30] for further bias field correction [31], averaging with a second anatomic T1 when available and then reprocessed using Freesurfer. All brain volumes were normalized to total intracranial volume and expressed as percentages of intracranial volume (% ICV).
2.4. Statistical analyses
Analyses were performed using SPSS 22 software. Results comparing demographic and OLTT data were considered significant if P ≤ .05 (two-tailed). Calibration phase data from one HYC and free recall data from one MCI patient could not be retrieved, which resulted in one fewer degree of freedom in those particular contrasts. Analysis of variance showed that there were no significant sex effects on any OLTT measure within the entire sample (all P ≥ .13) or each group (all P ≥ .07), which justified considering males and females together in the primary between-group analyses. There was no effect of test version on any of the outcome measures in the entire sample using one-way analysis of variance (calibration P = .85, free recall P = .78, cued recall P = .39, recognition P = .98). However, more HYC completed version A (n = 22) than B (n = 3) or C (n = 11), whereas the distribution was more even in the HOC (A = 8, B = 10, C = 13) and MCI groups (A = 19, B = 14, C = 14). Therefore, we covaried test version for subsequent analyses. Planned contrasts were used to examine age (i.e., HYC vs. HOC) and “disease” effects (i.e., HOC vs. MCI). The contrast of HYC versus MCI was not evaluated because it reflects both factors (i.e., age and disease).
To evaluate brain-behavior relationships related to our second goal, we performed partial correlations between each OLTT phase and volumes of the entorhinal cortex, parahippocampal gyrus, and hippocampus, separately for the left and right hemispheres. Covariates included OLTT version, age, and sex, of which the latter two can affect brain volumes. To provide an unbiased (i.e., data-driven) matrix of relationships, we performed exploratory analyses that included hippocampal subfields and all of the brain regions that FreeSurfer provides. Performances on the RBANS memory subtests were included in these exploratory analyses as accepted points of reference. We corrected these brain-behavior correlations for multiple comparisons using the false discovery rate as this method minimizes both type I and II errors [32], [33].
3. Results
3.1. Group characteristics
The control groups only differed by age. While the HOC and MCI groups were comparable demographically, MCI patients performed significantly worse on the Immediate and Delayed Memory Indices of the RBANS. Although MCI patients also performed significantly worse than HOC on the Attention (due to poorer performance on the Coding subtest) and Language (due to reduced semantic fluency) Indices, the group average was still within 1 SD of the mean (i.e., within normal limits). MCI patients also completed fewer sorts and had more errors during the Emory version of the Wisconsin Card Sorting Test compared to the HOC. Thus, the pattern of neuropsychological impairment in the MCI group is consistent with that of individuals who are at increased risk of converting to dementia, especially that due to AD [7] (Table 1).
3.2. OLTT performances
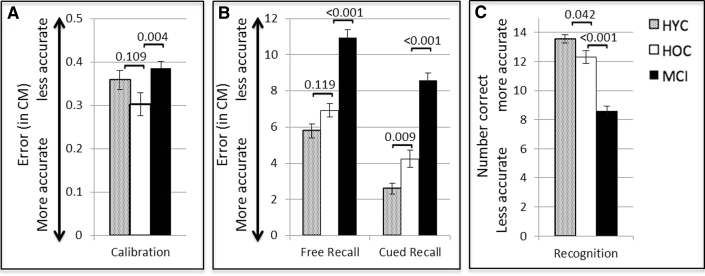
Calibration phase: There were no significant differences between the HYC and HOC during the calibration phase (t(65) = 1.62, P = .11). However, MCI patients were significantly less accurate than the HOC (t(75) = 2.05, P = .004), which justified using calibration accuracy as an additional covariate in free and cued recall analyses with these older groups (Fig. 2).
Fig. 2.
Performances on the OLTT during the Calibration (A), Free and Cued Recall (B), and Recognition phases (C). Error bars represent the standard error of measurement (SEM). Between group P-values are shown. Abbreviations: HYC, healthy young control; HOC, healthy older control; OLTT, Object Location Touchscreen Test; MCI, mild cognitive impairment.
Free recall: There were no significant differences between the HYC and HOC groups (F2,63 = 2.20, P = .12, pη2 = 0.07); however, the HOC outperformed the MCI patients (F3,73 = 15.26, P < .001, pη2 = 0.39).
