Abstract
Pancreatic cancer is estimated to be the 12th most common cancer in the United States in 2014 and yet this malignancy is the fourth leading cause of cancer-related death in the United States. Late detection and resistance to therapy are the major causes for its dismal prognosis. Apoptosis is an actively orchestrated cell death mechanism that serves to maintain tissue homoeostasis. Cancer develops from normal cells by accruing significant changes through one or more mechanisms, leading to DNA damage and mutations, which in a normal cell would induce this programmed cell death pathway. As a result, evasion of apoptosis is one of the hallmarks of cancer cells. PDAC is notoriously resistant to apoptosis, thereby explaining its aggressive nature and resistance to conventional treatment modalities. The current review is focus on understanding different intrinsic and extrinsic pathways in pancreatic cancer that may affect apoptosis in this disease.
Keywords: pancreatic cancer, apoptosis, signalling, therapy
Pancreatic ductal adenocarninoma (PDAC) is estimated to be the 12th most common cancer in the United States in 2014 and yet this malignancy is the fourth leading cause of cancer-related death in the United States. There were an estimated 46,420 new cases of pancreatic cancer and 39,590 deaths in the United States in 2014. The 5-year survival rate (2004–2010) is estimated at 6.7% which is not a substantial change from 6.2% in 2002 [Siegel et al., 2014]. Despite years of extensive research all over the world, the prognosis of pancreatic ductal adenocarcinoma (PDAC) is still dismal.
Apoptosis is an actively orchestrated cell death mechanism that serves to maintain tissue homoeostasis. Cancer develops from normal cells by accruing significant changes through one or more mechanisms, leading to DNA damage and mutations, which in a normal cell would induce this programmed cell death pathway. This would mean that most cancers, have mechanisms in place to evade apoptosis [Lowe et al., 2004; Arlt et al., 2013]. In fact, evasion of apoptosis is one of the hallmark characteristics of all cancers. In PDAC, this starts early, as the precursor lesions also lack apoptotic cells [Arlt et al., 2013; Hamacher et al., 2008]. In most treatment-responsive malignancies, the response to chemotherapy and radiotherapy is through the induction of apoptosis in the cancer cells [Schulze–Bergkamen and Krammer, 2004]. PDAC is notoriously resistant to apoptosis, thereby explaining its aggressive nature and resistance to conventional treatment modalities [Arlt et al., 2013].
ROLE OF APOPTOSIS IN BIOLOGY OF PDAC
Pancreatic ductal adenocarcinoma (PDAC) has a well worked out multi-step carcinogenesis theory. The cancer evolves through precursor lesions, known as pancreatic intraepithelial neoplasia (PanIN 1–3) to invasive ductal cancer [Hamacher et al., 2008]. PanIN 1 and 2 are hyperplastic lesions and PanIN 3 is carcinoma in situ [Ghaneh et al., 2007]. PanINs originate from the small pancreatic ducts, but the cell of origin of these PanINs or PDAC, whether ductal, acinar, or progenitor cell, still remains elusive [Reichert and Rustgi, 2011]. Gene expression from genetically engineered mouse models using mutant K-Ras supports the notion that acinar cells undergo acinar to ductal metaplasia (ADM), which functions as a precursor to PanINs [Means et al., 2005; Zhu et al., 2007; Houbracken et al., 2011]. However it is also possible that a progenitor population exists in the adult pancreas that forms PanINs with the mutant K-Ras without undergoing ADM [Rovira et al., 2010]. Different step-wise molecular alterations bring about the development of PDAC from PanINs. A gain of function mutation in K-Ras, found as early as PanIN 1, is probably the earliest event in the pathogenesis of PDAC. Other hits in the genome need to be sequentially accrued for the evolution of invasive cancer from the hyperplastic PanIN1. These include telomere shortening (PanIN1), loss of function of the tumor suppressor p16INK4a (PanIN2) and loss of function of tumor suppressors SMAD4 and TP53 (PanIN 3) [Bardeesy and DePinho, 2002; Ghaneh et al., 2007; Zhu et al., 2007]. These subcellular changes lead to upregulation of multiple prosurvival mechanisms and downregulation of apoptotic machinery as described below:
GROWTH FACTOR SIGNALING
Epidermal growth factor receptor (EGFR) family is particularly overexpressed in PDAC. EGFR is an oncogene, overexpression leads to increased cell proliferation, survival, and decreased apoptosis. These receptors are usually activated by EGF (epidermal growth factor) and TGF-α (transforming growth factor-α) in PDAC [Yarden and Sliwkowski, 2001]. On activation, they dimerize, autophosphorylating their tyrosine residues leading to activation of various intracellular pathways, namely Ras/Raf/MAPK, PI3K/AKT, and the JAK/STAT pathways [Ghaneh et al., 2007; Wong and Lemoine, 2009; Arlt et al., 2013]. These downstream changes lead to increased cell proliferation, tumor growth, and decreased apoptosis.
Ras/Raf/MAPK PATHWAY
Ras is one of the central downstream target of the EGFR family and KRAS is the most commonly mutated gene in PDAC and PanINs [Deramaudt and Rustgi, 2005]. Mutated Ras functions as an oncogene, causing continued cell proliferation, and resistance to apoptosis. Though the main downstream target of Ras is Raf leading to downstream activation of MEK1/2 and hence ERK1/2 MAPK, it also activates PI3K/Akt and PLCγ pathways [Arlt et al., 2013].
PI3k/Akt/mTOR PATHWAY
This cell survival pathway functions downstream of the EGFR or Ras protein as mentioned above. It may also be activated by other survival signals, like cytokines and hormones [Cohenuram and Saif, 2007]. It has a role to play in cell proliferation and survival and makes the cancer resistant to apoptosis [West et al., 2002]. While Akt2 gene amplification is reported in 11–20% of PDAC, upregulation of Akt pathway is present in up to 60% of PDAC [Cheng et al., 1996; Ruggeri et al., 1996; Altomare et al., 2002; Schlieman et al., 2003]. Akt once activated, acts on mTOR (mammalian target of rapamycin), c-myc, and NF-κB as its downstream targets [Hamacher et al., 2008; Wong and Lemoine, 2009]. For PDAC, this pathway is overactivated further because of PTEN (phosphatase and tensin homolog). PTEN is a tumor suppressor that functions as a negative regulator of PI3K/Akt pathway and is mutated or down regulated in 60% of PDAC [Asano et al., 2004; Xu et al., 2010].
NF-κB PATHWAY
NF-κB pathway includes dimers made of RELA, p50, and c-REL but the most common is the RELA-p50 dimer. This pathway is proinflammatory, primarily activated in response to cytokines like IL-1 and TNF α. The constitutive activation of this pathways is present in PDAC [Li et al., 2004]. Inhibition of this pathway leads to induction of apoptosis in PDAC and increases chemosensitivity [Li et al., 2004].
