Our understanding of the pathogenesis of pancreatic cancer and pancreatitis has advanced so greatly in recent years that it would hardly be an exaggeration to refer to the present time as the Pancreatic Renaissance. Unfortunately, the tangible benefits to patients have not been commensurate with the theoretical advances. In seeking to explain this disparity, most investigators view with suspicion the relevance of the models thus far used. For novel therapies to emerge, it is necessary that the animal models recapitulate the human disease with precision. This realization has engendered a vigorous search for better models, especially genetically engineered models. The key to progress lies in a sound understanding of the strengths, weaknesses, and applicability of the models in the investigator’s repertoire.
Animal Models in Pancreatic Cancer
Subcutaneous Xenograft Models
In this model, established pancreatic cancer cell lines or human pancreatic cancer tissue resected from patients are implanted subcutaneously in either nude mice or severe combined immunodeficient mice. Ease of initiation and monitoring the response to therapy by following up tumor size makes this the most simple of all models. With the use of cell lines there is concern that multiple passages of cell lines may have led to genetic alteration, thus leading to tumors that might be markedly different from real human disease. These concerns are alleviated with the use of human pancreatic tissue in which the histopathology of the xenografts maintains a phenotype identical to that of the original tumor including antigen expression and desmoplastic reaction.1 Although not studied specifically in the context of pancreatic cancer, the subcutaneous xenograft model established from human cancer has been shown to be of some value in predicting the efficacy of chemotherapeutic agents in trials.2,3 Use of immunocompromised mice is a drawback of these models because immunologic therapies such as vaccines or the immune system’s effect on the modulation of tumor growth cannot be evaluated in these models. There is hope that in the future, when an array of targeted therapies for pancreatic cancer will be available, subcutaneous xenografts created from the patient’s own resected tumor will provide a means of selecting and fine-tuning the antineoplastic agent appropriate for a given individual, and thus usher in an era of patient-targeted therapy.
Orthotopic Xenograft Models
Orthotopic models of pancreatic cancer, which use tissue grafted in the pancreas, are more complex and technically challenging to generate. However, this difficulty is offset more than adequately by its unique advantages in that the effect of the therapy on the tumor can be studied in its natural milieu. Furthermore, up to 60% of pancreatic cancer specimens in these models display locoregional spread and metastasis,4 thus closely mimicking human disease. Both pancreatic cancer cell lines and primary human pancreatic cancer tissue can be used in these models and offer distinct advantages. Tumors developed from cell lines do not show a dense stromal reaction as observed with human disease. Also, there is concern that these cells lines might have changed genetically over time. The use of human xenografts in the orthotopic setting, although labor intensive, has the advantage of using samples from real human disease and not merely cultured cells. In both systems, the use of immunodeficient mice remains an underlying disadvantage for reasons explained previously.
Genetically Engineered Models of Pancreatic Cancer
The proverbial differences between mice and men (such as their unique pharmacodynamics and pharmacokinetics), potential changes in genetic make-up of cancer with xenograft propagation, nonideal microenvironment of the xenograft, and lack of tumor–immune system interaction are some of the explanations that have been offered for the disparate therapeutic responses observed between human tumors in clinical trials and mouse xenografts.5 Efforts to address many of these shortcomings and to model pancreatic cancer genetics more precisely have led to the development of a plethora of genetically engineered mouse models of pancreatic cancer.5 The generation of these models essentially involves targeting a variety of genes to a range of cell targets (including ductal cells and acinar cells) in the pancreas to produce an array of neoplastic changes.6 Animals expressing mutant Kras in the pancreas develop a spectrum of preneoplastic changes in the form of pancreatic intraepithelial neoplasia.7 Targeting additional somatic alterations including inactivation of p16/CDKN2A,8 TP53,9 and transforming growth factor-β pathway components such as DPC4/SMAD410 in the background of mutant Kras leads to the development of tumors that recapitulate the full spectrum of pancreatic tumor development including invasive cancer, locoregional spread, and metastatic disease. Proponents of these models argue that they can be more reliable in predicting the efficacy of different therapies. For example, the combination of erlotinib and gemcitabine in a genetically engineered model of pancreatic cancer closely emulated results from a similar study in human beings.11 However, antihedgehog therapy that showed some promising results in genetically engineered models of pancreatic cancer12 has failed to provide any clinical benefit in a phase II study, suggesting that these models are not infallible.
