Presentation of Case
Dr. Lazaro V. Zayas (Psychiatry)
An 11-year-old girl was seen in an outpatient clinic of this hospital because of difficulty eating solid food and associated weight loss after an acute choking incident.
The patient had been in her usual health until 14 days before this evaluation, when a piece of meat became stuck in an orthodontic palate expander that had been inserted 19 days earlier and she thought she might choke. She was able to dislodge the meat shortly thereafter, but during the next 7 days, she reportedly had an intense fear of swallowing, ate very carefully, and had an associated decrease in her food intake. On the 7th day after the choking incident, she stopped eating most solid foods; thereafter, she consumed only milkshakes, liquid dietary supplements, and, occasionally, yogurt and oatmeal. She reported that she was unable to eat solid food because she was “afraid I can’t chew it up enough to swallow it so I don’t choke” and “I don’t really know how to chew like I used to.” One day before this evaluation, her orthodontist removed the palate expander. However, the swallowing difficulty persisted; she was unable to swallow small pieces of chicken or a single grain of rice. On the day of this evaluation, the patient consumed only a spoonful of oatmeal (which she had previously been willing to eat), a half can of a liquid dietary supplement, and a small amount of water and juice. Her parents noted that she chewed food with her front teeth but appeared to be unable to move the food from the front of her mouth to the back to swallow it. Her weight had decreased by 1.4 kg since an examination performed 2 months earlier, and the body-mass index (BMI; the weight in kilograms divided by the square of the height in meters) was below the 1st percentile. Her parents were worried about acute malnutrition and dehydration. They requested an urgent evaluation in the Eating Disorders Clinical and Research Program outpatient clinic at this hospital, and from there, the patient was referred to the Adolescent and Young Adult Medicine clinic at this hospital.
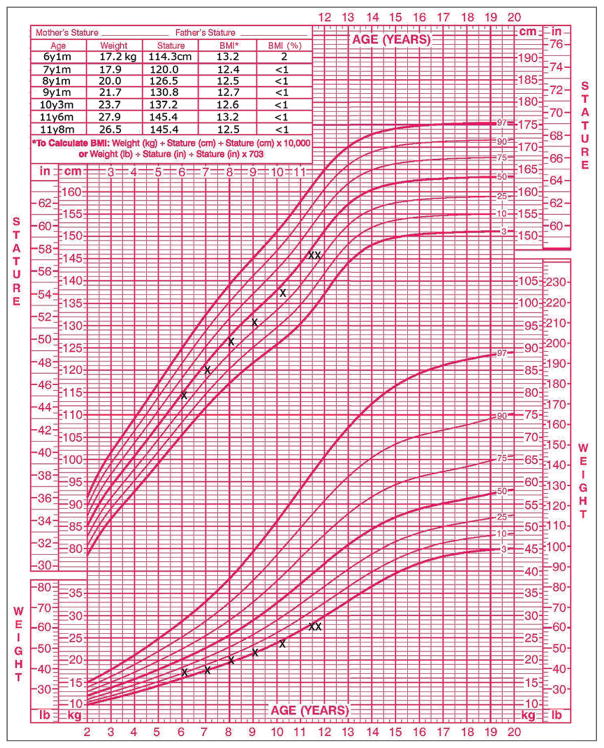
During a psychiatric evaluation of the patient, her parents reported that she had been a highly selective eater since infancy, had cried the first time she ate solid food, and had frequently required coaxing from her parents to complete meals. The patient disliked many foods (including artichokes, asparagus, meatballs, pasta sauce, hamburger buns, and sandwiches) because of their taste or texture or because they were “mixed together.” These strict preferences had severely limited the available food choices for the family’s dinners and affected the patient’s ability to socialize with friends. Her BMI had been as low as the one tenth percentile and had been consistently below the 1st percentile during recent years, including during her most recent examination 2 months earlier (Fig. 1). Cyproheptadine had been administered the previous year but was stopped after only transient improvement in her appetite because of lethargy. The patient did not report that she felt fat; she noted, “If anything, I worry that I’m too skinny. I’m one of the smallest in my class,” and she stated her desired weight to be average or a little lower than average. She reported no history of binge eating, self-induced vomiting, or use of laxatives, diuretics, or diet pills. She also reported no symptoms of pica or rumination disorder, depression, anxiety disorders, phobias (besides fear of choking), substance abuse, post-traumatic stress disorder, attention-deficit–hyperactivity disorder, or oppositional defiant disorder. As a young child, she had constipation, for which she had been seen by a gastroenterologist at another hospital and had briefly received treatment with polyethylene glycol, fiber, and a stimulant laxative. She had been seen intermittently by nutritionists at another hospital since she was 6 years of age. At the other hospital, testing for celiac disease was reportedly negative. She took no medications currently and was allergic to penicillin, which had caused hives. She lived with her parents and younger siblings; she was a student who did well academically and had many friends and hobbies. There was a family history of depression.