EMBRYONIC PATHWAYS
Sonic Hedgehog:—Three hedgehog proteins belong to this pathway, Sonic Hedgehog (SHH), Desert Hedgehog (DHH), and Indian Hedgehog (IHH). Among these, SHH, a ligand not expressed on pancreatic precursor cells or the mature pancreas, is expressed in about 70% of pancreatic cancers [Thayer et al., 2003; van den Brink, 2007]. SHH enhances proliferation of ductal epithelium and enhances K-RAS induced tumorigenesis by multiple pathways. It has been found to increase the expression of Cyclin D1 and decrease p21 in the ductal epithelium, thereby enhancing proliferation. At the same time, it makes the cells resistant to apoptosis by increasing the expression of anti-apoptotic molecules Bcl-xl and Bcl-2 [Morton et al., 2007]. SHH is found to be upregulated in pancreatic cancer stem cells [Li et al., 2009]. Recently, it has also been found that SHH leads to activation of pancreatic stellate cells and increases EMT markers like vimentin, desmin, and α-smooth muscle actin, hence causing desmoplasia [Bailey et al., 2008].
WNT pathway:—WNT/β-Catenin pathway has been realized to be important in the pathogenesis of PDAC. In the presence of WNT signal, cytoplasmic degradation of β-Catenin is inhibited, leading to increased transcription of WNT dependent genes, such as cyclin D1, c-myc, VEGF, MMP-7, and others [Freese et al., 2010]. In PDAC, this pathway has been shown to have roles in angiogenesis, resistance to apoptosis, cell cycle and maintenance of cancer stem cells [Cui et al., 2012]. Mechanistically, WNT activation, leads to increased cell proliferation by increasing the levels of cyclin D1 and c-myc. c-myc in turn further increases the expression of cyclin D1, cyclin A, CDK4, CDK2, and inhibits the cell cycle checkpoint protein p21 [Qi et al., 2007]. In addition, WNT activation inhibits apoptosis of these hyperproliferative cancer cells by increasing the expression of IAP (Inhibitor of Apoptosis Protein) and Survivin (BIRC5) [Guo et al., 2009]. Another anti-apoptotic mechanism involves WNT target MMP7, which inhibits extrinsic apoptosis by decreasing the expression of Fas receptor on the cancer cells [Almendro et al., 2009].
REGULATION OF APOPTOSIS IN PDAC
A cell can undergo apoptosis through three major pathways-Intrinsic mitochondrial, Extrinsic, or Intrinsic ER stress dependent. Cancer cells learn to evade all of them in their journey of oncogenic transformation by acquiring resistance mechanisms.
INTRINSIC MITOCHONDRIAL PATHWAY
This pathway may be activated in response to a number of intracellular derangements-DNA damage, oxidative stress, hypoxia, high intracellular calcium, to name a few. The primary organelle orchestrating this pathway is the mitochondria, which maintains a fine balance between the two types of Bcl-2 proteins, pro-apoptotic (Bax, Bad, Bim, Bak, Bid, Bik) and anti-apoptotic (Bcl-2, Bcl-XL, Mcl-1). Whenever the intracellular factors favoring apoptosis accumulate, the pro-apoptotic Bcl-2 proteins overwhelm the anti-apoptotic proteins, leading to increased permeability of the mitochondrial membrane, which causes the release of cytochrome-c and other factors like, AIF (Apoptosis Inducing Factor), Smac (Second Mitochondria-derived Activator of Caspase), DIABLO (Direct IAP Binding protein with Low pI), HtrA2 (Omi/High temperature requirement protein A) into the cytoplasm, initiating apoptotic cascade. Once in the cytoplasm, cytochrome-c binds to Apaf-1 (Apoptotic Protease Activating Factor-1) to form the multi-protein complex known as apoptosome. This complex cleaves pro-caspase 9 into its active enzyme, caspase-9. Caspase 9 furthers the cascade by cleaving procaspases 3 and 7 into active caspases 3 and 7 which are responsible for the morphological and biochemical changes we call apoptosis. Caspases are cysteine proteases, with a unique property of cleaving after aspartic acid residues. Caspase 2, 8, 9, 10, and 12 are the initiator caspases while Caspase 3, 6, and 7 are the effector caspases. The other released proteins, Smac, Diablo, and Omi/HtrA2 promote apoptosis by binding to the IAPs [Wong, 2011]. IAP family includes proteins having a BIR (Baculovirus IAP Repeat) domain which inhibits apoptosis by binding to caspases 3 and 9, thereby hindering their activation. IAP family includes proteins like C-IAP1 (BIRC2), C-IAP2 (BIRC3), X-IAP (BIRC4), Survivin (BIRC5) and many others.
PDAC CELLS ARE RESISTANT TO INTRINSIC APOPTOSIS
Anti-apoptotic/pro-apoptotic imbalance: Unlike other cancers, anti-apoptotic protein Bcl-2 is not frequently overexpressed in pancreatic cancer. In contrast, an imbalance between anti-apoptotic Bcl-XL, and pro-apoptotic Bax was found in the TGF-α murine model of pancreatic cancer, which closely simulates human cancer. In this model, the Bcl-XL activity was shown to be dependent upon activation of STAT3 and NF-κB. Hence the anti-apoptotic proteins have a role to play in precancerous lesions as well as in invasive pancreatic cancer [Greten et al., 2002].
Inhibitors of Apoptosis Proteins (IAPs): IAPs have been found to be overexpressed in preinvasive and invasive pancreatic cancer. Among the many IAPs, XIAP, cIAP, and Survivin are particularly upregulated in PDAC and precancerous lesions. Survivin, a recent addition to the IAP family, is absent from adult pancreas (normal, chronic pancreatitis) but is highly expressed in cancerous tissues. There is more frequent Survivin expression in cancerous lesions compared to preinvasive lesions [Satoh et al., 2001]. Interestingly, cIAP1 and cIAP2 overexpression have been found to correlate with chemoresistance in pancreatic cancer cells [Lopes et al., 2007]. NF–κB confers resistance to apoptosis in PDAC making them chemo and radio-resistant; one of the mechanisms is upregulation of the IAPs, especially the cIAPs and XIAP [Karin et al., 2002; Lin et al., 2010].
EXTRINSIC DEATH RECEPTOR PATHWAY
This pathway is activated in a cell in response to extracellular stimuli-binding of death ligands, TNF (Tumor Necrosis Factor), FasL, TRAIL (TNF-related apoptosis inducing ligand) to death receptors on the cell surface. These death receptors belong to the TNF Receptor family, including TNFR1, FAS (APO-1/CD95) and TRAIL (APO-2) receptor, the common thread being the intracellular domain these receptors share, known as the “death” domain. When the death ligands bind their respective receptors, adapter proteins like TRADD (TNF Receptor Associated Death Domain) and FADD (Fas Associated Death Domain) and procaspases 8, 10 are recruited to the death domain, thereby forming DISC (Death Inducing Signaling Complex). Formation of the DISC cleaves procaspase 8 into the active capsase 8, which proceeds to cleave the executioner caspases leading to apoptosis.
Despite the different mechanisms of initiation, there is a lot of cross talk among the intrinsic and extrinsic pathways. Cells are divided into two types, type I are only dependent on caspase 8 (extrinsic pathway) to cause apoptosis by itself while in type II, caspase 8 increases the permeability of the mitochondrial membrane, which then causes apoptosis. PDAC are type II cells [Hamacher et al., 2008]. Fas dependent apoptosis in such cells, hence is dependent on the cellular mitochondrial activity [Walczak and Krammer, 2000].
