Abstract
The inbred mouse strains, C57BL/6 and BALB/c have been used widely in preclinical psychiatric research. The differences in stress susceptibility of available strains has provided a useful platform to test pharmacological agents and behavioral responses. Previous brain gene profiling efforts have indicated that the inflammation and immune response gene pathway is the predominant gene network in the differential stress response of BALB/c and C57BL/6 mice. The implication is that a composite stress paradigm that includes a sequence of extended, varied and unpredictable stressors induces inflammation-related genes in the hippocampus. We hypothesized that the regulation of inflammation genes in the brain could constitute a primary stress response and tested this by employing a simple stress protocol, repeated exposure to the same stressor for 10 days, two hours of restraint per day. We examined stress-induced regulation of 13 proinflammatory cytokine genes in male BALB/c and C57BL/6 mice using quantitative PCR. Elevated cytokine genes included tumor necrosis factor alpha (TNFα), interleukin 6 (IL6), interleukin 10 (IL10), tumor necrosis factor (TNF) super family members and interleukin 1 receptor 1 (IL1R1). In addition, we examined restraint stress-induced regulation of 12 glutamate receptor genes in both strains. Our results show that restraint stress is sufficient to elevate the expression of inflammation-related genes in the hippocampus of both BABLB/c and C57BL/6 mice, but they differ in the genes that are induced and the magnitude of change. Cell types that are involved in this response include endothelial cells and astrocytes.
Keywords: Cytokines, Gene expression, Glutamate receptors, Hippocampus, Proinflammatory genes, Restraint stress
Introduction
Rodent stress models are widely used to investigate the neurobiological basis of psychiatric disorders. The use of stress models is supported by substantial evidence implicating stress as a precipitating factor for several neuropsychiatric disorders (Belmaker and Agam, 2008). However, individuals differ significantly in their behavioral response to stressors. Understanding the basis for susceptibility or resilience to stressful stimuli can shed light on important mechanistic underpinnings of behavioral deficits and psychiatric diseases.
The availability of rodent strains that exhibit differential behavioral responses to established stress paradigms are a valuable resource to examine the genetic factors involved in stress susceptibility. Two inbred mouse strains, C57BL/6 and BALB/c, have been critical in understanding the molecular and behavioral consequences of stress as they differ significantly in their stress responses. For example, despite similar basal levels of serum corticosterone, levels in BALB/c mice rise to 800–900 ng/mL in comparison to 450–500 ng/mL in C57BL/6 mice in response to restraint stress (Flint, 2001). Furthermore, differences in hippocampal gene profiles have been linked to differences in behavioral responses to chronic mild stress, where BALB/c mice show significantly more depressive-like symptoms than C57BL/6 mice (Liu et al., 2010, Malki et al., 2015). Thus, C57BL/6 mice are considered to be relatively stress resistant, whereas BALB/c mice are regarded as more stress-sensitive (Brinks et al., 2008, Brinks et al., 2007). Recent gene profiling experiments in the hippocampus of these strains highlighted the inflammation and immune response gene pathway in the stress vulnerability of BALB/c mice after exposure to chronic mild stress (Malki et al., 2015) and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway in sensitization to chronic restraint stress in C57BL/6 mice (Gray et al., 2014).
A growing understanding of the relationship between stress-induced glucocorticoid elevation and glutamate neurotransmission has indicated that genetic differences in stress susceptibility could be modulated by the regulation of glutamate receptors (Nasca et al., 2015a, Nasca et al., 2015b). Additionally, a direct relationship between inflammation and the glutamate system has been demonstrated (Erhardt et al., 2013, Haroon et al., 2016). To better understand the different molecular responses of C57BL/6 and BALB/c mice to stress in light of recent findings of altered inflammation cascades, and associations between inflammation and glutamatergic signaling, we examined the regulation of representative genes from these two categories. Our focus was on the hippocampus because its stress vulnerability is well established (Conrad, 2008, Kim and Diamond, 2002). We hypothesized that repeated exposure to a single stressor would be sufficient to alter the hippocampal expression of glutamatergic signaling and inflammation genes that generally are associated with complex and extended stress paradigms. We obtained quantitative gene expression data for individual genes and sought to phenotype the brain cells involved in the regulation of select targets.
Methods
Animals
Male C57BL/6 and BALB/c mice aged two months (Envigo/Harlan, Research Models and Services, Indianapolis, IN), 14 mice per strain, 22–25 g body weight were group-housed (3–4 per cage) in the same room in Techniplast blue line IVC cages with aspen chip bedding under standard conditions (20–23°C, 40–70% humidity, lights on at 06:00 h and off at 20:00 h) with unrestricted access to food and water. Mice were acclimatized undisturbed for 5 days prior to experimentation. All animal use procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at the University of South Dakota.
Restraint Stress
Mice were randomly assigned to one of two groups: restraint or control (n=7/group). Controls were handled for 10 min each day, which included transfer to a new cage and then returned to the home cage. Restraint stress using the Tailveiner Restrainer, length 10 cm, diameter 4 cm, slot ventilated (Braintree Scientific, Inc., Braintree, MA) began at approximately 14:00 h each day for a duration of 2 hours. Mice were exposed to only one restraint stress exposure per day. Mice were returned to the home cage after each daily restraint exposure. Three hours after the last exposure mice were killed by rapid decapitation as per American Veterinary Medical Association guidelines (Leary et al., 2013). The brains were hemisected and one hemisphere from each mouse was rapidly frozen on dry ice for immunohistochemical analyses. The entire hippocampus was dissected from the other half (Hunsberger et al., 2005, Newton et al., 2003), rapidly frozen on dry ice and stored at −80°C until used for RNA isolation.