Figure 1. The Patient’s Growth Chart and Body-Mass Index.
The chart shows the patient’s height and weight (top and bottom, respectively) relative to average values among girls in her age group. The table inset shows the patient’s body-mass index, which is below the 1st percentile.
On medical examination, the temperature was 38°C, the respiratory rate 18 breaths per minute, and the oxygen saturation 97% while the patient was breathing ambient air; the blood pressure and pulse were 107/70 mm Hg and 114 beats per minute while she was in the supine position and 95/72 mm Hg and 123 beats per minute while she was standing. The weight was 26.5 kg, and the BMI 12.5. There were no oral petechiae, and sexual development was classified as Tanner stage 2 (with stages ranging from 1 to 5 and higher stages indicating more advanced pubertal development); the remainder of the examination was normal. The hemoglobin level, hematocrit, platelet count, red-cell indexes, and results of renal- and liver-function tests were normal, as were blood levels of glucose, calcium, magnesium, total protein, globulin, and thyrotropin; other test results are shown in Table 1. Urinalysis revealed a specific gravity of higher than 1.030, a pH of 6.0, and 4+ ketones and 1+ protein by dipstick. An electrocardiogram showed sinus tachycardia at a rate of 110 beats per minute, with a PR interval of 116 msec, a corrected QT interval of 422 msec, and evidence of an incomplete right bundle-branch block.
Table 1.
Laboratory Data.
| Variable | Reference Range, Age-Adjusted* | On Admission |
|---|---|---|
| White-cell count (per mm3) | 4500–13,500 | 9200 |
| Differential count (%) | ||
| Neutrophils | 40–59 | 74.8 |
| Lymphocytes | 33–48 | 18.7 |
| Monocytes | 4–11 | 5.5 |
| Eosinophils | 0–8 | 0.4 |
| Basophils | 0–3 | 0.4 |
| Sodium (mmol/liter) | 135–145 | 136 |
| Potassium (mmol/liter) | 3.4–4.8 | 5.2 |
| Chloride (mmol/liter) | 100–108 | 100 |
| Carbon dioxide (mmol/liter) | 23.0–31.9 | 21.0 |
| Plasma anion gap (mmol/liter) | 3–15 | 15 |
| Albumin (g/dl) | 3.3–5.0 | 5.5 |
| Phosphorus (mg/dl)† | 4.5–5.5 | 4.4 |
Reference values are affected by many variables, including the patient population and the laboratory methods used.
The ranges used at Massachusetts General Hospital are age-adjusted for patients who are not pregnant and do not have medical conditions that could affect the results. They may therefore not be appropriate for all patients.
To convert the values for phosphorus to millimoles per liter, multiply by 0.3229.
The patient was admitted to this hospital. A diagnosis and management decisions were made.
Differential Diagnsis
Medical Perspective
Dr. Kathryn S. Brigham
One of the striking features of this case is the presence of acute food refusal superimposed over a chronic failure to gain weight. Multiple diagnoses could explain the chronic and acute conditions (Table 2).
Table 2.