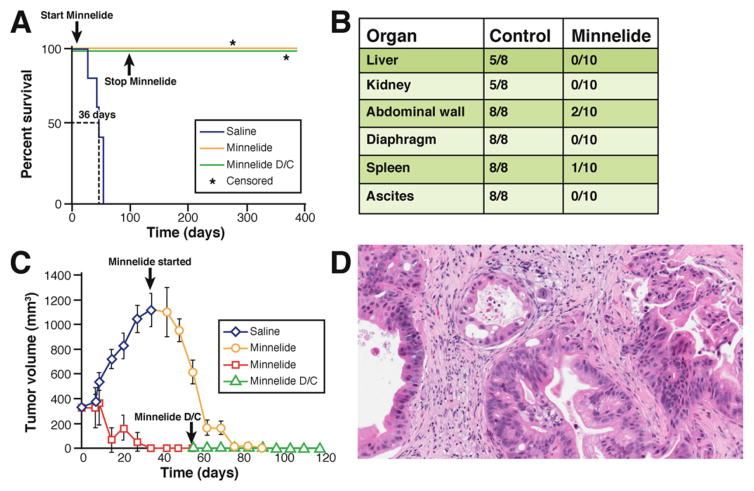
In summary, multiple mouse models of pancreatic cancer exist (Table 1). All have unique advantages and pitfalls. For this reason, novel therapies should be evaluated in more than one model. The results and conclusions gained from testing drugs in multiple complementary models will be more reliable and will have a significantly greater chance of being extrapolated to clinical studies. By using such an approach we recently evaluated the efficacy of Minnelide, an analog of the natural compound triptolide,13 in multiple animal models (orthotopic, human xenografts, and genetically engineered mice) with simulation of a variety of clinical situations and were rewarded with very promising results14 (Figure 1). Minnelide will soon be evaluated in a phase I clinical trial against pancreatic cancer.
Table 1.
Strengths and Limitations of Various Models of Pancreatic Cancer and Pancreatitis
| Pancreatic cancer models | ||
|---|---|---|
|
| ||
| Model | Strengths | Limitations |
| Subcutaneous xenograft model | Ease of establishment Ease of monitoring the tumor size Recapitulation of human tumor histology (eg, desmoplasia) with use of human pancreatic cancer xenografts Potential use of subcutaneous xenograft with pancreatic cancer tissue from patients in selecting therapy tailored to patient’s tumor |
Unnatural milieu Lack of modulation of tumor growth by immune system Concerns regarding real representation of human disease by cell lines Lack of metastasis and locoregional spread |
| Orthotopic model | Tumor growth in natural milieu Locoregional and metastatic spread with aggressive cell lines Recapitulation of human tumor histology (eg, desmoplasia) with use of human pancreatic cancer xenografts |
Technically challenging Difficult to monitor tumor size requiring advanced techniques such as ultrasound Lack of modulation of tumor growth by immune system |
| Genetically engineered model | Recapitulation of genetic changes of pancreatic cancer Use of immune-competent mice enables evaluation of effect of immune-surveillance on tumor growth |
Concerns regarding relevance to human disease because the tumor’s genetic make-up is that of mouse Cost of establishment of mouse colonies Difficulty of monitoring tumor growth Long delay in development of tumors |
|
| ||
| Models of pancreatitis | ||
|
| ||
| Cerulein model | Convenient Rapid induction Reproducible Similar histologic changes as human disease Availability of corresponding in vitro model |
Unknown clinical relevance Less severity |
| Choline-deficient ethionine supplementation diet model | Convenient Noninvasive |
Unknown clinical relevance Contribution of hepatic and central nervous system changes to mortality, which is unchanged with pancreas-targeted therapies Lack of corresponding in vitro model |
| Arginine model | Convenient Rapid induction |
Unknown clinical relevance Lack of corresponding in vitro model |
| Duct obstruction model | Rapid induction Clinically relevant |
Invasive Mild severity |
| Duct infusion model | Rapid induction Clinically relevant Recapitulation of human disease in terms of severity and systemic changes Infusion of different component of bile can help dissect their role in acute pancreatitis |
Invasive Difficult to establish the model |
Figure 1.