Quantitative PCR Analysis
A hypothesis driven approach was employed to develop a target list of 13 inflammation genes and 12 glutamate receptor genes. The inflammation genes include IL1a, IL1b, IL1R1, IL2Rb, IL6, IL10, IL10RA, IL10RB, NF-kB, TNFa, TNFSF4, TNFSF6 and TNFSF10. The glutamate system genes were drawn from the N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and metabotropic classes of glutamate receptor genes, Gria1, Gria2, Gria3, Gria4, Grin1, Grin2A, Grin2B, Grm1, Grm2, Grm3, Grm4 and Grm5. Quantitation of relative gene expression was performed as previously described (Sathyanesan et al., 2015). Briefly, RNA was isolated using the RNAqueous kit (Ambion) and quantitated using the Nanodrop spectrophotomter (Thermo). Quality was confirmed using the Nanoassay on the Bioanalyzer (Agilent). Reverse transcribed cDNA was utilized for PCR amplification employing Sybr Green chemistry (Qiagen) and gene-specific primers in the Realplex Mastercycler realtime PCR machine (Eppendorf). Specificity of product was determined by melt curve analysis. Data were normalized using the housekeeping genes cyclophilin, β-actin and GAPDH. Primer sequences are provided in the supplement.
Immunohistochemistry
Immunohistochemical studies were performed using cryocut coronal sections (16 µm) as previously described (Sathyanesan et al., 2015). Sections were incubated with different primary antibody combinations in antibody solution, 2.5% bovine serum albumin (BSA) in phosphate buffered saline (PBS), at 4°C overnight (IL6, 1:200; IL10, 1:150; glial fibrillary acidic protein [GFAP], 1:1000; alpha smooth muscle actin [SMA], 1:150, Abcam, Cambridge, MA, USA). Tomato lectin (Vector labs, Burlingame, CA) staining was used to mark vasculature. Antibodies were used as per manufacturer’s instructions and specificity was tested using incubation in antibody solutions lacking primary antibody. Following primary antibody incubation, slides were washed in 1xPBS three times for 5 minutes each at room temperature. Slides were then incubated in appropriate fluorescent secondary antibody (1:500, Alexa-488 and 594, Life Technologies) in 2.5% BSA in PBS for 2 hours at room temperature. The slides were then rinsed in 1x PBS three times for five minutes each and coverslipped using VectaMount (Vector Labs). Three sections from each mouse were analyzed. Sections were viewed by an unbiased observer to evaluate differences and images were captured using a Nikon Eclipse Ni microscope equipped with a DS-Qi1 monochrome, cooled digital camera and NIS-AR 4.20 Elements imaging software. Objective magnifications that were used include 20x, 40x and 60X. Sections from stressed and control mice were captured using identical exposure settings.
Statistical Analysis
Relative gene expression using quantitative PCR was calculated using the ΔΔCt method and the data were statistically analyzed using the t-test in SigmaStat 4.0. Gene expression comparisons were considered statistically significant at p < 0.05. Significant effects were subjected to the Benjamini Hochberg correction for multiple comparisons. Results were replicated in an independent cohort. Data are presented as mean +/− s.e.m.
Results
We examined stress-induced hippocampal gene regulation in BALB/c and C57BL/6 mice using quantitative PCR, focusing our attention on inflammation and glutamate receptor genes.
mRNA expression – inflammation genes
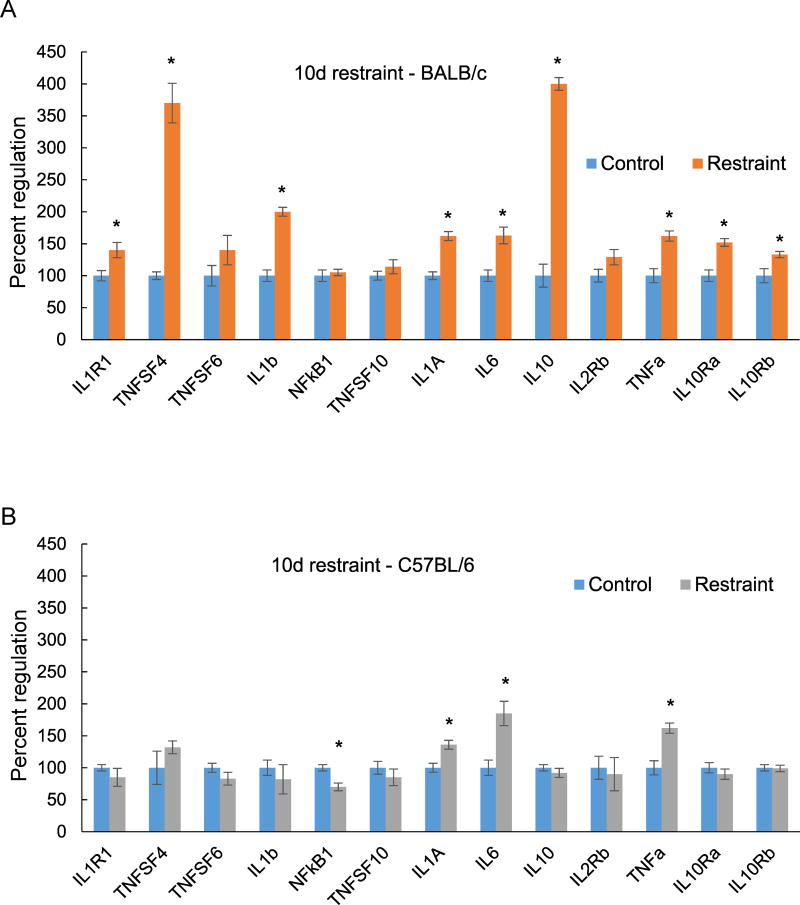
Ten days restraint stress exposure upregulated 9 genes associated with inflammation in BALB/c (Fig. 1A) and 3 genes in C57BL/6J mice (Fig. 1B). The only downregulated inflammation gene was NF-kB, which was decreased by 30% by stress in the C57BL/6 strain. Tnfsf4 and IL10 were the most highly induced genes with both elevated over 300% compared to controls (Fig. 1A). Genes that were upregulated in both strains are IL1A, IL6 and TNFa. IL6 and TNFa gene expression was more highly regulated in C57BL/6 than in BALB/c mice. Expression of the IL1 and IL10, IL1R1, IL10Ra and IL10Rb receptor genes was elevated in BALB/c mice in addition to a strong increase in IL10 receptor gene expression (Fig. 1A).