Cued recall: HYC outperformed HOC (F2,63 = 5.09, P = .009, pη2 = 0.14), and HOC outperformed MCI (F3,73 = 15.3, P < .001, pη2 = 0.39).
Recognition: HYC outperformed HOC (F2,63 = 3.34, P = .04, pη2 = 0.10), and HOC outperformed MCI (F3,73 = 23.55, P < .001, pη2 = 0.39).
3.3. Correlations
3.3.1. OLTT & RBANS
There were significant relationships between OLTT memory phases and the RBANS memory subtests; however, approximately 68–88% of the variance in test performances was unaccounted for, suggesting that these measures assess different aspects of memory (Table 2). A critical question, addressed below, is whether OLTT performance is related to medial temporal lobe volume (Table 3).
Table 2.
Partial correlations between OLTT phases and RBANS memory subtests (controlling for age, sex, and OLTT version)
| OLTT Free Recall | OLTT Cued Recall | OLTT Recognition | RBANS List Recall | RBANS List Recognition | RBANS Story Recall | RBANS Figure Recall | |
|---|---|---|---|---|---|---|---|
| OLTT Calibration | .201 (.09) | .198 (.10) | −.144 (.23) | −.221 (.06) | −.173 (.15) | −.204 (.09) | −.330 (.005) |
| OLTT Free Recall | .823 (<.001) | −.765 (<.001) | −.562 (<.001) | −.445 (<.001) | −.488 (<.001) | −.435 (<.001) | |
| OLTT Cued Recall | −.900 (<.001) | −.559 (<.001) | −.424 (<.001) | −.514 (<.001) | −.454 (<.001) | ||
| OLTT Recognition | .538 (<.001) | .384 (<.001) | .500 (<.001) | .347 (<.001) | |||
| RBANS List Recall | .573 (<.001) | .737 (<.001) | .521 (<.001) | ||||
| RBANS List Recognition | .545 (<.001) | .422 (<.001) | |||||
| RBANS Story Recall | .512 (<.001) |
Abbreviations: OLTT, Object Location Touchscreen Test; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
NOTE: OLTT Calibration, Free Recall, and Cued Recall used error scores where higher values represented worse performance. All df = 107. Bolded values indicate correlations surpassing the FDR corrected P ≤ .0258.
Table 3.
Left and right hemisphere partial correlation results (Pearson's r) for the three medial temporal lobe regions of interest controlling for age, sex, and test version for the OLTT while RBANS tests controlled for age and sex
| OLTT Calibration∗ | OLTT Free Recall∗ | OLTT Cued Recall | OLTT Recognition | RBANS List recall | RBANS List Recognition | RBANS Story Recall | RBANS Figure Recall | |
|---|---|---|---|---|---|---|---|---|
| Left hemisphere | ||||||||
| Entorhinal | −.165 (.17) | −.143 (.23) | −.312 (.008) | .239 (.043) | .156 (.18) | .257 (.026) | .213 (.07) | .291 (.01) |
| Parahippocampal | −.141 (.24) | −.216 (.07) | −.291 (.013) | .151 (.20) | .219 (.06) | .278 (.016) | .223 (.055) | .303 (.008) |
| Hippocampus | −.195 (.10) | −.225 (.058) | −.298 (.01) | .217 (.07) | .270 (.019) | .281 (.015) | .301 (.009) | .409 (<.001) |
| Right hemisphere | ||||||||
| Entorhinal | −.050 (.68) | −.393 (.001) | −.494 (<.001) | .404 (<.001) | .345 (.002) | .255 (.027) | .291 (.01) | .216 (.06) |
| Parahippocampal | −.266 (.024) | −.190 (.11) | −.267 (.023) | .174 (.14) | .243 (.035) | .216 (.06) | .341 (.003) | .360 (.002) |
| Hippocampus | −.184 (.12) | −.195 (.10) | −.255 (.03) | .153 (.20) | .282 (.014) | .370 (.001) | .259 (.025) | .466 (<.001) |
Abbreviations: OLTT, Object Location Touchscreen Test; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status.
NOTE: Bolded values surpassed the FDR correction threshold of P ≤ .026. Uncorrected P-values are in parentheses.