PDAC CELLS ARE RESISTANT TO EXTRINSIC APOPTOSIS
FAP-1:—PDAC seems resistant to the death receptor pathway, despite the presence of death receptors at the cell surface and intact downstream pathways. One of the culprits implicated is FAP-1 (Fas-associated phosphatase-1), which is overexpressed in pancreatic cancer. FAP-1 decreases the Fas-FasL interaction by inhibiting Fas translocation towards the membrane, thereby effectively downregulating membrane receptor activity [Ungefroren et al., 2001].
Decoy receptors:—Decoy receptors are identical to the death receptors, but lack the “death” domain. The most common decoy receptor overexpressed in PDAC is DcR3 (Decoy Receptor 3) which leads to decreased binding of the death ligands (FasL) to death receptors (Fas) with functional downstream activity, thereby inhibiting apoptosis [Elnemr et al., 2001].
c-FLIPL:—This molecule is structurally identical to caspase-8, but lacks the amino acids essential for caspase activity. Interestingly, c-FLIPL is overexpressed in pancreatic cancer, competing with procaspase-8 for participating in DISC formation [Elnemr et al., 2001]. Similar to DcR3, its overexpression leads to decreased apoptosis by decreasing death receptor signaling.
TRAF2:—A death receptor adaptor, upregulated in PDAC, links NF–κB with extrinsic apoptosis. TRAF2 belongs to the TNF receptor associated factor (TRAF) protein family. TRAF2 functions to inhibit TNF receptor associated death signal and instead activates NF-κB signaling. Thus upregulated TRAF2 inhibits death receptor pathway induced apoptosis and makes cancer cells more invasive by increasing NF–κB and its downstream targets IL-8 and MMP-9 [Trauzold et al., 2005].
INTRINSIC ENDOPLASMIC RETICULUM PATHWAY
This mode of apoptosis is believed to be activated when ER is injured by cellular stresses like hypoxia, free radicals, and glucose deprivation. It functions independent of the mitochondria. The initiator is thought to be caspase 12, activated in response to severe ER injury [Wong, 2011]. ER is the organelle responsible for protein folding and assembly and allows only correctly folded proteins to exit into the cytosol. It is particularly sensitive to intracellular perturbations, like DNA damage, oxidative stress, decreased ER calcium levels-all these interfere with protein folding in the ER and lead to “ER stress”. The cell deals with this stress by upregulating UPR (Unfolded Protein Response) signaling in the ER. This signaling has an important role in tumorigenesis, as cancer cells are under “physiological ER stress” because of the high replicative rate. It has been shown that UPR plays a survival role in a number of types of cancer [Healy et al., 2009; Li and Lee, 2006]. Our group, interestingly, found GRP78 (Glucose Regulated protein 78kDa), an integral survival protein in the UPR response, to be upregulated in pancreatic cancer cell lines as well as human tumor samples. GRP78 is required for activation of ER stress sensors. Most of the GRP78 resides within the ER lumen, but a minor fraction functions as a transmembrane protein, which plays a role in apoptosis. It has been found that GRP78 can bind and inhibit caspase-7 and pro-apoptotic proteins like BIK and BAX.
ROLE OF APOPTOSIS IN TREATMENT OF PANCREATIC CANCER
The currently available or investigational chemotherapies exert their anticancer effects via apoptosis. These drugs can be classified into two broad classes: Broad spectrum agents that affect multiple pathways and targeted therapies with well-defined targets.
BROAD SPECTRUM ANTICANCER AGENTS
Antimetabolites
5-FU, a nucleoside analogue, was the standard therapy for advanced pancreatic cancer (Table I) prior to the FDA approval of gemcitabine. Gemcitabine is another nucleoside analogue which is the current standard of care. After phosphorylation, gemcitabine, gets incorporated into replicating DNA resulting in premature chain termination and apoptosis [Abbruzzese, 2002]. The 1 year survival rates in a phase III trial with 126 patients were 18% for patients treated with gemcitabine vs 2% in patients treated with 5-FU [Burris et al., 1997]. However, pancreatic tumors show initial sensitivity to gemcitabine followed by the rapid development of resistance. Several genetic and epigenetic changes have been associated with this resistance that are involved in gemcitabine transport and metabolism. Alterations in hENT-1 (nucleoside transporter-1), DCK (deoxycytidine kinase), and RNR-M1 and M2 (ribonucleotide reductase), have been shown to promote gemcitabine resistance. Overexpression of prosurvival pathways like S100A4-BNIP3, NF-κB, and PI3-AKT have been implicated in gemcitabine resistance. Interestingly, all these resistance markers lead to progression towards a mesenchymal phenotype and increase in stem cell markers [Kim and Gallick, 2008].
TABLE I.
Newer Therapies for Pancreatic Cancer and Their Molecular Targets
| Treatment target | Drug description | Mechanism of action |
|---|---|---|
| 1 Fas-FasL interaction | Synthetic phosphodiester oligodeoxynucleotide | Modulate Fas-FasL expression |
| Antibodies and antisense oligonucleotide | Decrease FasL | |
| 2 Death receptors | TRAIL (TNF related apoptosis inducing ligand) and its structural modifications | Bind and activate DR4 and DR5 receptors |
| RO5458640 (humanized monoclonal antibody) | Inhibit binding of TWEAK (TNF-like weak inducer of apoptosis) ligand to its receptor (Fn14) | |
| Mapatumumab, Conatumumab/AMG 655 | Agonistic DR4/DR5 antibody | |
| 3 Bcl-2 family of proteins | Antisense oligonucleotides | Inhibit overexpression of Bcl-2 and BclXL. |
| Small molecule inhibitors (Gossypol-AT101, TW-37, PPARγ-inactive TZD derivatives, Apogossypolone, ABT 737, Obatoclax, ABT 199) | Inhibit the interaction of various anti-apoptotic Bcl-2 members to pro-apoptotic members increasing the activity of latter. BH3 mimetics | |
| 4 Inhibitor of apoptosis proteins (IAPs) | SMAC mimetics (JP 1201, TL32711 (Birinapant), LCL161, SM-164,) | Inhibit IAPs after binding to their BIR3 domain |
| Antisense oligonucleotides (AEG 35156, Embelin, Xantag) | Functional depletion of XIAP | |
| 5 Survivin | YM 155 (sepantronium bromide) | Transcriptional repressor |
| Antisense oligonucleotides (ISIS23722, EZO-3042), siRNAs | Functional depletion of survivin | |
| Hsp90 inhibitors (shepherdin, AICAR) | Interfere with survivin folding and stability | |
| 6 Caspases | Gambogic acid and its derivatives (MX-2167) | Directly activate caspase-3 |
| Reolysin (human reovirus) | Target transformed KRAS cancer cells and activate caspase-4 | |
| 7 EGFR/Ras/Raf/MAPK | EGFR inhibitors Tyrosine kinase inhibitors (Erlotinib, Geftinib) | Interfere with ATP binding site of EGFR tyrosine kinase |
| Monoclonal antibodies (Cetuximab, Matuzumab, Trastuzumab) | Inhibit EGF ligand binding to EGFR | |
| Farnesyl transferase inhibitors (Tipifarnib, Lonafarnib) | Inhibit isoprenylation of KRAS | |
| 8 PI3K/Akt/mTOR | Natural compounds (curcumin, triptolide, resveratrol) | Possible transcriptional inhibition. |
| HIV protease inhibitors (Nelfinavir), Metformin | Inhibit activation of Akt | |
| mTOR inhibitors (Everolimus, Ridaforolimus, Temsirolimus) | Bind to FKBP12 and inhibit mTOR | |
| 9 NF-κB | Natural compounds (triptolide, luteolin, curcumin, EGCG) | Possible transcriptional inhibition |
| IKK inhibitors (BAY-11–7082, BAY-11–7085) | Prevent degradation of IκB | |
| Proteasome inhibitors (bortezomib) | Prevent degradation of IκBα by inhibiting 20S proteasome. | |
| DNA binding inhibitor (SN-50) | Inhibit NF-κB nuclear translocation | |
| 10 HDAC | Class I inhibitors (Depsipeptide) | Bind to zinc containing catalytic domain of HDACs |
| Class I and II inhibitors (Vorinostat, panabinostat, abexinostat, valproic acid) |
Platinums
Oxaliplatin has been approved as a second line drug to be used in combination either with gemcitabine or FOLFIRINOX regimens. Platins interact with DNA to form DNA adducts that are primarily intrastrand crosslinks, which ultimately lead to apoptosis. However, these apoptotic signals can be attenuated and resistance to platinums is a common phenomenon in pancreatic cancer. Resistance mechanisms are due to intracellular changes that prevent platins from interacting with DNA, interfere with DNA damage signals from activating the apoptotic machinery or both. More specifically, these changes include increased DNA adduct repair by nucleotide excision repair, loss of damage recognition, activation of PI3/Akt pathway, loss of p53 function, overexpression of anti-apoptotic proteins, and decreased caspase activation [Siddik, 2003].