Figure 1.
Changes in hippocampal expression of inflammation genes measured by QPCR after 10 days of restraint stress (A) BALB/c mice. (B) C57BL/6 mice. Each bar represents the mean of n=5 mice. Error bars are +/− SEM; *p < 0.05 vs. Control, , t-test, IL1R1- interleukin 1 receptor type 1, TNFSF 4, 6 and 10 – tumor necrosis factor super family 4, 6 and 10, IL1B – interleukin 1 beta, Nfkb1 - nuclear factor kappa B subunit 1, IL1A – interleukin 1 alpha, IL6 – interleukin 6, IL10- interleukin 10, IL2RB – interleukin 2 receptor subunit beta, TNF a- tumor necrosis factor alpha, IL10Ra – interleukin 10 receptor subunit alpha, IL10Rb – interleukin 10 receptor subunit beta.
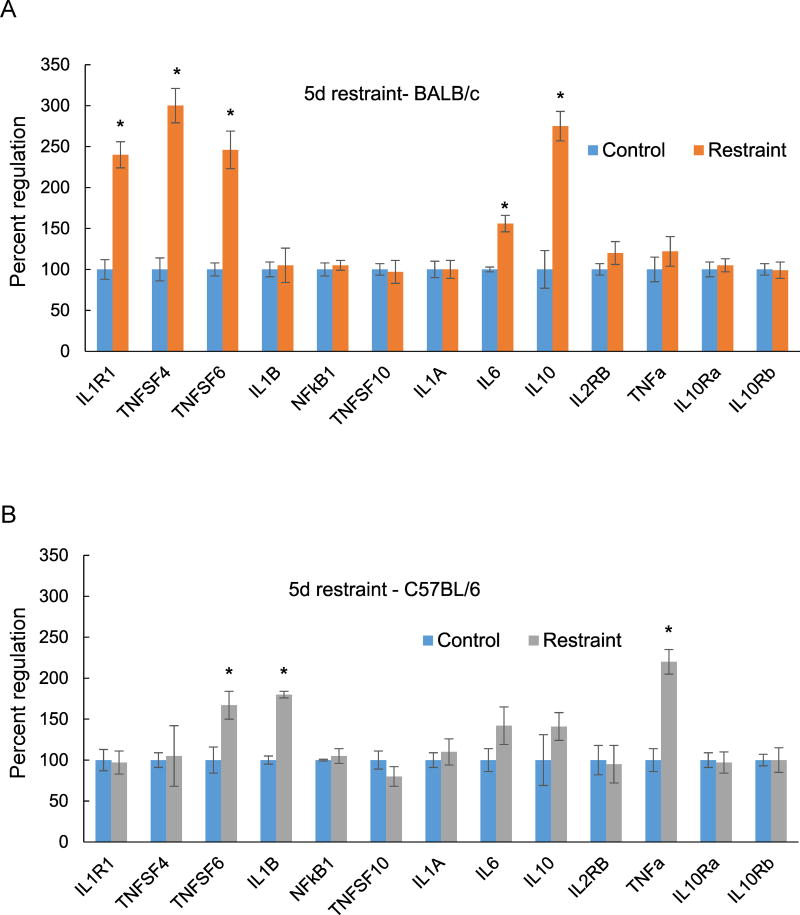
We also examined the regulation of inflammation genes with a shorter stress exposure of 5 days (Fig. 2). In BALB/c mice there was overlap between the genes induced by restraint exposure for 10 days and 5 days. Four of the five genes elevated at 5 days, IL1R1, TNFSF4, IL6 and IL10 (Fig. 2A), were also significantly elevated at 10 days (Fig. 1A). TNFSF4 and IL10 were the most highly induced genes with restraint stress for both 5 and 10 days. In C57BL/6 mice, 3 genes were elevated after 5 days of stress (Fig. 2B) and only TNFa was also significantly induced at 10 days, although the level of expression declined sharply with longer stress exposure (Fig. 1B). TNFSF6 had a similar expression pattern in both strains, elevated after 5 days (Fig. 2 A, B) but returned to baseline at 10 days (Fig. 1 A, B). IL1A exhibited comparable expression in both strains, but was induced only with exposure for 10 days but not 5 days.