Causes of Acute Food Refusal and Chronic Failure to Gain Weight.
| Type of Cause | Acute Food Refusal | Chronic Failure to Gain Weight |
|---|---|---|
| Neurologic | Acute oromotor dysfunction (e.g., due to head trauma or hypoxic injury) | Chronic oromotor dysfunction (e.g., due to muscular dystrophy or pseudobulbar palsy) |
| Gastrointestinal | Foreign body in the gastrointestinal tract Ulceration Other acute causes of abdominal pain |
Achalasia Inflammatory bowel disease Celiac disease Other chronic causes of abdominal pain |
| Endocrine | — | Hyperthyroidism Addison’s disease Type 1 diabetes mellitus |
| Infectious | — | Tuberculosis Human immunodeficiency virus |
| Psychosocial | Child abuse and neglect | Insufficient food availability Child abuse and neglect |
| Psychiatric | Anorexia nervosa Avoidant–restrictive food intake disorder Obsessive–compulsive disorder Pediatric acute-onset neuropsychiatric syndrome Globus hystericus |
Anorexia nervosa Avoidant–restrictive food intake disorder Obsessive–compulsive disorder Stimulant use |
| Other | Cancer (e.g., lymphoma or rhabdomyosarcoma) Adenotonsillar hypertrophy Medication use Chronic pulmonary, cardiac, or renal disease (e.g., cystic fibrosis and renal tubular acidosis) |
Both acute oromotor dysfunction (e.g., due to head trauma or hypoxic injury) and chronic oromotor dysfunction (e.g., due to muscular dystrophy or pseudobulbar palsy) can affect swallowing and thus affect weight. However, this patient had no history of difficulty swallowing (dysphagia), so oromotor dysfunction is an unlikely cause in this case. Other acute causes of oropharyngeal dysphagia include cancer (e.g., lymphoma or rhabdomyosarcoma) and adenotonsillar hypertrophy,1 but the history and findings on examination were not suggestive of these causes.
Many gastrointestinal problems can lead to the acute and chronic conditions seen in this patient. Food allergies, celiac disease, inflammatory bowel disease, constipation, and other sources of abdominal pain can cause a child to be chronically underweight. Achalasia can lead to dysphagia of both solids and liquids; although this disease is very rare in children, there have been some documented cases of achalasia that were initially misdiagnosed as an eating disorder. However, this patient had previously had a very thorough work-up by a pediatric gastroenterologist that had confirmed the presence of constipation and ruled out other diseases. Pain with eating, swallowing, or digestion can lead to an acute decrease in oral intake, but the history and findings on examination did not suggest a cause of pain, such as recent overuse of nonsteroidal antiinflammatory drugs, oral ulcers, or poor dentition.
In this patient, as in any patient who has a near choking event, it is worth considering whether a foreign body is preventing her from swallowing normally. However, the descriptions from the patient’s parents suggested that she was having more difficulty with chewing food and moving it to the back of her mouth than with swallowing, so the presence of a foreign body is unlikely.
Psychosocial factors, such as child abuse and neglect and lack of food availability, must also be considered, but this family was able to provide sufficient food and there were no “red flags” for abuse and neglect. Picky eating alone is rarely a cause of chronic underweight, because children who are picky eaters tend to eat enough of the foods that they do like.2
Many chronic illnesses — such as malignant tumors or chronic cardiovascular, pulmonary, endocrine, or renal conditions — may lead to anorexia, increased metabolic demand, or both. However, with the exception of this patient’s chronic low weight, she had been a healthy child, with no signs of chronic illness. Chronic infectious illnesses, such as human immunodeficiency virus and tuberculosis, can lead to wasting, but on the basis of her history, she was at relatively low risk for these conditions. Medications for chronic conditions, such as stimulants for attention-deficit disorder, can adversely affect appetite, but this patient had never received any stimulants.