Taxanes
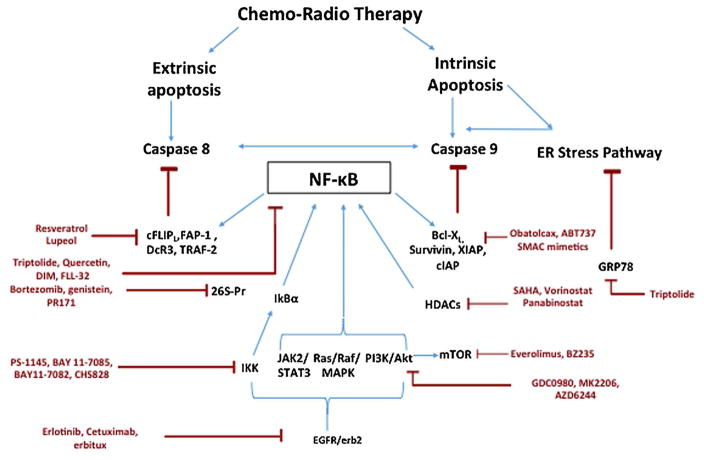
Taxanes were being used as adjunct chemotherapy in treatment of pancreatic cancer with no definitive regimen until the advent of nab-paclitaxel. This nanoparticle-based albumin-bound water soluble formulation of paclitaxel was approved as a first line drug for metastatic pancreatic cancer with gemcitabine after a successful phase III trial in 2013 [Von Hoff et al., 2013]. Taxanes cause mitotic arrest in cancer cells by disrupting microtubule function leading to apoptosis. However, PDAC cells exhibit resistance to taxanes by upregulating NF–κB [Dong et al., 2002], overexpressing G2/M phase transition proteins like polo like kinases and aurora kinases or altering the paclitaxel binding site of β-tubulin (Fig. 1 ).
Fig. 1.
Mechanisms contributing to apoptosis evasion in pancreatic cancer.
Novel therapeutic agents
Minnelide
Triptolide, derived from the Chinese herb tripterygium wilfordii, is a potent anticancer compound but is poorly soluble in water, limiting its clinical use. Minnelide, a water soluble prodrug of triptolide has been shown to be very effective against PDAC in various preclinical models and is currently undergoing phase I trials [Chugh et al., 2012]. It acts by inhibiting multiple pathways in cancer such as Sp1 [Banerjee et al., 2013], NF-κB [Liu et al., 2014] and Hsp 70 [Phillips et al., 2007], thus activating apoptotic and autophagic cascades leading to cancer cell death [Mujumdar et al., 2010]. We also found that triptolide, decreases GRP78 in pancreatic cancer cell lines, thereby activating ER stress which further augments apoptotic response [Mujumdar et al., 2010]. Although it is effective as a monotherapy in PDAC, triptolide sensitized resistant pancreatic cancer cell lines to cisplatin [Zhu et al., 2012] and TRAIL [Chen et al., 2014]. It induced cell death by downregulating Bcl-2, Bcl-XL, COX2, cyclin D1, and survivin in vitro. Our group has also shown that Minnelide synergizes with paclitaxel, oxaliplatin and TRAIL in abolishing pancreatic cancer and prolonging the survival of tumor bearing mice (unpublished data).
Curcumin
Curcumin is an inhibitor of several molecules involved in proliferation of cancer cells. It also alters the expression of microRNAs in pancreatic cancer cells [Lev-Ari et al., 2007]. Phase 2 trials of curcumin alone or in combination with gemcitabine showed that curcumin might have some biological activity in PDAC [Dhillon et al., 2008].
TARGETED THERAPIES
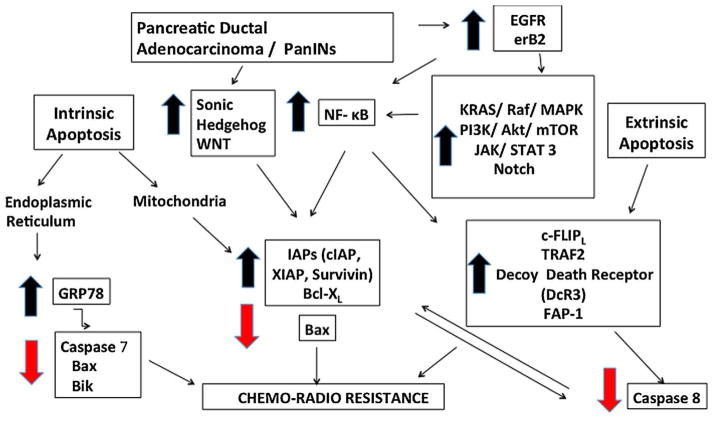
Although laboratory results of targeted therapies have been impressive, until now only erlotinib, an EGFR tyrosine kinase inhibitor, has demonstrated marginal survival benefit in combination with gemcitabine in phase III clinical trials. Even though the failures of targeted therapies in the clinical setting are discouraging for PDAC, it is reasonable to surmise that major progress will evolve as the molecular biology of apoptotic pathways in pancreatic adenocarcinoma continues to be unraveled (Fig. 2).
Fig. 2.
Novel targeted therapies in pancreatic cancer.