Figure 2.
Changes in hippocampal expression of inflammation genes measured by QPCR after 5 days of restraint stress. (A) BALB/c mice; (B) C57BL/6 mice. Each bar represents the mean of n=5 mice. Error bars are +/− SEM; *p < 0.05 vs Control, t-test. IL1R1- interleukin 1 receptor type 1, TNFSF 4, 6 and 10 – tumor necrosis factor super family 4, 6 and 10, IL1B – interleukin 1 beta, Nfkb1 - nuclear factor kappa B subunit 1, IL1A – interleukin 1 alpha, IL6 – interleukin 6, IL10- interleukin 10, IL2RB – interleukin 2 receptor subunit beta, TNF a- tumor necrosis factor alpha, IL10Ra – interleukin 10 receptor subunit alpha, IL10Rb – interleukin 10 receptor subunit beta.
mRNA expression – glutamate receptor genes
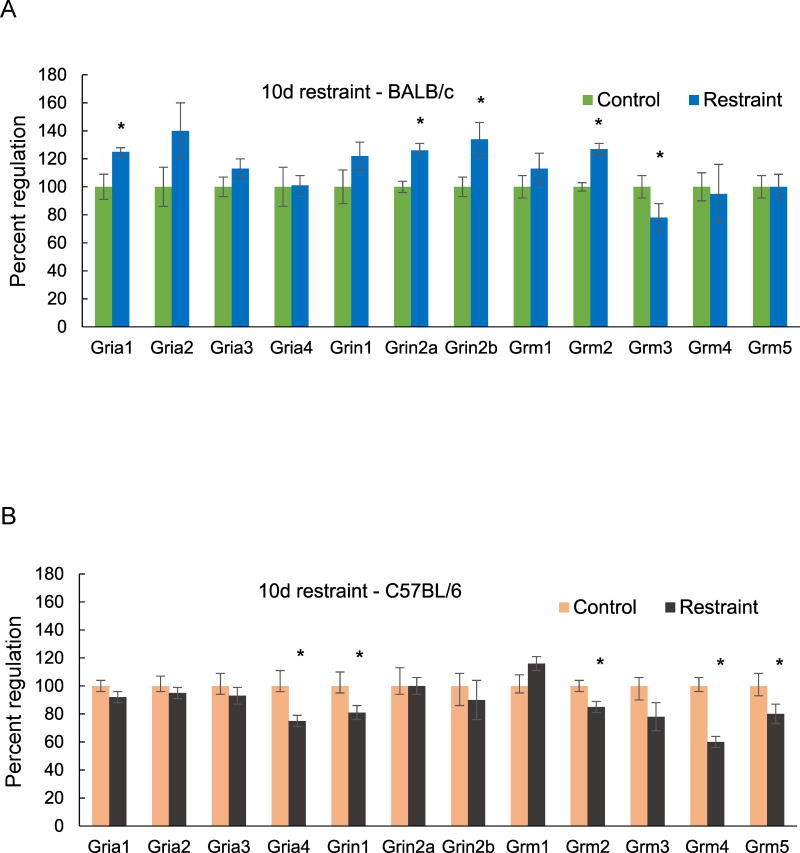
Restraint stress for 10 days produced more upregulated than downregulated glutamate receptor genes in BALB/c mice (Fig. 3A). The 4 significantly upregulated genes were Gria1, Grin2a, Grin2b (NMDAR2A, NMDAR2B) and Grm2 (mGluR2). Expression of Grin2a and Grin 2b was elevated by 26 and 34% respectively. Grm3 expression was downregulated by 22% compared to unstressed controls. In C57BL/6 mice, the only significant effect of stress for 10 days was down-regulation of expression of several glutamate receptor genes (Fig. 3B). GluR4 (Gria4, AMPA 4) expression was reduced by 25% and Grin1 (NMDAR1) expression by 20%. There was a 15% decrease in Grm2 (mGluR2) but no regulation of Grm3 (mGluR3). The most strongly downregulated gene in C57BL/6 mice was Grm4 (mGluR4), which exhibited a 40% reduction due to stress exposure (Fig. 3B); this contrasted with no change in Grm4 gene expression in BALB/c mice after stress for 10 days (Fig. 3A).
Figure 3.
Changes in hippocampal expression of glutamate receptor genes measured by QPCR after 10 days of restraint stress. (A) BALB/c mice; (B) C57BL/6 mice. Each bar represents the mean of n=5 mice. Error bars are +/− SEM; * = p < 0.05, vs Control, t-test. Gria1 (glutamate AMPA receptor 1), Gria2 (AMPAR 2), Gria3 (AMPAR 3), Gria4 (AMPAR 4), Grin1 (glutamate NMDAR 1), Grin2a (NMDAR 2a), Grin2b (NMDAR 2b), Grm1 (metabotropic glutamate receptor 1), Grm2 (metabotropic glutamate receptor 2), Grm3 (metabotropic glutamate receptor 3), Grm4 (metabotropic glutamate receptor 4), Grm5 (metabotropic glutamate receptor 5).