Calibration and free recall data were lost for one participant, which resulted in df = 71; df = 72 for all other OLTT correlations; df = 73 for all RBANS correlations.
3.3.2. Brain-behavior relationships
Overall, performance on OLTT Cued Recall was robustly associated with the volume of the three key medial temporal lobe regions, especially that of the entorhinal cortex bilaterally. In contrast, OLTT Free Recall and Recognition phases were significantly related to only the right entorhinal cortex volume. Given the observed partial correlations with entorhinal volume, we tested the equality of the correlation coefficients between the OLTT Cued Recall condition with Free Recall and Recognition using an online calculator ([34]; also see [35]). Significance was assessed at P < .05 (one-tailed) because the relationship was known to be larger for cued recall relative to any other memory measure, which supports directional testing. These comparisons revealed that cued recall was significantly more highly associated with entorhinal volume compared to free recall (z = 2.42, P = .008) or recognition (z = 1.96, P = .025), whereas these latter two phases were not significantly different (z = 0.81, P = .21). Likewise, cued recall was significantly more related to entorhinal volume than RBANS list (z = 1.75, P = .04) and story recall (z = 1.64, P = .05), with trends also evident relative to list recognition (z = 1.42, P = .08) and figure recall (z = 1.52, P = .06) (Table 3).
Supplementary Tables 1 and 2 show OLTT and RBANS correlations with the full set of FreeSurfer brain volumes. Within the left cerebral hemisphere, OLTT Cued Recall performance was significantly related to the inferior lateral ventricles (positive relationship), entorhinal and parahippocampal cortices (inverse relationships), and the hippocampus (whole and CA2/3, CA4/dentate gyrus, and subiculum; inverse relationships). OLTT Cued Recall performance exhibited a significant inverse relationship with only one region outside of the medial temporal lobe: the superior temporal gyrus. Note that inverse relationships are due to the use of error scores in the OLTT. Performance on the RBANS Figure recall subtest demonstrated similar (positive) medial temporal relationships but extensive and nonspecific neocortical (superior temporal sulcus, lateral occipital, pars orbitalis, and precentral gyrus) and subcortical (anterior cingulate, thalamus, and amygdala) relationships. The other OLTT phases and RBANS subtests were generally related to subicular volume but often fell short of the false discovery rate–corrected significance threshold for other medial temporal structures.
A similar pattern emerged in the right cerebral hemisphere as OLTT cued recall performance was associated with medial temporal lobe volume (entorhinal, parahippocampal, inferior lateral ventricle, presubiculum, and subiculum) and insular volume. OLTT Free Recall (entorhinal cortex, and subiculum) and recognition (entorhinal, inferior lateral ventricle, and insula) performances were also related to some of these regions. RBANS figure recall performance again demonstrated diffuse positive relationships with neocortical, subcortical, and medial temporal lobe volumes, though not the entorhinal cortex or inferior lateral ventricle. Performances on the other RBANS subtests were variably related to medial temporal and neocortical regions.
Thus, the OLTT cued recall and RBANS figure recall were the measures most highly reflective of medial temporal lobe volume. However, these relationships were quite selective in the case of the OLTT cued recall (especially with entorhinal cortex), whereas RBANS figure recall demonstrated diffuse relationships with multiple neocortical and subcortical regions.