THERAPIES DIRECTLY TARGETING APOPTOTIC PATHWAYS
Death receptoreextrinsic apoptosis pathway
Systemic administration of TNFα and CD95L is associated with severe adverse effects precluding their therapeutic use. TRAIL, on the other hand has a greater selectivity for cancer cells via binding to DR4 and DR5 receptors. Unfortunately, a majority of PDAC cells are resistant to TRAIL mediated apoptosis due to upregulated anti apoptotic pathways. TRAIL and DR4/DR5 agonistic antibodies (Conatumumab, Tigatuzumab) in PDAC cells activate prosurvival pathways like NF-κB, ERK and PKC [Trauzold et al., 2005; Khanbolooki et al., 2006], thereby causing a paradoxical increase in tumor growth and invasiveness of PDAC as shown in an orthotopic murine model of PDAC [Trauzold et al., 2001]. Resistant pancreatic cancer cells can be sensitized to death receptor mediated apoptosis by inhibiting the prosurvival pathway NF-κB or by decreasing the expression of anti-apoptotic proteins as shown by various studies [Arlt et al., 2013].
Triptolide, as shown previously by our group effectively leads to inhibition of NF-κB pathway, augments TRAIL induced lysosomal membrane permeabilization and inhibits cFLIP, XIAP, and induces cleavage of Bid to sensitize PDAC cells to TRAIL mediated apoptosis [Chen et al., 2014]. In vivo, Minnelide synergizes with TRAIL to abolish PDAC tumors in a xenograft mouse model of TRAIL resistant PDAC cells (unpublished data). Multiple other molecules targeting this apoptotic pathway are currently being developed [Werner et al., 2011].
Mitochondrial/intrinsic apoptosis pathway
The Bcl-2 family members play the major role in this pathway. Multiple therapies ranging from antisense oligonucleotides to anti Bcl-2 antibody, to ribozymes have been tested against PDAC, but none of these have proven beneficial in clinic. On the other hand, substantial efforts have been made to develop natural or semisynthetic SMIs (small molecule inhibitors) of Bcl-2 proteins. Gossypol and its derivatives that is TW-37, AT-101 are pan Bcl-2 inhibitors that are undergoing clinical trials for various visceral and hematological malignancies. They bind to the hydrophobic groove of Bcl-2 and Bcl-xL and prevent their heterodimerization with the pro-apoptotic proteins of the same family. Other SMIs, like ABT-737 and its oral analogue ABT 269, developed by using NMR technology mimic the BH-3 domain of BAD and inhibit the anti-apoptotic Bcl-2 protein family except for Mcl-1, which is upregulated. Synergistic antitumor activity have been achieved using TRAIL and these SMIs [Wong, 2011; Masood et al., 2011; Arlt et al., 2013]. Another SMI developed via high throughput screening of natural compounds is obatoclax (indole bipyrrole), that disrupts interactions between Mcl-1 and Bak and induces Bax mediated apoptosis in solid organ malignancies. It has also been tested with TRAIL in PDAC [Wong, 2011; Masood et al., 2011].
Common apoptosis pathway
The central component for any cell undergoing apoptosis via any pathway is a proteolytic system involving caspases. Targeting these terminal components could lead to a more effective direct therapy for cancer. Several pharmacological and biological agents have been designed to synthetically activate caspases in pancreatic cancer. One example is caspase modulator I–1541, which stabilizes procaspase-3 in an “on-state” conformation that facilitates its subsequent autoactivation. It was able to induce apoptosis in multiple cell lines with defects in various apoptotic pathways upstream of caspase activation [Wolan et al., 2009]. Gambogic acid and its derivative, MX-2167, have been shown to directly activate caspase-3 and induce apoptosis in most solid organ cancers. A receptor based approach using somatostatin receptor subtype 2 was shown to activate caspase-3 and induce apoptosis in pancreatic cancer cells [Vernejoul et al., 2002]. A reovirus based therapy, reolysin, targets transformed KRas pancreatic cancer cells leading to ER stress mediated apoptosis caused by cleavage of caspase-4 [Carew et al., 2013].
IAP and survivin inhibitors
Smac/DIABLO is an intrinsic antagonist of IAPs and promotes apoptosis by antagonizing IAP-mediated caspase inhibition. Its structural analysis led to the recognition of IAP binding motif (IBM) and have helped in developing Smac mimetics/peptides and non-peptide inhibitors (SM-164) that enhance apoptosis and sensitize cells to chemotherapeutics drugs and radiation [LaCasse et al., 2008; Owens et al., 2013]. Several of these Smac mimetics are currently in phase I trials. Single stranded antisense oligonucleotides against XIAP (AEG35156), have been employed in combination with conventional chemotherapy but with little or no benefit in patients with PDAC. Others (TWX006, Xantags, and Embelin) are still being investigated [LaCasse et al., 2008; Mahadevan et al., 2013]. Survivin is one of the ubiquitous proteins found to be upregulated in human cancers and several strategies targeting survivin have been used to sensitize resistant pancreatic cancer cells to apoptosis. Antisense oligonucleotides (ISIS23722), transcriptional repressors (YM155), HSP90 antagonists (shepherdin, AICAR) and immune based anti-survivin antibodies lead to functional or chemical depletion of survivin and are being investigated in multiple solid organ cancers including pancreatic cancer [LaCasse et al., 2008; Owens et al., 2013].
SIGNAL TRANSDUCTION PATHWAYS
Epidermal growth factor receptor pathway
EGF receptor can be targeted therapeutically by monoclonal antibodies or small molecule tyrosine kinase inhibitors (TKI). Monoclonal antibodies interact with the extracellular domain of EGFR, inhibiting EGF ligand binding. The small molecule TKIs act intracellularly by interfering with the ATP binding site of the EGFR tyrosine kinase. Both the monoclonal antibodies and the small molecule TKIs efficiently block the EGFR signal transduction pathways in-vitro, inducing tumor cell cycle arrest and potentiating apoptosis. Cetuximab, matuzumab, and trastuzumab have been tested in clinical trials with mixed results [Bayraktar and Rocha-Lima, 2010]. Erlotinib, which has been FDA approved for advanced PDAC and geftinib, which is undergoing trials, are EGFR tyrosine kinase inhibitors. These TKIs also have a chemo and radiosensitizing effect as they suppress the DNA repair mechanisms induced by these conventional therapies [Bayraktar and Rocha-Lima, 2010].
Ras/Raf/MAPK pathway
Farnesyltransferase inhibitors like tipifarnib and lonafarnib that target the isoprenylation of K-Ras though very effective in in vitro studies, were not very effective in animal studies due to activation of the secondary prenylation pathway via geranylgeranyltransferase. Drugs targeting the downstream Raf kinases like sorafenib have also been tested in clinical trials but did not achieve a very meaningful response [Siu et al., 2006], which points towards other important pathways downstream to Ras in PDAC like YAP and STK33 [Scholl et al., 2009; Zhang et al., 2014]. Nevertheless, there are some exciting therapies targeting K-Ras in different ways such as SMIs that either block SOS-mediated nucleotide exchange or allosterically shift the affinity of KRAS to favor GDP over GTP [Maurer et al., 2012]. Suppression of oncogenic RAS signaling by interfering with its localization to endomembrane has also shown interesting results in human PDAC cells in vitro and in vivo [Spiegel et al., 2014].