Immunohistochemistry
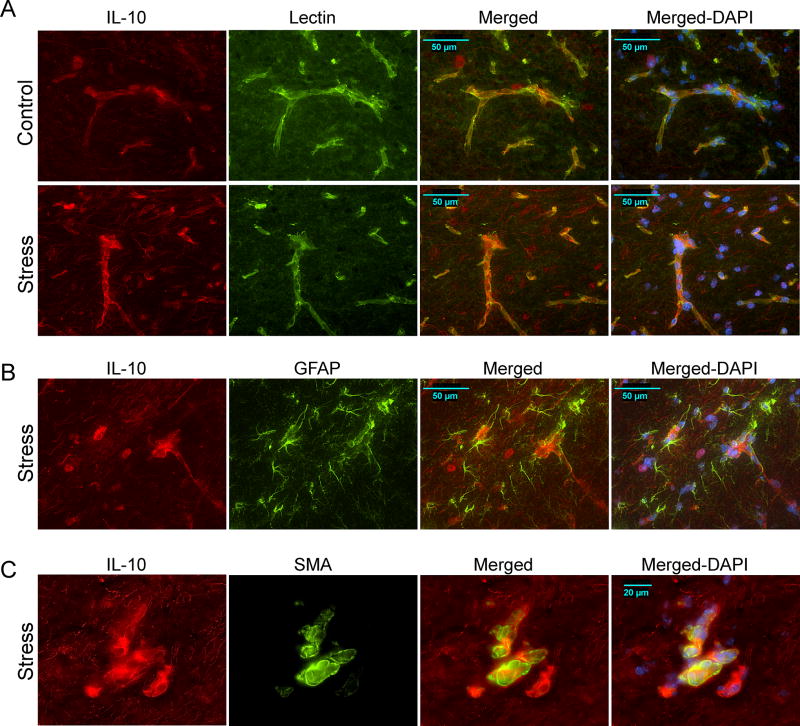
Immunohistochemical analysis of IL10 protein expression in BALB/c following 10 days of restraint indicated that stress-induced elevation was in blood vessels (Fig. 4). The expression pattern of IL10 protein was similar in both controls and stressed mice, with detectable expression in neurons and blood vessels. However, vasculature in the stratum lacunosum moleculare (SLM) region of the hippocampus displayed higher restraint-induced expression (Fig. 4A lower panel). Co-labeling with lectin showed that endothelial cells are the likely source of stress-induced elevation of IL10 in BALB/c mice. As the SLM has high glial density we performed colocalization analysis with glial fibrillary acidic protein (GFAP) as an astrocytic marker (Fig. 4B). Although IL10 signal was close to GFAP labeled cells no colocalization was evident. The signal proximity is likely due to glial end-feet wrapping around blood vessels expressing IL10. Prominent restraint-induced IL10 expression appeared to arise from large diameter vessels in the SLM (Fig 4C). Co-labeling with alpha smooth muscle actin (αSMA) confirmed that IL10 is elevated in these larger SMA-positive blood vessels. IL10 is therefore expressed in both smaller lectin-positive capillaries (Fig. 4A), and larger arterioles invested with smooth muscle cells (Fig. 4C). We examined restraint-induced IL6 regulation using triple labeling IHC (Fig. 5). IL6 was expressed at low levels in control mice (Fig. 5, top panel) and was upregulated in the GFAP-positive astrocytic processes in the dentate gyrus in stressed mice.
Figure 4.
Immunohistochemical analysis of interleukin 10 (IL10) protein expression in the hippocampus of BALB/c mice after 10 days of restraint stress. (A) IL10 colocalization with lectin (vascular marker)- stained capillaries in the SLM, stratum lacunosum moleculare, region of the hippocampus of BALB/c controls (top row) and stressed (lower row). (B) IL10 colocalization with glial fibrillary acidic protein (GFAP)-positive astrocytes in the SLM of stressed BALB/c mice. (C) IL10 colocalization with alpha smooth muscle actin (SMA)- labeled blood vessels in the hippocampus of stressed BALB/c mice. a DAPI – 4’, 6-Diamidino-2-phenylindole.
Figure 5.
Immunohistochemical analysis of interleukin 6 (IL6) protein expression in the hippocampus of BALB/c mice after 10 days of restraint stress. IL6 colocalization with glial fibrillary acidic protein (GFAP)- stained astrocyte processes in the dentate gyrus. Expression in a control mouse is shown in the top row and a stressed mouse is shown in the lower panel. DG- dentate gyrus.
Discussion
Our study examined the regulation of specific classes of genes in the hippocampus as a function of stress in two behaviorally distinct inbred mouse strains. Restraint stress significantly increased the expression of several proinflammatory genes in both strains, but the effects were more pronounced in BALB/c than in C57BL/6 mice. BALB/c and C57BL/6 mice exhibit distinct differences in behavioral assessments of cognitive function and fear response in both basal and stressed conditions (Brinks et al., 2008, Brinks et al., 2007). As stress models are widely used to obtain insight into neuropsychiatric disorders and therapeutic agents, understanding the molecular basis for the differential response to stress in commonly used inbred mouse lines can shed light on key cellular signaling pathways that are involved.
mRNA expression – inflammation genes
A recent whole genome gene profiling study examining the effects of unpredictable chronic mild stress on the hippocampus in BALB/c versus C57BL/6 strains implicated a proinflammatory hub, centered on NF-kB, in the stress sensitivity of BALB/c mice (Malki et al., 2015). Furthermore, all the signaling pathways that emerged from their analyses are associated with inflammation and immune responses. Our focused gene expression results, aimed at determining relative levels of specific inflammation genes, are broadly consistent with the previous findings as the quantitative PCR data indicate that restraint stress induces more hippocampal inflammatory genes, and at higher levels, in BALB/c than in C57BL/6 mice. Also, increasing the exposure period from 5 to 10d resulted in more genes being induced in BALB/C but not in C57BL/6 mice.