3.4. Discriminant function analyses
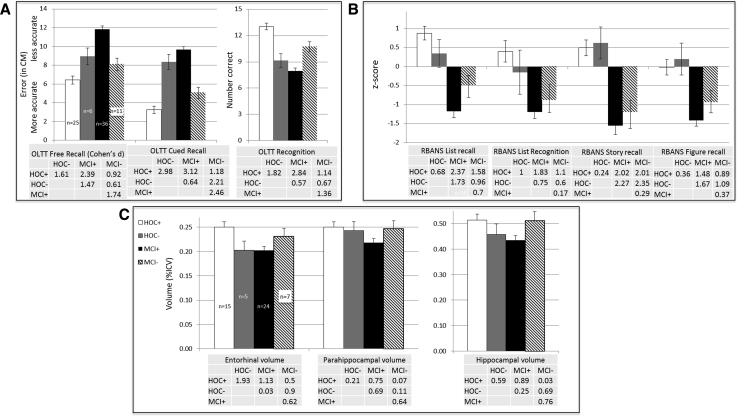
Given the robust associations between OLTT Cued Recall and medial temporal lobe integrity, we performed an exploratory discriminant function analysis using cued recall accuracy to identify older participants (i.e., HOC and MCI) whose OLTT performance was atypical of their diagnostic group. This approach allowed us to agnostically classify participants based on OLTT performance. The resulting Wilks' Lambda of 0.629 was significant (P < .001) and yielded an overall classification accuracy of 78.2% with 80.6% sensitivity and 76.6% specificity. Of particular interest were the 6 HOC classified as MCI (i.e., HOC−) and the 11 MCI classified as HOC (i.e., MCI−). As can be seen in Fig. 3, the HOC− group performed more similarly to the “true” MCI patients (MCI+) on the OLTT, but not the RBANS subtests, and also demonstrated comparable entorhinal and hippocampal volumes. The “false” MCI group (MCI−) generally performed between the MCI+ and “true” HOC (HOC+) groups and demonstrated brain volumes that were more similar to the HOC+ (Fig. 3).
Fig. 3.
Group performances on the OLTT (A) and RBANS memory subtest performances (B) based on discriminant function analyses of older participants (HC & MCI). (C) Brain volumes (in percent of intracranial volume) are shown for key medial temporal lobe regions that were correlated with OLTT performance. Tables show the effect size (in Cohen's d) between the groups in lieu of formal statistics. Error bars represent the SEM. Abbreviations: OLTT, Object Location Touchscreen Test; MCI, mild cognitive impairment; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; HOC, healthy older controls; ICV, intracranial volume.
4. Discussion
The present study had two primary aims. First, we evaluated and found significant effects of both age and MCI on cognitive performance using a novel OL memory test. Age effects were evident during the associative portions of the task (i.e., cued recall and recognition) with additional effects of MCI superimposed across all task conditions. The robust effect of MCI replicates prior work demonstrating that OL tasks are sensitive to memory impairment in patients with MCI [12], [13], [14], [15]. While we posited that the novel continuous measure of memory (i.e., OLTT Free and Cued Recall phases) would be more sensitive to memory decline than the traditional dichotomous (i.e., recognition) format, the effect sizes between MCI and HOC were actually comparable. However, our predictions were supported by the significant relationships between cued recall and the hypothesized medial temporal lobe regions, whereas only a single region was related to recognition performance (discussed in more detail below). Like prior work [14], we found a significant, but incomplete (i.e., non- one-to-one), relationship between an OL task and the standard memory test formats (RBANS; see Table 2). Such results suggest that evaluating OL memory, especially using the cued recall condition, may yield important new clinical information that is not captured by traditional testing formats.
With regard to our second goal of examining the relationship between performance and brain volumes, our study is the first to reveal that OLTT performance showed consistent associations with medial temporal lobe structures that are meaningful from both theoretical and disease-specific standpoints. Of particular interest were the findings of significantly higher relationships between OLTT Cued Recall and entorhinal volume relative to all other OLTT conditions and at least some RBANS measures. As discussed in the Introduction, the entorhinal cortex is the “gateway” into the hippocampus and is affected early in the course of AD [5], [6]. Extrapolating from neurophysiologic reports of continuous activity in the entorhinal cortex during time delays [23], we posit that reduced volume adversely affects one's ability to mentally hold and transfer OL knowledge to the hippocampus for binding.
The hemispheric relationships with OLTT performance may be important to consider because Postma et al. [16] posited that the right hemisphere preferentially mediates the spatial aspects of OL memory, whereas the left hemisphere mediates “fine-grain” contextual/environmental associations. Thus, the selective relationship between spatially demanding OLTT Free Recall performance and the right entorhinal and subiculum volumes is consonant with this model. Likewise, OLTT Cued Recall relied on both spatial processing and fine-grain knowledge of the object's location within the environment. These latter processes presumably account for relationships between cued recall and the volumes of the left entorhinal and parahippocampal cortices and hippocampus (including the subregions receiving direct projections from the entorhinal cortex). Although cued recall and recognition ostensibly engage the same cognitive processes, there were distinct differences in the brain-behavior relationships that may indicate distinct processing demands. Specifically, recognition accuracy was related to the right entorhinal and insular cortices, and to the inferior lateral ventricle volume bilaterally; findings that reinforce our call for novel methods of evaluating memory (i.e., moving beyond the traditional dichotomous response format). Importantly, these patterns emerged following the unbiased analyses of all FreeSurfer regions and stood in contrast to the diffuse relationships that emerged for some RBANS subtests (e.g., Figure Recall).