PI3K/Akt/mTOR pathway
Despite the proven protumorigenic role of Akt signaling in PDAC only few specific inhibitors of this pathway have been tested in clinical setting. Drugs known to inhibit this pathway, albeit non-exclusively, are natural compounds (curcumin, resveratrol, and triptolide), HIV protease inhibitor nelfinavir and metformin [Arlt et al., 2013]. Several inhibitors targeting mTOR have been tested and several more are being developed against multiple cancer types including PDAC. Some of these that have been used in preclinical trials are everolimus, ridaforolimus, temsirolimus, and GDC-0980, with everolimus having completed a phase II trial with no significant anti-tumor effect [Wolpin et al., 2009].
NF-κB INHIBITORS
Constitutive and cancer induced NF-κB activation is the major factor responsible for chemoresistance in PDAC [Carbone and Melisi, 2012]. It has been proved that inhibition of NF-κB in cancer cells is possible and multiple modalities have been developed to either prevent the drug induced NF-κB activation or to suppress the constitutional NF-κB activity in pancreatic cancer cells [Carbone and Melisi, 2012]. Multiple approaches to inhibit NF-κB have been developed, namely IKK inhibitors (BAY-11-7082 BAY-11-7085, CHS828), proteasome inhibitors (bortezomib, RP-171, carfilzomib) and DNA binding inhibitors (SN-50). Other drug classes that exhibit a strong NF-κB inhibition are NSAIDs (aspirin, indomethacin, COX-2 inhibitors), naturally occurring compounds (triptolide, curcumin, resveratrol, epigallocatechin gallate (EGCG), and luteolin) which block this pathway at various steps [Lin et al., 2010]. As NF-κB is required for various cellular and physiological responses like immune system, persistent suppression is undesirable. Intermittent inhibition of NF-κB to overcome the chemoresistance in PDAC is one way to ensure a desirable antitumor effect.
HDAC INHIBITORS
Histone acteylases (HATs) and deacetylases (HDACs) are enzymes that affect the expression of multiple genes via altering various histone and non-histone proteins and chromatin modifications [Bernstein et al., 2007]. These enzymes regulate multiple cellular functions including proliferation and apoptosis by maintaining a fine balance between acetylated and deacetylated chromatin [Marks and Xu, 2009]. HDAC inhibition leads to a reversal of epigenetic changes associated with PDAC and induces cellular differentiation and apoptosis. HDAC inhibitors can be classified into six groups but all of them have a characteristic zinc binding moiety that inhibits HDACs.
HDAC inhibitors sensitize pancreatic cancer cells to both extrinsic and intrinsic apoptotic pathways by downregulating their inhibitors (c-FLIP, XIAP, Bcl-2, Mcl-1) and upregulating death receptors (DR4, DR5) [Koutsounas et al., 2013; Feng et al., 2014]. Multiple studies have shown the synergistic anti-tumor effects of HDAC inhibitors with TRAIL, ABT-737, and other conventional chemotherapies [Koutsounas et al., 2013; Feng et al., 2014]. In addition to caspase dependent apoptosis HDAC inhibitors also induce caspase independent apoptosis by DNA fragmentation through the increased nuclear translocation of Bax-AIF-serine protease complex [Garcia–Morales et al., 2005].
HDAC inhibitors in combination therapy have been shown to synergize with multiple conventional anticancer agents such as gemcitabine, topoisomerase inhibitors, and proteasome inhibitors.
IMMUNOTHERAPY
Immune therapies hold promise as an alternative treatment modality with significantly less toxicity. There are four main categories of immunotherapy: checkpoint inhibitors, vaccines, monoclonal antibodies, and cytokines. They work either by activating T cells or inducing humoral immune response to tumor specific antigens resulting in cell mediated or antibody dependent cellular cytotoxicity in cancer cells [Laheru and Jaffee, 2005]. Checkpoint inhibitors block the T-cell inhibitory signals to enhance pre-existing anti-cancer immune responses. Agents targeting these molecules like CTLA-4 (ipilimumab: phase I), programmed cell death-1 (PD-1) (pembrolizumab: phase IB, nivolumab: phase I/II) are being tested in combination to further enhance the inherent anti-cancer immune response [Arslan and Yalcin, 2014].
Multiple types of vaccines have been tested in PDAC namely whole cell recombinant vaccine, dendritic cell vaccine, DNA, and peptide vaccines. The most studied and clinically tested vaccines in PDAC are whole cell vaccines, namely Algenpantucel-L based on allogeneic pancreatic cancer cells expressing αGal epitope and GVAX based on irradiated tumor cells expressing murine GM-CSF are undergoing phase III and II trials respectively [Gunturu et al., 2013]. Other vaccines based on telomerase (GV1001), K-Ras, mesothelin (CRS-207), heat shock protein (HSPPC-96) and antigen-pulsed dendritic cells +/− lymphokine activated killer therapy are also undergoing clinical trials.
CONCLUSION
There is abundant evidence suggesting that dysregulation of the apoptotic pathway plays a major role in pancreatic cancer carcinogenesis and that targeting apoptosis for treatment of this deadly cancer is feasible. Many such agents are undergoing either clinical or preclinical testing alone or in combination with conventional anticancer drugs. However, most of these agents have failed to translate into meaningful clinical benefit for patients suffering from pancreatic cancer. There are a multitude of reasons that could explain failure of these earlier therapies-lack of suitable preclinical models mimicking human disease, incomplete understanding of PDAC pathobiogenesis and unclear role of tumor microenvironment to name a few. However, with evolving research and better collaboration, we hope pancreatic cancer will no longer remain a death sentence.
Acknowledgments
Grant sponsor: NIH; Grant numbers: R01-CA170946, CA124723; Grant sponsor: NIH; Grant number: R01-CA184274.
This study was funded by NIH grants R01-CA170946 and CA124723 (to AKS); NIH grant R01-CA184274 (to SB); Katherine and Robert Goodale foundation support (to AKS) and Minneamrita Therapeutics LLC (to AKS).
Footnotes
Conflict of interest: University of Minnesota has filed a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. AKS is the co-founder and the Chief Scientific Officer of this company.