These results are interesting because restraint stress is a simple model in comparison to the chronic mild stress paradigm which employs over 10 different stressors that are administered in an unpredictable sequence for several weeks. The finding that the same class of genes are induced with a limited and predictable stress exposure for a few days indicates that the induction of inflammation genes, predominantly proinflammatory cytokine signaling molecules, could constitute a primary stress response. It is interesting to note that the interleukins IL10 and IL6, the genes for which were elevated in BALB/c mice at 5 and 10d, were also increased at the protein level by chronic stress in stimulated lymphocytes of BALB/c but not C57BL/6 mice (Palumbo et al., 2010). Precisely how stress exposure leads to an alteration in the expression of inflammatory genes within the hippocampus is a topic worthy of investigation.
IL10 is known to be anti-inflammatory and potently suppresses proinflammatory cytokines in macrophages (Moore et al., 2001). A previous restraint stress (6h/day) study in female C57BL/6 mice reported increased IL6 but downregulation of IL10 mRNA in the cortex and hippocampus (Voorhees et al., 2013). We observed a robust induction of IL10 gene expression only in BALB/c but not in C57BL/6 mice. It is currently unclear if gender influences IL10 regulation as our study was conducted in males. IL10 is known to temporally suppress the expression of TNFa in monocytes (Rajasingh et al., 2006), and its increase in BALB/c mice could be responsible for the lower levels of TNFa gene induction in comparison to C57BL/6 mice, particularly at the 5d time point. Although IL10 is considered a beneficial molecule that can counter the actions of proinflammatory cytokines, overexpression of IL10 as a therapeutic strategy in an Alzheimer’s disease mouse model resulted in a change of immune gene expression towards a M2-like activated state and a deterioration in cognitive function (Chakrabarty et al., 2015). Identifying the cells involved in the inflammatory stress response is essential to developing an integrated understanding of stress-induced gene expression and modulation of behavior. Stress-induced elevation of IL10 protein was primarily in the blood vessels of the SLM region of the hippocampus. This vascular elevation could serve to inhibit macrophage interactions at the blood brain barrier. In contrast to vascular expression of IL10 in the molecular layer, IL6 protein expression was elevated in the astrocytic processes in the dentate gyrus.
TNFSF 4 and 6 belong to the tumor necrosis factor cytokine superfamily and function by binding to specific TNF receptors. These ligands have been shown to be involved in immune response, proinflammatory and apoptotic cell death mechanisms (Karulf et al., 2010, Milanovic et al., 2016). The robust association between inflammation and apoptosis is well known, and it is thus not surprising that these dual actions are represented by the TNFSF genes. A comparative analysis of the effects of chronic mild stress in C57BL/6 and BALB/c mice reported significant cell loss, specifically in the hippocampus of BALB/c mice (Palumbo et al., 2010). The TNFSF genes could be potentially involved in cell loss mechanisms. In the present study, NFkb1 gene expression was modestly downregulated, but in a statistically significant manner in C57BL/6 mice after stress for 10 days. This is intriguing as previous work highlighted the Nfkb1 gene hub as influencing the heightened inflammatory response of BABLB/c over C57BL/6 mice in response to chronic mild stress (Malki et al., 2015). The decrease in Nfkb1 gene expression could be an adaptive response that lowers the magnitude of the inflammatory response in C57BL/6 mice and could influence stress resilience. We also noted that C57BL/6 mice adapt to restraint exposures after 5 days, causing us to speculate that behavioral deficits could be more pronounced with 5 days of stress exposure rather 10 days.