The above noted findings suggest that the OLTT may hold particular promise from an early detection standpoint. For example, a recent review found that early change in the entorhinal cortex is a sensitive biomarker that distinguishes MCI from healthy controls, reflects disease severity, and may be more sensitive to subsequent conversion to AD than the hippocampus [36]. Likewise, the rate of entorhinal atrophy was a better predictor of conversion from MCI to dementia relative to other common candidate regions (e.g., the hippocampus or prefrontal cortex) [37], [38], [39]. The exploratory discriminant function analyses are interesting within this context because the entorhinal, but not hippocampal or parahippocampal, volumes were indistinguishable between the low-performing HOC (i.e., HOC−) and the MCI + groups. In contrast, the high-performing MCI− group had volumes that were more similar to the HOC + group. Although we cannot verify the presence of AD in our MCI cohort, the relative volumetric preservation in the MCI− group raises the possibility of a non-AD etiology and/or nominal disease burden in the medial temporal lobes of these participants. It is important to note that these subgroups were not readily apparent using the RBANS subtests (Fig. 3B). We should note that the RBANS subtests all showed significant relationships with hippocampal volume bilaterally (also see [40], [41]), whereas OLTT Cued Recall was only significantly related to the volume of the left hippocampus. If replicated, such findings could allow providers to use a range of memory tests to evaluate the relative functioning of medial temporal lobe or other disease-relevant brain regions [42] in a more sensitive and specific manner.
The current cross-sectional findings require replication, and future research should use a longitudinal approach to determine whether OLTT performance truly portends cognitive decline or merely reflects normal variation in task performance and neuroanatomy. Integrating “gold standard” biomarkers of AD pathology, such as amyloid imaging or cerebrospinal fluid markers, would be particularly helpful in this regard. Visualizing tau deposition in the entorhinal cortex would be ideal and is becoming more feasible given recent ligand developments [43], [44]. Such studies will be possible in the near future because we have included the OLTT in the evaluation of a longitudinally followed cohort of older adults of varying stages and types of dementia at the Michigan Alzheimer's Disease Core Center. Finally, the continuous nature of the OLTT recall phases may make it sensitive to both pharmacologic and nonpharmacologic interventions, as we recently demonstrated using transcranial direct current stimulation [26]. Prior to such use, however, it will be important to establish psychometric properties and to evaluate the OLTT using rigorous methods like the COSMIN guidelines [45], which were beyond the scope of the present study. Planned studies will also evaluate the ecological validity of the OLTT by comparing performance with comparable tasks in the patient's home. Thus, the OLTT may join other recently developed memory tests [42], [46] as “next generation” measures that are sensitive to early cognitive decline and perhaps allow for a more targeted evaluation of biologically relevant areas.
Research in Context.
-
1.
Systematic review: The authors reviewed the literature using traditional (e.g., Web of Science, Pubmed) sources and cited relevant references appropriately. The cognitive demands of, and neuroanatomic structures supporting, object location (OL) memory may explain this commonly reported difficulty in patients with mild cognitive impairment.
-
2.
Interpretation: Our findings demonstrate that OL memory is sensitive to cognitive decline in mild cognitive impairment and appears more specific to medial temporal lobe integrity compared with standard memory test formats (e.g., word lists). Moreover, the use of continuous, rather than dichotomous, measures of memory may better reflect disease progression.
-
3.
Future directions: The sensitivity and specificity of OL memory should be evaluated using a) longitudinal data to establish its predictive capacity, b) “gold standard” biomarkers such as amyloid and tau imaging to clearly link performance with disease pathology. Continuous measures of memory may also hold promise for evaluating the effects of both pharmacologic and nonpharmacologic intervention.
Acknowledgments
Those interested in using the OLTT for academic and additional validation purposes are encouraged to contact the corresponding author. This work was supported by the Department of Veterans Affairs (B6366 W and IRX001534 to BMH) and partially by the National Institute on Aging funded Emory Alzheimer's Disease Research Center (2P50AG025688) and Michigan Alzheimer's Disease Core Center (5P30AG053760). The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2017.10.007.