References
- Abbruzzese JL. Past and present treatment of pancreatic adenocarcinoma: Chemotherapy as a standard treatment modality. Semin Oncol. 2004;29(6Suppl 20):2–8. doi: 10.1053/sonc.2002.37382. [DOI] [PubMed] [Google Scholar]
- Almendro V, Ametller E, Garcia-Recio S, Collazo O, Casas I, Auge JM, Maurel J, Gascon P. The role of MMP7 and its cross-talk with the FAS/FASL system during the acquisition of chemoresistance to oxaliplatin. PLoS ONE. 2009;4(3):e4728. doi: 10.1371/journal.pone.0004728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Tanno S, Skele KL, Hoffman JP, Testa JR. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem. 2002;87(4):470–476. doi: 10.1002/jcb.10287. [DOI] [PubMed] [Google Scholar]
- Arlt A, Muerkoster SS, Schafer H. Targeting apoptosis pathways in pancreatic cancer. Cancer Lett. 2013;332(2):346–358. doi: 10.1016/j.canlet.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Arslan C, Yalcin S. Current and future systemic treatment options in metastatic pancreatic cancer. J Gastrointest Oncol. 2014;5(4):280–295. doi: 10.3978/j.issn.2078-6891.2014.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23(53):8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clinl Cancer Res. 2008;14(19):5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Sangwan V, McGinn O, Chugh R, Dudeja V, Vickers SM, Saluja AK. Triptolide-induced cell death in pancreatic cancer ismediated byO-GlcNAc modification of transcription factor Sp1. J Biol Chem. 2013;288(47):33927–33938. doi: 10.1074/jbc.M113.500983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2(12):897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- Bayraktar S, Rocha-Lima CM. Advanced or metastatic pancreatic cancer: Molecular targeted therapies. Mt Sinai J Med. 2010;77(6):606–619. doi: 10.1002/msj.20217. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128(4):669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- Carbone C, Melisi D. NF-kappaB as a target for pancreatic cancer therapy. Expert Opin Ther Targets. 2012;16(Suppl2):S1–10. doi: 10.1517/14728222.2011.645806. [DOI] [PubMed] [Google Scholar]
- Carew JS, Espitia CM, Zhao W, Kelly KR, Coffey M, Freeman JW, Nawrocki ST. Reolysin is a novel reovirus-based agent that induces endoplasmic reticular stress-mediated apoptosis in pancreatic cancer. Cell Death Dis. 2013;4:e728. doi: 10.1038/cddis.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Sangwan V, Banerjee S, Chugh R, Dudeja V, Vickers SM, Saluja AK. Triptolide sensitizes pancreatic cancer cells to TRAIL-induced activation of the death receptor pathway. Cancer Lett. 2014;348(1–2):156–166. doi: 10.1016/j.canlet.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci USA. 1996;93(8):3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4(156) doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohenuram M, Saif MW. Epidermal growth factor receptor inhibition strategies in pancreatic cancer: Past, present and the future. JOP. 2007;8(1):4–15. [PubMed] [Google Scholar]
- Cui J, Jiang W, Wang S, Wang L, Xie K. Role of Wnt/beta-catenin signaling in drug resistance of pancreatic cancer. Curr Pharma Des. 2012;18(17):2464–2471. doi: 10.2174/13816128112092464. [DOI] [PubMed] [Google Scholar]
- Deramaudt T, Rustgi AK. Mutant KRAS in the initiation of pancreatic cancer. Biochimica et Biophysica Acta. 2005;1756(2):97–101. doi: 10.1016/j.bbcan.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clinl Cancer Res. 2008;14(14):4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Dong QG, Sclabas GM, Fujioka S, Schmidt C, Peng B, Wu T, Tsao MS, Evans DB, Abbruzzese JL, McDonnell TJ, Chiao PJ. The function of multiple IkappaB: NF-kappaB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene. 2002;21(42):6510–6519. doi: 10.1038/sj.onc.1205848. [DOI] [PubMed] [Google Scholar]
- Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Fujimura T, Ninomiya I, Fushida S, Nishimura GI, Shimizu K, Miwa K. Human pancreatic cancer cells disable function of Fas receptors at several levels in Fas signal transduction pathway. Int J Oncol. 2001;18(2):311–316. doi: 10.3892/ijo.18.2.311. [DOI] [PubMed] [Google Scholar]
- Feng W, Zhang B, Cai D, Zou X. Therapeutic potential of histone deacetylase inhibitors in pancreatic cancer. Cancer Lett. 2014;347(2):183–190. doi: 10.1016/j.canlet.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Freese JL, Pino D, Pleasure SJ. Wnt signaling in development and disease. Neurobiol Dis. 2010;38(2):148–153. doi: 10.1016/j.nbd.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Morales P, Gomez-Martinez A, Carrato A, Martinez-Lacaci I, Barbera VM, Soto JL, Carrasco-Garcia E, Menendez-Gutierrez MP, Castro-Galache MD, Ferragut JA, Saceda M. Histone deacetylase inhibitors induced caspase-independent apoptosis in human pancreatic adenocarcinoma cell lines. Mol Cancer Ther. 2005;4(8):1222–1230. doi: 10.1158/1535-7163.MCT-04-0186. [DOI] [PubMed] [Google Scholar]
- Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Gut. 2007;56(8):1134–1152. doi: 10.1136/gut.2006.103333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, Schmid RM. Stat3 and NF-kappaB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123(6):2052–2063. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- Gunturu KS, Rossi GR, Saif MW. Immunotherapy updates in pancreatic cancer: Are we there yet? Ther Adv Med Oncol. 2013;5(1):81–89. doi: 10.1177/1758834012462463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Chen Y, Zhang B, Kang M, Xie Q, Wu Y. Potentiation of the effect of gemcitabine by emodin in pancreatic cancer is associated with survivin inhibition. Biochem Pharmacol. 2009;77(11):1674–1683. doi: 10.1016/j.bcp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Hamacher R, Schmid RM, Saur D, Schneider G. Apoptotic pathways in pancreatic ductal adenocarcinoma. MoL Cancer. 2008;7:64. doi: 10.1186/1476-4598-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625(1–3):234–246. doi: 10.1016/j.ejphar.2009.06.064. [DOI] [PubMed] [Google Scholar]
- Houbracken I, de Waele E, Lardon J, Ling Z, Heimberg H, Rooman I, Bouwens L. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology. 2011;141(2):731–741. doi: 10.1053/j.gastro.2011.04.050. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: From innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Khanbolooki S, Nawrocki ST, Arumugam T, Andtbacka R, Pino MS, Kurzrock R, Logsdon CD, Abbruzzese JL, McConkey DJ. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5(9):2251–2260. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- Kim MP, Gallick GE. Gemcitabine resistance in pancreatic cancer: Picking the key players. Clin Cancer Res. 2008;14(5):1284–1285. doi: 10.1158/1078-0432.CCR-07-2247. [DOI] [PubMed] [Google Scholar]
- Koutsounas I, Giaginis C, Theocharis S. Histone deacetylase inhibitors and pancreatic cancer: Are there any promising clinical trials? World J Gastroenterol. 2013;19(8):1173–1181. doi: 10.3748/wjg.v19.i8.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27(48):6252–6275. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer-science driving clinical progress. Nat Rev Cancer. 2005;5(6):459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, Aderka D, Ben-Yosef R. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest. 2007;25(6):411–418. doi: 10.1080/07357900701359577. [DOI] [PubMed] [Google Scholar]
- Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6(1):45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101(10):2351–2362. doi: 10.1002/cncr.20605. [DOI] [PubMed] [Google Scholar]
- Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol. 2009;568:161–173. doi: 10.1007/978-1-59745-280-9_10. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bai L, Chen W, Xu S. The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14(1):45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Salnikov AV, Bauer N, Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Schemmer P, Werner J, Herr I. Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-kappaB downregulation. Int J Cancer. 2014;134(10):2489–2503. doi: 10.1002/ijc.28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes RB, Gangeswaran R, McNeish IA, Wang Y, Lemoine NR. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int J Cancer. 2007;120(11):2344–2352. doi: 10.1002/ijc.22554. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432(7015):307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Mahadevan D, Chalasani P, Rensvold D, Kurtin S, Pretzinger C, Jolivet J, Ramanathan RK, Von Hoff DD, Weiss GJ. Phase I trial of AEG35156 an antisense oligonucleotide to XIAP plus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma. Am J Clin Oncol. 2013;36(3):239–243. doi: 10.1097/COC.0b013e3182467a13. [DOI] [PubMed] [Google Scholar]
- Marks PA, Xu WS. Histone deacetylase inhibitors: Potential in cancer therapy. J Cell Biochem. 2009;107(4):600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood A, Azmi AS, Mohammad RM. Small molecule inhibitors of bcl-2 family proteins for pancreatic cancer therapy. Cancers. 2011;3(2):1527–1549. doi: 10.3390/cancers3021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, Fauber BP, Pan B, Malek S, Stokoe D, Ludlam MJ, Bowman KK, Wu J, Giannetti AM, Starovasnik MA, Mellman I, Jackson PK, Rudolph J, Wang W, Fang G. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucleotide exchange activity. Proc Natl Acad Sci USA. 2012;109(14):5299–5304. doi: 10.1073/pnas.1116510109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ, Jr, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132(16):3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M, Lewis BC. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA. 2007;104(12):5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujumdar N, Mackenzie TN, Dudeja V, Chugh R, Antonoff MB, Borja-Cacho D, Sangwan V, Dawra R, Vickers SM, Saluja AK. Triptolide induces cell death in pancreatic cancer cells by apoptotic and autophagic pathways. Gastroenterology. 2010;139(2):598–608. doi: 10.1053/j.gastro.2010.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujumdar N, Banerjee S, Chen Z, Sangwan V, Chugh R, Dudeja V, Yamamoto M, Vickers SM, Saluja AK. Triptolide activates unfolded protein response leading to chronic ER stress in pancreatic cancer cells. Am J Physiol Gastrointest Liver Physiol. 2014;306(11):G1011–G1020. doi: 10.1152/ajpgi.00466.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens TW, Gilmore AP, Streuli CH, Foster FM. Inhibitor of apoptosis proteins: Promising targets for cancer therapy. J Carcinog Mutagen. 2013:14. doi: 10.4172/2157-2518.S14-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67(19):9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- Qi Y, Tu Y, Yang D, Chen Q, Xiao J, Chen Y, Fu J, Xiao X, Zhou Z. Cyclin A but not cyclin D1 is essential for c-myc-modulated cell-cycle progression. J Cell Physiol. 2007;210(1):63–71. doi: 10.1002/jcp.20816. [DOI] [PubMed] [Google Scholar]
- Reichert M, Rustgi AK. Pancreatic ductal cells in development, regeneration, and neoplasia. J Clin Invest. 2011;121(12):4572–4578. doi: 10.1172/JCI57131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA. 2010;107(1):75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21(2):81–86. [PubMed] [Google Scholar]
- Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92(2):271–278. doi: 10.1002/1097-0142(20010715)92:2<271::aid-cncr1319>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89(11):2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl C, Frohling S, Dunn IF, Schinzel AC, Barbie DA, Kim SY, Silver SJ, Tamayo P, Wadlow RC, Ramaswamy S, Dohner K, Bullinger L, Sandy P, Boehm JS, Root DE, Jacks T, Hahn WC, Gilliland DG. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell. 2009;137(5):821–834. doi: 10.1016/j.cell.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Schulze-Bergkamen H, Krammer PH. Apoptosis in cancer-implications for therapy. Seminars Oncol. 2004;31(1):90–119. doi: 10.1053/j.seminoncol.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- Siu LL, Awada A, Takimoto CH, Piccart M, Schwartz B, Giannaris T, Lathia C, Petrenciuc O, Moore MJ. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res. 2006;12(1):144–151. doi: 10.1158/1078-0432.CCR-05-1571. [DOI] [PubMed] [Google Scholar]
- Spiegel J, Cromm PM, Zimmermann G, Grossmann TN, Waldmann H. Small-molecule modulation of Ras signaling. Nat Cheml Biol. 2014;10(8):613–622. doi: 10.1038/nchembio.1560. [DOI] [PubMed] [Google Scholar]
- Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425(6960):851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauzold A, Roder C, Sipos B, Karsten K, Arlt A, Jiang P, Martin-Subero JI, Siegmund D, Muerkoster S, Pagerols-Raluy L, Siebert R, Wajant H, Kalthoff H. CD95 and TRAF2 promote invasiveness of pancreatic cancer cells. FASEB J. 2005;19(6):620–622. doi: 10.1096/fj.04-2984fje. [DOI] [PubMed] [Google Scholar]
- Trauzold A, Wermann H, Arlt A, Schutze S, Schafer H, Oestern S, Roder C, Ungefroren H, Lampe E, Heinrich M, Walczak H, Kalthoff H. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene. 2001;20(31):4258–4269. doi: 10.1038/sj.onc.1204559. [DOI] [PubMed] [Google Scholar]
- Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, Emme D, Roder C, Kalthoff H, Wajant H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25(56):7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- Ungefroren H, Kruse ML, Trauzold A, Roeschmann S, Roeder C, Arlt A, Henne-Bruns D, Kalthoff H. FAP-1 in pancreatic cancer cells: Functional and mechanistic studies on its inhibitory role in CD95-mediated apoptosis. J Cell Sci. 2001;114(Pt15):2735–2746. doi: 10.1242/jcs.114.15.2735. [DOI] [PubMed] [Google Scholar]
- Vernejoul F, Faure P, Benali N, Calise D, Tiraby G, Pradayrol L, Susini C, Buscail L. Antitumor effect of in vivo somatostatin receptor subtype 2 gene transfer in primary and metastatic pancreatic cancer models. Cancer Res. 2002;62(21):6124–6131. [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256(1):58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- Werner K, Ruckert F, Saeger HD, Grutzmann R, Pilarsky C. Recent patents concerning targeted therapy of apoptosis resistance in pancreatic cancer. Recent Patents DNA Gene Sequences. 2011;5(1):28–34. doi: 10.2174/187221511794839237. [DOI] [PubMed] [Google Scholar]
- West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Update. 2002;5(6):234–248. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- Wolan DW, Zorn JA, Gray DC, Wells JA. Small-molecule activators of a proenzyme. Science. 2009;326(5954):853–858. doi: 10.1126/science.1177585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP, Fuchs CS. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27(2):193–198. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HH, Lemoine NR. Pancreatic cancer: Molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol. 2009;6(7):412–422. doi: 10.1038/nrgastro.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RS. Apoptosis in cancer: From pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ehdaie B, Ohara N, Yoshino T, Deng CX. Synergistic action of Smad4 and Pten in suppressing pancreatic ductal adenocarcinoma formation in mice. Oncogene. 2010;29(5):674–686. doi: 10.1038/onc.2009.375. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, Chetram M, Joshi M, Wang F, Kallakury B, Toretsky J, Wellstein A, Yi C. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7(324):ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171(1):263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Li J, Wu S, Li S, Le L, Su X, Qiu P, Hu H, Yan G. Triptolide cooperates with Cisplatin to induce apoptosis in gemcitabine-resistant pancreatic cancer. Pancreas. 2012;41(7):1029–1038. doi: 10.1097/MPA.0b013e31824abdc0. [DOI] [PubMed] [Google Scholar]
- van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87(4):1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]