Potential anticancer therapies should be evaluated in multiple animal models. Thus, novel therapies should be evaluated in more than one model because the results and conclusions gained from testing drugs in multiple complementary models will be more reliable. For example, Minnelide,14 a novel water-soluble analog of a natural compound triptolide,13 was evaluated in multiple animal models in preclinical studies. (A) Kaplan–Meier curve showing that Minnelide increased survival of mice with pancreatic cancer in an orthotopic xenograft model with pancreatic cancer cell line AsPc-1. In this model AsPC-1 cells (2 × 105) were injected into the tail of the pancreas of athymic nu/nu mice. On day 7, mice were randomized to Minnelide (0.42 mg/kg) or the saline group. On day 100, the Minnelide-treated cohort (n = 10) was divided into 2 groups. One group (n = 5) continued to receive Minnelide (0.42 mg/kg), but treatment was terminated in the second group (n = 5; D/C; stop Minnelide). All the animals in the Minnelide-treated group were alive at day 385 when the experiment was terminated. Survival lines are superimposed. *Censored because deaths were unrelated to pancreatic cancer. (B) In another similar orthotopic xenograft model with pancreatic cancer cell line S2-013, Minnelide markedly reduced locoregional spread. Macroscopic analysis of metastasis in control and Minnelide-treated groups is shown. (C) Minnelide decreases tumor size in a human subcutaneous xenograft model of pancreatic cancer. A de-identified patient pancreatic tumor was implanted into severe combined immunodeficient animals that were randomized to Minnelide (0.42 mg/kg) or the saline group when the tumor reached a size of 300 mm3. In the Minnelide group the treatment was stopped on day 55 (Minnelide D/C). Even when Minnelide was stopped, the tumor did not recur. In the saline group, when the tumor reached 1000 mm3 the animals were started on Minnelide. This was associated with marked decreases in the tumor volume. (D) Tumor in the subcutaneous human xenograft model retains its original architecture. The neoplastic glands are surrounded by fibrous stromal tissue.
Animal Models of Acute Pancreatitis and Their Relevance to Human Disease
Any discussion of pancreatitis models would be incomplete without mention of the in vitro cerulein hyperstimulation acinar cell model of acute pancreatitis. Experiments in this relatively simple model have elucidated many important concepts in the pathogenesis of pancreatitis including cytosolic calcium changes, co-localization, intra-acinar zymogen activation, nuclear factor-κB activation, and inhibition of secretion, to name a few.15 Furthermore, acini from genetically engineered mice can provide important information on the role of different genes in acinar cell injury. For example, intra-acinar activation of trypsinogen has been considered the central event leading to acinar injury seen in acute pancreatitis, but conclusive evaluation of this paradigm has been elusive to date. Only recently have experiments with acinar cells from trypsinogen knockout mice largely resolved this issue.16 Although they are very useful, the relevance of these in vitro models to human disease is unclear and confirmation of their validity is required. This comes usually in the form of studies on normal human pancreatic tissue taken from the margins of pancreatic resections. However, such efforts are hampered by the contamination of these samples with tumor cells and their limited availability. The latter problem has been ameliorated considerably in recent times owing to the advent of endocrine pancreas transplantation programs, which have made healthy human exocrine pancreatic tissue from donor organs available for in vitro experiments. Seminal concepts in our understanding of acute pancreatitis such as stimulus-secretion coupling, early events during acute pancreatitis (specifically activation of digestive enzymes, cytosolic calcium changes, and co-localization of zymogens and lysosomes), and novel concepts such as direct effect of biliary salts on acini should be evaluated in these humanized models before the data can be extrapolated to human disease.
Many in vivo models of acute pancreatitis are available (Table 1). The cerulein hyperstimulation model is most widely used because of its ease of induction, noninvasiveness, and reproducibility. Although this model has been very useful for elucidation of the early events in pancreatitis, its relevance to human disease is unclear because the presence of cholecystokinin receptors on human acinar cells has been called into question. The choline-deficient ethionine-supplementation model is another simple and noninvasive model induced by feeding mice a diet rich in ethionine but deficient in choline. A severe form of pancreatitis with 80%–100% mortality after 2– 8 days is observed in this model. However, primary (not owing to multi-organ failure) changes in liver and brain are observed in this model, which contributes to mortality. Thus, interventions that limit the severity of pancreatitis may not impact mortality, limiting the usefulness of this model. The arginine-induced pancreatitis model is another relatively noninvasive model in which 1–2 injections of arginine are able to induce pancreatitis.17,18 However, the clinical relevance of this model is unclear because with the exception of case reports hyperargininemia is not a common cause of pancreatitis in human beings.