Supplementary data
References
- 1.Jack C.R., Vemuri P., Wiste H.J., Weigand S.D., Lesnick T.G., Lowe V. Shapes of the trajectories of 5 major biomarkers of Alzheimer disease. Arch Neurol. 2012;69:856–867. doi: 10.1001/archneurol.2011.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H., Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 3.Braak H., Braak E. The human entorhinal cortex–normal morphology and lamina-specific pathology in various diseases. Neurosci Res. 1992;15:6–31. doi: 10.1016/0168-0102(92)90014-4. [DOI] [PubMed] [Google Scholar]
- 4.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer's disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small S.A., Schobel S.A., Buxton R.B., Witter M.P., Barnes C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canto C.B., Wouterlood F.G., Witter M.P. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008;2008:1–18. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 8.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derouesne C., Dealberto M.J., Boyer P., Lubin S., Sauron B., Piette F. Empirical evaluation of the ‘Cognitive Difficulties Scale’ for assessment of memory complaints in general practice: A study of 1628 cognitively normal subjects aged 45-75 years. Int J Geriatr Psychiatry. 1993;8:599–607. [Google Scholar]
- 10.Farias S.T., Mungas D., Harvey D.J., Simmons A., Reed B.R., DeCarli C. The measurement of everyday cognition: Development and validation of a short form of the Everyday Cognition scales. Alzheimers Dement. 2011;7:593–601. doi: 10.1016/j.jalz.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farias S.T., Mungas D., Cahn-Weiner D., Baynes K., Reed B.R., Jagust W. The measurement of Everyday Cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troyer A.K., Murphy K.J., Anderson N.D., Hayman-Abello B.A., Craik F.I.M., Moscovitch M. Item and associative memory in amnestic mild cognitive impairment: Performance on standardized memory tests. Neuropsychology. 2008;22:10–16. doi: 10.1037/0894-4105.22.1.10. [DOI] [PubMed] [Google Scholar]
- 13.Kulzow N., Kerti L., Witte V.A., Kopp U., Breitenstein C., Floel A. An object location memory paradigm for older adults with and without mild cognitive impairment. J Neurosci Methods. 2014;237:16–25. doi: 10.1016/j.jneumeth.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Kessels R.P.C., Nys G.M.S., Brands A.M.A., Van den Berg E., Van Zandvoort M.J.E. The modified Location Learning Test: Norms for the assessment of spatial memory function in neuropsychological patients. Arch Clin Neuropsychol. 2006;21:841–846. doi: 10.1016/j.acn.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Hampstead B.M., Stringer A.Y., Stilla R.F., Amaraneni A., Sathian K. Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia. 2011;49:2349–2361. doi: 10.1016/j.neuropsychologia.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postma A., Kessels R.P.C., van Asselen M. How the brain remembers and forgets where things are: The neurocognition of object-location memory. Neurosci Biobehav Rev. 2008;32:1339–1345. doi: 10.1016/j.neubiorev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Gillis M., Garcia S., Hampstead B. Working memory contributes to the encoding of object location associations: support for a 3-part model of object location memory. Behav Brain Res. 2016;311:192–200. doi: 10.1016/j.bbr.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 18.Murray E.A., Wise S.P. Why is there a special issue on perirhinal cortex in a journal called hippocampus? The perirhinal cortex in historical perspective. Hippocampus. 2012;22:1941–1951. doi: 10.1002/hipo.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Epstein R., Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 21.Mayes A., Montaldi D., Migo E. Associative memory and the medial temporal lobes. Trends Cogn Sci. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Baddeley A. Working memory: Looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 23.Ranganath C. Working memory for visual objects: Complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience. 2006;139:277–289. doi: 10.1016/j.neuroscience.2005.06.092. [DOI] [PubMed] [Google Scholar]