Because there was no evidence to support any other underlying cause of this patient’s condition, a psychiatric cause seemed likely. Obsessive–compulsive disorder can be associated with food and eating rituals that lead to weight loss, but neither the patient nor her parents endorsed any rituals. Acute eating restrictions can be one of the presenting symptoms of the pediatric acute-onset neuropsychiatric syndrome (PANS), but the patient did not have additional neuropsychiatric symptoms or any evidence of a preceding infection, which typically occurs with PANS. Globus hystericus can lead to a fear of swallowing, particularly with solid or lumpy foods,2 but the major issue in this patient was with chewing, not swallowing.
Ultimately, a feeding or eating disorder seemed to be the most likely cause. Bulimia nervosa was unlikely, given that the patient did not have a history of binge eating or purging. Her low BMI would fit with a diagnosis of anorexia nervosa, and some patients with anorexia nervosa have acute food refusal. However, the patient did not report having a fear of weight gain or endorse body-image disturbance, and many of her favorite foods were high-fat foods, which are not typically favored by persons with anorexia nervosa. Instead, the patient’s presentation was most consistent with avoidant–restrictive food intake disorder (ARFID). Similar to many patients with ARFID, this patient had a long-term failure to gain weight appropriately and now had more acute weight loss.3 In addition, her eating habits were causing disruption in her family and she needed to use nutritional supplements, and these features also fit well with the diagnosis of ARFID.3
The medical complications of eating disorders are well established in the medical literature.4,5 This patient was ketotic and dehydrated and essentially refused to eat, and thus she met the criteria for medical hospitalization for eating disorders that are published by the American Academy of Pediatrics5 and the Society for Adolescent Health and Medicine.6 The mild hyperkalemia that was initially seen in this patient was most likely a result of laboratory error (i.e., hemolysis), because analysis of a second sample revealed a normal potassium level. Regardless of the patient’s psychiatric diagnosis, by the time of her presentation, she had decompensation that necessitated medical hospitalization.
Speech Pathological Perspective
Ms. Sarah T. Sally
Once the patient was hospitalized, Speech Pathology was consulted to conduct an inpatient clinical evaluation of feeding and swallowing because of her dysphagia.7 One of the primary goals of this assessment was to determine whether the feeding difficulty was primarily a swallowing deficit and, if it was, to determine which phase of swallowing was impaired.8 In assessing for the presence of oropharyngeal dysphagia, the possibility of structural or physiological issues was considered before the possibility of a behavioral component. The clinical examination began with observation of posture, level of alertness, and cognitive functioning; all these features were at an appropriate level for oral feeding. An examination of oral anatomy was then conducted, with observation of the lips, tongue, palate, jaw, facial symmetry, dentition, and oral secretions; all the findings were indicative of normal functioning. Oral motor control was evaluated through observation of the range, rate, and accuracy of movements of the lips and tongue, soft palate, and pharyngeal walls during speech, reflexive activity, and swallowing.7 The patient had an adequate ability to produce intelligible speech, swallow saliva, and perform oral motor tasks, such as lateralization of the tongue and elevation of the tongue tip to the alveolar ridge. There was no reported history of oral motor deficits related to eating or drinking, such as difficulty with biting, chewing, or drinking from age-appropriate cups.
A trial of oral intake was the next step of this assessment. First, water was provided through a straw, since the patient identified substances of this consistency as the easiest to swallow. She readily drank consecutive sips without any clinical signs of oropharyngeal dysphagia. Next, purees were provided, since the patient identified substances of this consistency as more challenging to swallow. The patient fed herself a bolus that was one quarter teaspoon in size. She had evidence of diminished oral motor skills; she stripped the bolus off the spoon in an incomplete manner and held it in her oral cavity for seconds before she initiated oral transfer in preparation for swallowing. The most notable feature was the anterior position of her tongue during swallowing, which resulted in expulsion of a portion of the bolus past the lips. The patient stated that she “forgot how to swallow” and explained that when her palate expander was in place, she had learned to swallow in a new way, by placing her tongue in this anterior position. This altered oral motor pattern had persisted after removal of the dental appliance. A verbal and visual explanation of how to achieve accurate placement of the tongue tip on the alveolar ridge for swallowing was provided, and then the patient practiced this skill. Multiple boluses of applesauce were provided, and oral motor coordination improved. The patient did not report odynophagia. There were no clinical signs of pharyngeal issues — such as a significant delay in the production of a swallow, gurgling voice quality, cough, or increased respiratory effort or respiratory distress7 — and thus instrumental assessment (e.g., a videofluoroscopic swallowing study) was deferred. The medical team was not concerned about esophageal issues, and therefore, a barium swallow examination was not recommended.