mRNA expression – glutamate receptor genes
Glutamate neurotransmission in the hippocampus is altered by stress exposure and produces behavioral changes relevant to psychiatric disorders (Sanacora et al., 2012). Here, stress differentially regulated the different classes of glutamate receptors in the two strains. Restraint significantly elevated two NMDA receptor subunit genes in BALB/c mice, Grin2a (NR2A) and Grin2b (NR2B). Grin1 (NR1) was not regulated in BABL/c but was decreased in C57BL/6 mice, indicating that even within the NMDA class of glutamate receptors stress differentially alters gene expression based on genetic background. Chronic corticosterone administration, which simulates the primary endocrine response to stress, has been shown to mediate its deleterious effects on the hippocampus by increasing Grin2a and Grin2b gene expression (Weiland et al., 1997). As BALB/c mice exhibit 2–3 fold higher corticosterone levels in response to stress than C57BL/6 mice (Brinks et al., 2007) it is possible that this differential glucocorticoid response influences the greater induction of NMDA receptor genes in BALB/c mice. Grin2a appears to play an important role in mediating the behavioral effects of restraint stress as deletion of this gene was sufficient to prevent stress-induced anxiety (Mozhui et al., 2010). The NMDA receptor genes are also interesting from the standpoint of antidepressant activity as NMDA antagonists have been found to produce robust and fast acting antidepressant effects in preclinical and clinical tests (Li et al., 2010, Zarate et al., 2006). An increase in Grin2a and Grin2b can be expected to elicit a pro-depressive behavioral phenotype. However, the potential compensatory changes modulated by signaling via other glutamate receptors has to be taken into consideration. The role of hippocampal Grm2 (mGluR2) gene expression in glutamate tone and stress sensitivity was recently investigated after chronic (21d) restraint stress in C57BL/6 mice (Nasca et al., 2015a). The authors noted that stress decreased Grm2 gene expression, and that lower Grm2 levels increased vulnerability to stress. We found Grm2 gene expression to be downregulated in C57BL/6 but upregulated in BALB/c mice. The downregulation of Grm3 (mGluR3) gene expression appears counterintuitive as it is in the same class of group II metabotropic glutamate receptors. However, unlike Grm2 which is neuronal, Grm3 is present on both neurons and glia. Given the deleterious effects of stress on glial structure and function (Liu et al., 2011), the reduction in Grm3 could be indicative of glial pathology. Grm4 (mGluR4), a member of the class III mGluRs, was the most downregulated gene in C57BL/6 mice. It has been considered as a useful target for psychiatric drug development but results have been mixed. Knockout mice exhibited slight improvement in spatial working memory but major deficits in prepulse inhibition (Sagata et al., 2010). Maternal separation stress significantly reduced gene expression of mGluR4 in the rat hippocampus, but so did ketamine and electroconvulsive treatment in naïve rats (O' Connor et al., 2013). Although earlier studies indicated that mGlu4 has a protective role against NMDA excitotoxicity (Bruno et al., 2000), a selective mGluR4 agonist increased behavioral despair in the forced swim and tail suspension tests (Podkowa et al., 2015). It is uncertain if the Gria4 reduction that we observed in C57BL/6 mice could have behavioral consequences.
Immunohistochemistry
Inflammation and glutamate receptor genes signify two key mechanisms that are actively investigated to obtain insight into the pathophysiology of neuropsychiatric disorders. The regulation of IL10 in blood vessels and IL6 in astrocytes indicates that the cellular effects of stress are not limited to neuronal cells and could cause perturbations in the neurovascular unit. IL-1β has been studied as an inflammatory cytokine responsible for the anhedonic consequences of stress (Koo and Duman, 2008), presumably via neuroinflammation. Interestingly, anxiogenic behaviors precipitated by social defeat stress could be suppressed by specific knockdown of the IL-1β receptor in endothelial cells (Wohleb et al., 2014). This highlights the intricacies of the stress response and the multiple cell types that are involved in the signaling interactions that produce behavioral deficits. Understanding how stress influences functionally distinct cell types in the brain can shed light on the complexity of psychiatric diseases and provide directions for innovative therapeutic approaches. In future studies it will be important to examine whether males and females differ in their response to stress exposure at the level of the neurovascular unit.
Supplementary Material
Acknowledgments
This work is supported by U.S. Public Health Service grant MH106640, USD Center for Brain and Behavioral Research and the South Dakota Board of Regents.
Footnotes
Declaration of interest
The authors declare that there are no competing interests.
Contributor Information
Monica Sathyanesan, Division of Basic Biomedical Sciences, Sanford School of Medicine, University of South Dakota, Vermillion, SD 57069.
Jacob M Haiar, Division of Basic Biomedical Sciences, Sanford School of Medicine, University of South Dakota, Vermillion, SD 57069.
Michael J Watt, Division of Basic Biomedical Sciences, Sanford School of Medicine, University of South Dakota, Vermillion, SD 57069.
Samuel S Newton, Division of Basic Biomedical Sciences, Sanford School of Medicine, University of South Dakota, Vermillion, SD 57069.