- 24.Hampstead B.M., Sathian K., Phillips P.A., Amaraneni A., Delaune W.R., Stringer A.Y. Mnemonic strategy training improves memory for object location associations in both healthy elderly and patients with amnestic mild cognitive impairment: a randomized, single-blind study. Neuropsychology. 2012;26:385–399. doi: 10.1037/a0027545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampstead B.M., Khoshnoodi M., Yan W., Deshpande G., Sathian K. Patterns of effective connectivity during memory encoding and retrieval differ between patients with mild cognitive impairment and healthy older adults. Neuroimage. 2016;124:997–1008. doi: 10.1016/j.neuroimage.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.England H.B., Fyock C., Gillis M.M., Hampstead B.M. Transcranial direct current stimulation modulates spatial memory in cognitively intact adults. Behav Brain Res. 2015;283:191–195. doi: 10.1016/j.bbr.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 27.Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B., Salat D.H., van der Kouwe A.J.W., Makris N., Segonne F., Quinn B.T. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Van Leemput K., Bakkour A., Benner T., Wiggins G., Wald L.L., Augustinack J. Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus. 2009;19:549–557. doi: 10.1002/hipo.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y.Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 32.Storey J.D. The positive false discovery rate: A Bayesian interpretation and the q-value. Ann Stat. 2003;31:2013–2035. [Google Scholar]
- 33.Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 34.Lee I., Preacher K. 2013. Calculation for the Test of the Difference Between Two Dependent Correlations With One Variable in Common [Computer Software]http://quantpsy.org Available at: [Google Scholar]
- 35.Steiger J. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–251. [Google Scholar]
- 36.Zhou M.X., Zhang F., Zhao L., Qian J., Dong C.B. Entorhinal cortex: a good biomarker of mild cognitive impairment and mild Alzheimer's disease. Rev Neurosci. 2016;27:185–195. doi: 10.1515/revneuro-2015-0019. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigue K.M., Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J Neurosci. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desikan R.S., Cabral H.J., Fischl B., Guttmann C.R.G., Blacker D., Hyman B.T. Temporoparietal MR imaging measures of atrophy in subjects with mild cognitive impairment that predict subsequent diagnosis of Alzheimer disease. AJNR Am J Neuroradiol. 2009;30:532–538. doi: 10.3174/ajnr.A1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devanand D.P., Bansal R., Liu J., Hao X.J., Pradhaban G., Peterson B.S. MRI hippocampal and entorhinal cortex mapping in predicting conversion to Alzheimer's disease. Neuroimage. 2012;60:1622–1629. doi: 10.1016/j.neuroimage.2012.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.England H.B., Gillis M.M., Hampstead B.M. RBANS memory indices are related to medial temporal lobe volumetrics in healthy older adults and those with mild cognitive impairment. Arch Clin Neuropsychol. 2014;29:322–328. doi: 10.1093/arclin/acu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stelmokas J., Yassay L., Giordani B., Dodge H.H., Dinov I.D., Bhaumik A. Translational MRI volumetry with NeuroQuant: Effects of version and normative data on relationships with memory performance in healthy older adults and patients with mild cognitive impairment. J Alzheimers Dis. 2017;60:1499–1510. doi: 10.3233/JAD-170306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loewenstein D.A., Curiel R.E., Greig M.T., Bauer R.M., Rosado M., Bowers D. A novel cognitive stress test for the detection of preclinical Alzheimer disease: discriminative properties and relation to amyloid load. Am J Geriatr Psychiatry. 2016;24:804–813. doi: 10.1016/j.jagp.2016.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jonasson M., Wall A., Chiotis K., Saint-Aubert L., Wilking H., Sprycha M. Tracer Kinetic Analysis of (S)-F-18-THK5117 as a PET Tracer for Assessing Tau Pathology. J Nucl Med. 2016;57:574–581. doi: 10.2967/jnumed.115.158519. [DOI] [PubMed] [Google Scholar]
- 44.Scholl M., Lockhart S.N., Schonhaut D.R., O'Neil J.P., Janabi M., Ossenkoppele R. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mokkink L.B., Terwee C.B., Patrick D.L., Alonso J., Stratford P.W., Knol D.L. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63:737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Papp K.V., Amariglio R.E., Dekhtyar M., Roy K., Wigman S., Bamfo R. Development of a psychometrically equivalent short form of the Face-Name Associative Memory Exam for use along the early Alzheimer's disease trajectory. Clin Neuropsychol. 2014;28:771–785. doi: 10.1080/13854046.2014.911351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.