On the basis of this evaluation, structural and physiological causes of feeding or swallowing difficulty were ruled out. The findings, coupled with signs of anxious behavior in the patient, led us to conclude that the dysphagia was behavioral.
Psychiatric Perspective
Dr. Kamryn T. Eddy
The patient’s presentation was characterized by the sudden onset of food refusal and weight loss after a traumatic choking incident, which, in a patient with an already slender frame, resulted in a serious case of low body weight. The two key psychiatric causes in the differential diagnosis were a specific phobia of choking and anorexia nervosa.
The patient attributed her inability to eat solid foods to her being scared of choking and “forgetting” how to chew and swallow. A specific phobia of choking may result in avoidance of a number of situations in which choking is possible, including eating. When difficulty eating and its associated consequences become the principal focus of clinical attention, a diagnosis of ARFID is appropriate.
Similar to ARFID, anorexia nervosa is defined by the presence of undereating or food restriction and accompanying weight loss, low weight, failure to make expected weight gains, or a combination of these conditions. However, in anorexia nervosa, restrictive eating is accompanied by an overvaluation of thinness, an intense fear of fatness, and a disturbance in body image. In some young patients with anorexia nervosa, these features can be challenging to detect, because the patients have limited insight into their motives for restriction and may have difficulty appreciating the consequences of low weight.9–11 In fact, a subset of those with anorexia nervosa are described as having a “non–fat phobic” presentation, in which they do not express a frank fear of weight gain but instead endorse other rationales for restriction.12 Thus, young patients with suspected anorexia nervosa may require longitudinal assessment and ongoing reevaluation. This patient never expressed anxiety about or unwillingness to gain weight and instead seemed comfortable and pleased with weight gain and growth over time; these features support the diagnosis of ARFID.
ARFID is defined by the presence of avoidant or restrictive eating that results in persistent failure to meet nutritional needs; evidence of ARFID includes low weight or failure to have expected gains or growth, nutritional deficiencies, reliance on nutritional supplements or enteral feeding, psychosocial impairment, or a combination of these features.13 Restrictive eating may be motivated by low appetite or lack of interest in eating, sensitivities to certain sensory aspects of foods, or fear of adverse consequences of eating, such as choking or vomiting.14 The restriction is not due to cultural practices or lack of available food, body-image disturbance is not present, and anorexia nervosa or bulimia nervosa cannot be diagnosed concurrently. Furthermore, if another medical or psychiatric condition is present, the eating behavior and sequelae require independent clinical attention. The key features of the patient’s presentation — including the food avoidance driven by anxiety about choking and the associated nutritional sequelae — overlapped with features of choking phobia and anorexia nervosa but were most consistent with the diagnosis of ARFID. These key features suggested behavioral treatment targets.
Clinical Diagnosis
Avoidant–restrictive food intake disorder (ARFID).
Discussion of Management
Pharmacologic Perspective
Dr. Eric P. Hazen
ARFID is a relatively new diagnosis, and few published data are available to guide pharmacologic treatment. Currently, the best clinical approach is to target specific symptoms that may contribute to the condition and to identify and treat coexisting psychiatric conditions that may be exacerbating the patient’s symptoms of restrictive eating and overall distress.
Targeted symptoms that may be treated with medication include severe situational anxiety about eating, as well as reduced appetite or early satiety resulting from chronic undernourishment. Food-related anxiety can be addressed through the short-term use of benzodiazepines, such as lorazepam, before meals. Appetite stimulants, such as cyproheptadine, may be helpful for some patients. However, these agents should be used thoughtfully and in combination with behavioral treatments, because if a patient’s appetite is stimulated before he or she has learned skills to master the food aversion, further frustration and distress could ensue.