References
- Belmaker RH, Agam G. Major Depressive Disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Brinks V, de Kloet ER, Oitzl MS. Strain specific fear behaviour and glucocorticoid response to aversive events: modelling PTSD in mice. Prog Brain Res. 2008;167:257–261. doi: 10.1016/S0079-6123(07)67019-8. [DOI] [PubMed] [Google Scholar]
- Brinks V, van der Mark M, de Kloet R, Oitzl M. Emotion and cognition in high and low stress sensitive mouse strains: a combined neuroendocrine and behavioral study in BALB/c and C57BL/6J mice. Front Behav Neurosci. 2007;1:8. doi: 10.3389/neuro.08.008.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno V, Battaglia G, Ksiazek I, van der Putten H, Catania MV, Giuffrida R, Lukic S, Leonhardt T, Inderbitzin W, Gasparini F, Kuhn R, Hampson DR, Nicoletti F, Flor PJ. Selective activation of mGlu4 metabotropic glutamate receptors is protective against excitotoxic neuronal death. J Neurosci. 2000;20(17):6413–6420. doi: 10.1523/JNEUROSCI.20-17-06413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty P, Li A, Ceballos-Diaz C, Eddy JA, Funk CC, Moore B, DiNunno N, Rosario AM, Cruz PE, Verbeeck C, Sacino A, Nix S, Janus C, Price ND, Das P, Golde TE. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85(3):519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19(6):395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Traskman-Bendz L, Guillemin GJ, Brundin L. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38(5):743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint MST SS. C57BL/6 Mice Are Resistant to Acute Restraint Modulation of Cutaneous Hypersensitivity. Toxicol Sci. 2001;62:250–256. doi: 10.1093/toxsci/62.2.250. [DOI] [PubMed] [Google Scholar]
- Gray JD, Rubin TG, Hunter RG, McEwen BS. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry. 2014;19(11):1171–1178. doi: 10.1038/mp.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, Hu XP, Miller AH. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 2016;21(10):1351–1357. doi: 10.1038/mp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger JG, Bennett AH, Selvanayagam E, Duman RS, Newton SS. Gene profiling the response to kainic acid induced seizures. Brain Res Mol Brain Res. 2005;141(1):95–112. doi: 10.1016/j.molbrainres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol. 2010;185(8):4856–4862. doi: 10.4049/jimmunol.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3(6):453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, Greenacre C, Gwaltney-Brant S, McCrackin MA, Meyer R, Miller D, Shearer J, Yanong R. AVMA Guidelines for the Euthanasia of Animals:2013 Edition. 2013 [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li B, Zhu HY, Wang YQ, Yu J, Wu GC. Glia atrophy in the hippocampus of chronic unpredictable stress-induced depression model rats is reversed by electroacupuncture treatment. J Affect Disord. 2011;128(3):309–313. doi: 10.1016/j.jad.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang N, Zuo P. cDNA microarray analysis of gene expression in the cerebral cortex and hippocampus of BALB/c mice subjected to chronic mild stress. Cell Mol Neurobiol. 2010;30(7):1035–1047. doi: 10.1007/s10571-010-9534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malki K, Mineur YS, Tosto MG, Campbell J, Karia P, Jumabhoy I, Sluyter F, Crusio WE, Schalkwyk LC. Pervasive and opposing effects of Unpredictable Chronic Mild Stress (UCMS) on hippocampal gene expression in BALB/cJ and C57BL/6J mouse strains. BMC Genomics. 2015;16:262. doi: 10.1186/s12864-015-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanovic D, Pesic V, Loncarevic-Vasiljkovic N, Pavkovic Z, Popic J, Kanazir S, Jevtovic-Todorovic V, Ruzdijic S. The Fas Ligand/Fas Death Receptor Pathways Contribute to Propofol-Induced Apoptosis and Neuroinflammation in the Brain of Neonatal Rats. Neurotox Res. 2016 doi: 10.1007/s12640-016-9629-1. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30(15):5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 2015a;20(6):755–763. doi: 10.1038/mp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca C, Zelli D, Bigio B, Piccinin S, Scaccianoce S, Nistico R, McEwen BS. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci U S A. 2015b;112(48):14960–14965. doi: 10.1073/pnas.1516016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Collier EF, Hunsberger J, Adams D, Terwilliger R, Selvanayagam E, Duman RS. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23(34):10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. Epub 2003/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor RM, Pusceddu MM, Dinan TG, Cryan JF. Impact of early-life stress, on group III mGlu receptor levels in the rat hippocampus: effects of ketamine, electroconvulsive shock therapy and fluoxetine treatment. Neuropharmacology. 2013;66:236–241. doi: 10.1016/j.neuropharm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Palumbo ML, Canzobre MC, Pascuan CG, Rios H, Wald M, Genaro AM. Stress induced cognitive deficit is differentially modulated in BALB/c and C57Bl/6 mice: correlation with Th1/Th2 balance after stress exposure. J Neuroimmunol. 2010;218(1–2):12–20. doi: 10.1016/j.jneuroim.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Podkowa K, Rzezniczek S, Marciniak M, Acher F, Pilc A, Palucha-Poniewiera A. A novel mGlu4 selective agonist LSP4-2022 increases behavioral despair in mouse models of antidepressant action. Neuropharmacology. 2015;97:338–345. doi: 10.1016/j.neuropharm.2015.05.039. [DOI] [PubMed] [Google Scholar]
- Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G, Goukassian D, Zhu Y, Losordo DW, Kishore R. IL-10-induced TNF-alpha mRNA destabilization is mediated via IL-10 suppression of p38 MAP kinase activation and inhibition of HuR expression. FASEB J. 2006;20(12):2112–2114. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- Sagata N, Iwaki A, Aramaki T, Takao K, Kura S, Tsuzuki T, Kawakami R, Ito I, Kitamura T, Sugiyama H, Miyakawa T, Fukumaki Y. Comprehensive behavioural study of GluR4 knockout mice: implication in cognitive function. Genes Brain Behav. 2010;9(8):899–909. doi: 10.1111/j.1601-183X.2010.00629.x. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62(1):63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanesan M, Girgenti MJ, Warner-Schmidt J, Newton SS. Indomethacin induced gene regulation in the rat hippocampus. Mol Brain. 2015;8:59. doi: 10.1186/s13041-015-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, Eubank TD, Marsh CB. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS One. 2013;8(3):e58488. doi: 10.1371/journal.pone.0058488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland NG, Orchinik M, Tanapat P. Chronic corticosterone treatment induces parallel changes in N-methyl-D-aspartate receptor subunit messenger RNA levels and antagonist binding sites in the hippocampus. Neuroscience. 1997;78(3):653–662. doi: 10.1016/s0306-4522(96)00619-7. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci. 2014;34(7):2583–2591. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.