The duct obstruction model of acute pancreatitis mimics gallstone obstruction-induced acute pancreatitis in the clinical setting. It is quick and does not require a sophisticated surgical technique. However, at least in rats, duct ligation produces a mild pancreatitis without extensive necrosis or infectious complications, and thus is unsuitable to evaluate these clinically important features. In the duct perfusion model, pancreatitis is induced by infusion of various noxious stimuli such as bile salts or alcohol into the pancreatic duct. Importantly, the severity of pancreatitis in this model can be modulated by controlling the concentration, volume, infusion pressure, and time of the agent being used. The pancreatitis in this model tends to be patchy and limited to the head of the pancreas. A modification of this approach in which the intraductal bile acids are combined with intravenous cerulein shows many similarities to severe human pancreatitis in terms of morphologic changes in the pancreas, systemic signs (capillary leak, organ dysfunction, and infectious complications), and mortality, thus making it clinically relevant.19
Although some of the findings from studies of acute pancreatitis in these animal models have found their way into clinical practice (isovolemic resuscitation in early phase of acute pancreatitis, use of antibiotics in severe acute pancreatitis, and jejunal enteral feedings),20 most of the specific therapies (anti–platelet activating factor,21 antiprotease22) that have shown promise in animal models have not lived up to expectations in clinical trials. These disheartening results possibly could be explained by incorrect modeling of human disease and by incorrect use of available models. An ideal animal model should reproduce the disease in terms of etiology, symptomatology, histopathology, pathophysiology, and treatment effectiveness. The aforementioned animal models recapitulate human disease in terms of histopathology (mild edematous pancreatitis vs pancreatic necrosis), pathophysiology (trypsin activation, intracellular calcium changes, co-localization, immune cell infiltration, and cytokine storm), and outcomes (mortality and morbidity). However, with respect to the etiology of pancreatitis, the modeling of human disease in animals has been less than perfect. Gallstone disease and alcohol abuse are the most common causes of acute pancreatitis in human beings. Although duct injection or duct alteration models may have some relevance to gallstone pancreatitis, none of the models recapitulate alcoholic pancreatitis. Ethanol feeding alone in animals causes a mild and variable pathologic response in the pancreas, making investigations into the cellular and molecular mechanisms of ethanol’s effect exceedingly difficult. Most of the current knowledge regarding the pathogenesis of alcohol-induced acute pancreatitis has evolved from models in which a combination of ethanol and another agent such as cerulein has induced pancreatitis.
There is also a dearth of good models for chronic pancreatitis in mice. Animal models of chronic pancreatitis aim to recapitulate the key histopathologic features such as fibrosis and atrophy. The pancreatic duct obstruction model of chronic pancreatitis is used infrequently. The pancreatic duct ligation tends to induce atrophy and the development of fibrosis is delayed and species specific.23 One of the most commonly used models for the study of chronic pancreatitis is one in which repeated pancreatic injury induced by cerulein injection leads to pancreatic fibrosis simulating chronic pancreatitis.24 This mimics the clinical scenario in which repeated episodes of acute pancreatitis eventually lead to atrophy and fibrosis. Although exocrine insufficiency is observed in this model, for endocrine insufficiency additional stimuli are needed.25 Pancreatic fibrogenesis in this model can be enhanced further by combining it with agents such as lipopolysaccharides, ethanol, and cyclosporine A. Interestingly, long-term administration of alcohol, the most common cause of chronic pancreatitis in human beings, only leads to mild functional insufficiency and not morphologic changes. Ethanol feeding is used more commonly in combination with additional agents such as cerulein or lipopolysaccharide to develop models of chronic pancreatitis.25
There is a genuine need to simulate clinical scenarios in experimental models. When the patient presents at the hospital with acute pancreatitis, the early events are clearly underway or long past. On the other hand, in the laboratory, most studies evaluate the impact of potential therapies on the course of acute pancreatitis when they are administered either as prophylaxis (ie, before administration of pancreatic injury– causing stimuli) or during early events of pancreatitis. Thus, in future studies the impact of therapies potentially should be evaluated in models with severe pancreatitis, when administered at stages when pancreatic necrosis already has occurred, or when the cytokine storm is at its peak. This will permit a more faithful modeling of the presentation of acute pancreatitis in human beings.