Coexisting psychiatric conditions appear to be common among patients with ARFID. Concurrent anxiety disorders are the most prevalent; they occur in more than 70% of patients in some clinical samples.15 Rates of concurrent generalized anxiety disorder in particular have been estimated to be 50% among patients who are being treated for ARFID.16 Major depressive disorder is also common among patients with ARFID, although it appears to occur less commonly in association with this condition than with anorexia nervosa and bulimia nervosa.15
In general, the treatment of coexisting conditions in patients with ARFID, including the decision to treat with medication and the choice of agent, should follow established standards of care. Selective serotonin-reuptake inhibitors are usually the first-line pharmacologic treatment for anxiety disorders and major depressive disorder in pediatric patients. However, additional consideration may be given to choosing an agent with a side-effect profile that will not exacerbate feeding issues and will possibly improve them. For example, this patient’s anxiety was treated with mirtazapine, a serotonergic anxiolytic and antidepressant medication with anticholinergic and antihistaminergic effects. The common side effects of increased appetite and weight gain were an advantage in this patient, whose appetite had waned as she adjusted over time to minimal food intake. Since the medication was started as she was learning skills to overcome her aversion to eating, it was able to work in concert with the cognitive behavioral therapy she was receiving to reduce her anxiety and increase her will to eat. Although there have been no controlled studies of mirtazapine for ARFID, at least one published case report supports its use for similar clinical conditions.17
Psychiatric Perspective
Dr. Jennifer J. Thomas
After discharge from the hospital, this patient began to receive psychological treatment that targeted her fear of adverse consequences of eating (i.e., her choking phobia), her lack of interest in eating (which led her to skip meals and not meet overall calorie needs), and her food selectivity due to sensory sensitivity (which led her to eat a very limited variety of foods). Because there is not yet an evidence-based psychological treatment for ARFID, we designed an individualized cognitive behavioral intervention that was based on empirically supported approaches for the treatment of specific phobia, eating disorders in low-weight patients, and selective eating. Members of our team have recently written a book outlining these interventions.18
Treatment for Choking Phobia
Because this patient was not regularly consuming solid food, the most important first step was to help her reinitiate oral intake. On the basis of previous case studies that suggested a benefit from gradual exposure to feared foods in patients with a choking phobia,19,20 I worked with the patient and her family to create a list of foods for exposure. The list started with the foods that she perceived to be least likely to cause choking and ended with the same type of meat that was involved in the initial choking incident. Because the patient had concerns about her oral motor skills, I followed a progression of food exposure that was based on texture; I began with purees and soft foods and then introduced crunchy and fibrous foods (Fig. 2). Both before and after the patient consumed 5 to 10 bites of a food, I asked her to estimate the likelihood (on a scale ranging from 0 to 100) that she would choke. Her ratings decreased across sessions until she was successfully able to consume the same food that was involved in the initial incident and to predict that choking was very unlikely.
Figure 2.
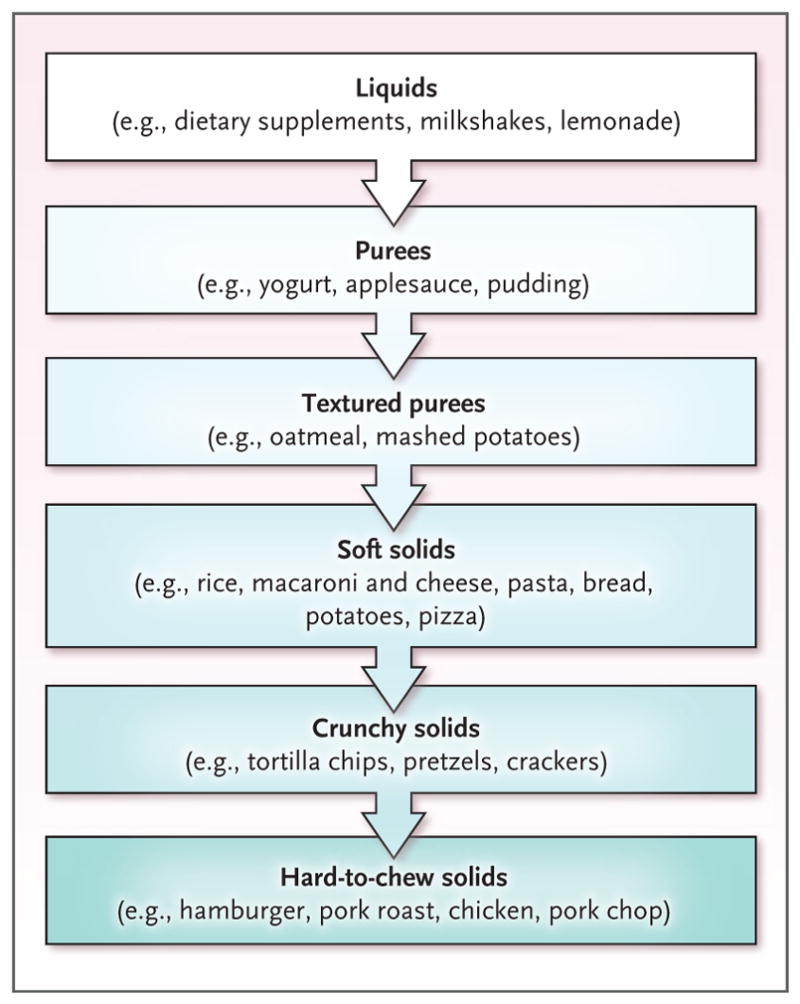
Progression of Food Exposure in Patients with a Choking Phobia.
Treatment for Low Weight
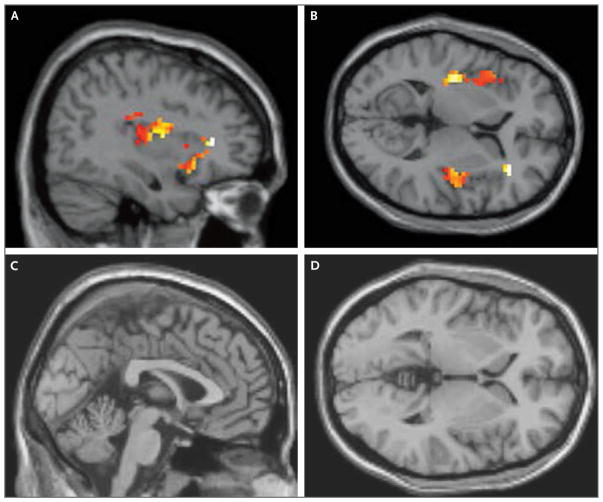
Once this patient had started to consume most of the foods that she had eaten before the initial choking incident, the next goal was to restore her weight. Adolescent Medicine set a target weight that was based on the patient’s individual growth chart (Fig. 1). Since the efficacy of family-based treatment for young patients with anorexia nervosa is established,21 we encouraged the patient’s parents to take charge of renourishment by increasing supervision during meals and snacks. They also began to serve more energy-dense foods that were among the few foods that the patient preferred (e.g., pasta and milkshakes). Although the patient was initially reluctant to eat more, her parents asserted firm but gentle pressure until she increased her intake. One intervention that was particularly helpful for the family was sharing with them the results of the patient’s participation in our team’s ongoing research study (“Homeostatic and Hedonic Food Motivation Underlying Eating Disorder Trajectories,” unpublished data). In a healthy control participant, activation of the anterior insula was significantly higher in response to images of high-calorie foods than in response to images of objects (P<0.001 with the use of a t-test), but in this patient, no such difference was observed (P>0.05 with the use of a t-test) (Fig. 3). After receiving this information and seeing functional magnetic resonance imaging scans, the patient’s parents expressed increased motivation to assist the patient in renourishment, given their new understanding that the patient had diminished activation of her appetite-regulating circuitry in response to palatable foods and that this might prevent her from seeking out those foods independently.
Figure 3. Functional MRI Scans of the Brain That Show Patient Responses to Images of High-Calorie Foods.