In summary, a variety of models of acute pancreatitis are available. Despite this, it is necessary to increasingly use human pancreatic tissue for confirming seminal observations and to elucidate novel mechanisms, to develop new models that will correctly represent the etiology of human pancreatic disease, and to model the clinical presentation of human disease in currently available models of pancreatitis.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Capella G, Farre L, Villanueva A, et al. Orthotopic models of human pancreatic cancer. Ann N Y Acad Sci. 1999;880:103–109. doi: 10.1111/j.1749-6632.1999.tb09514.x. [DOI] [PubMed] [Google Scholar]
- 2.Fiebig HH, Maier A, Burger AM. Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer. 2004;40:802–820. doi: 10.1016/j.ejca.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JI, Decker S, Zaharevitz D, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loukopoulos P, Kanetaka K, Takamura M, et al. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas. 2004;29:193–203. doi: 10.1097/00006676-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 6.Ding Y, Cravero JD, Adrian K, et al. Modeling pancreatic cancer in vivo: from xenograft and carcinogen-induced systems to genetically engineered mice. Pancreas. 2010;39:283–292. doi: 10.1097/MPA.0b013e3181c15619. [DOI] [PubMed] [Google Scholar]
- 7.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 8.Qiu W, Sahin F, Iacobuzio-Donahue CA, et al. Disruption of p16 and activation of Kras in pancreas increase ductal adenocarcinoma formation and metastasis in vivo. Oncotarget. 2011;2:862–873. doi: 10.18632/oncotarget.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Kojima K, Vickers SM, Adsay NV, et al. Inactivation of Smad4 accelerates Kras(G12D)-mediated pancreatic neoplasia. Cancer Res. 2007;67:8121–8130. doi: 10.1158/0008-5472.CAN-06-4167. [DOI] [PubMed] [Google Scholar]
- 11.Singh M, Lima A, Molina R, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- 12.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips PA, Dudeja V, McCarroll JA, et al. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 14.Chugh R, Sangwan V, Patil SP, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4:156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saluja AK, Lerch MM, Phillips PA, et al. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- 16.Dawra R, Sah RP, Dudeja V, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217. e2. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czako L, Takacs T, Varga IS, et al. Involvement of oxygen-derived free radicals in L-arginine-induced acute pancreatitis. Dig Dis Sci. 1998;43:1770–1777. doi: 10.1023/a:1018839821176. [DOI] [PubMed] [Google Scholar]
- 18.Dawra R, Sharif R, Phillips P, et al. Development of a new mouse model of acute pancreatitis induced by administration of L-arginine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1009–G1018. doi: 10.1152/ajpgi.00167.2006. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt J, Rattner DW, Lewandrowski K, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foitzik T, Hotz HG, Eibl G, et al. Experimental models of acute pancreatitis: are they suitable for evaluating therapy? Int J Colorectal Dis. 2000;15:127–135. doi: 10.1007/s003840000216. [DOI] [PubMed] [Google Scholar]
- 21.Abu-Zidan FM, Windsor JA. Lexipafant and acute pancreatitis: a critical appraisal of the clinical trials. Eur J Surg. 2002;168:215–219. doi: 10.1080/11024150260102816. [DOI] [PubMed] [Google Scholar]
- 22.Singh VP, Chari ST. Protease inhibitors in acute pancreatitis: lessons from the bench and failed clinical trials. Gastroenterology. 2005;128:2172–2174. doi: 10.1053/j.gastro.2005.03.087. [DOI] [PubMed] [Google Scholar]
- 23.Churg A, Richter WR. Early changes in the exocrine pancreas of the dog and rat after ligation of the pancreatic duct. A light and electron microscopic study. Am J Pathol. 1971;63:521–546. [PMC free article] [PubMed] [Google Scholar]
- 24.Neuschwander-Tetri BA, Burton FR, Presti ME, et al. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci. 2000;45:665–674. doi: 10.1023/a:1005423122127. [DOI] [PubMed] [Google Scholar]
- 25.Aghdassi AA, Mayerle J, Christochowitz S, et al. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair. 2011;4:26. doi: 10.1186/1755-1536-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]