Sagittal and transverse images obtained from a healthy control participant (Panels A and B, respectively) show activation of the anterior insula in response to images of high-calorie foods. Sagittal and transverse images obtained from this patient, who has avoidant–resistant food intake disorder (Panels C and D, respectively), show no activation. Responses to images of high-calorie foods were measured relative to responses to images of objects.
Increasing Dietary Variety
Once the patient was nearing her target weight, the final priority was increasing dietary variety. In collaboration with her family, the patient and I made a list of foods that the family often consumed, foods that are commonly served in social settings, and foods that contain nutrients that were not already provided in her preferred foods. Because previous studies have suggested that repeated exposure promotes acceptance of new foods in children,22,23 we created a tasting chart on which the patient received a point for each taste of a new food, and she could earn a reward after she had accumulated a predetermined number of points. This direct approach was successful for some foods but not all of them. In addition, the patient became more willing to try new foods when she witnessed her peers consuming them in social settings. By the end of treatment, she had expanded the variety of her diet such that she was consuming at least 10 fruits or vegetables, 10 grain-based foods, and 10 protein-based foods.
One year after the patient’s initial assessment, she did not report having any fear of choking and had reincorporated all previously consumed solid foods into her diet. She had gained 6.4 kg and had grown 8 cm in height, and her BMI was just below the 3rd percentile. Although her diet remained somewhat limited, she had increased the variety to the extent that it was no longer affecting her physical or psychosocial functioning.
Final Diagnosis
Avoidant–restrictive food intake disorder (ARFID).
Acknowledgments
We thank Dr. Lazaro V. Zayas for his contributions to the case history, and Dr. Thilo Deckersbach, Dr. Elizabeth A. Lawson, Dr. Madhusmita Misra, and Rachel Franklin for their preparation of the functional magnetic resonance imaging studies shown in Figure 3.
Footnotes
This case was presented at Psychiatry Grand Rounds.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Durvasula VS, O’Neill AC, Richter GT. Oropharyngeal dysphagia in children: mechanism, source, and management. Otolaryngol Clin North Am. 2014;47:691–720. doi: 10.1016/j.otc.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Ornstein RM, Katzman DK. Child and adolescent feeding and eating disorders and the DSM-5: a brave new world. Adolesc Med State Art Rev. 2014;25:360–76. [PubMed] [Google Scholar]
- 3.Katzman DK, Stevens K, Norris M. Redefining feeding and eating disorders: what is avoidant/restrictive food intake disorder? Paediatr Child Health. 2014;19:445–6. doi: 10.1093/pch/19.8.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein MA, Dechant EJ, Beresin EV. Eating disorders. Pediatr Rev. 2011;32:508–21. doi: 10.1542/pir.32-12-508. [DOI] [PubMed] [Google Scholar]
- 5.Rosen DS. Identification and management of eating disorders in children and adolescents. Pediatrics. 2010;126:1240–53. doi: 10.1542/peds.2010-2821. [DOI] [PubMed] [Google Scholar]
- 6.Golden NH, Katzman DK, Sawyer SM, et al. Update on the medical management of eating disorders in adolescents. J Adolesc Health. 2015;56:370–5. doi: 10.1016/j.jadohealth.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Logemann JA. Evaluation and treatment of swallowing disorders. 2. Austin, TX: Pro-Ed; 1998. [Google Scholar]
- 8.Arvedson JC, Brodsky L. Pediatric swallowing and feeding: assessment and management. 2. Albany, NY: Singular Publishing Group; 2002. [Google Scholar]
- 9.Becker AE, Eddy KT, Perloe A. Clarifying criteria for cognitive signs and symptoms for eating disorders in DSM-V. Int J Eat Disord. 2009;42:611–9. doi: 10.1002/eat.20723. [DOI] [PubMed] [Google Scholar]
- 10.Bravender T, Bryant-Waugh R, Herzog D, et al. Classification of child and adolescent eating disturbances. Int J Eat Disord. 2007;40(Suppl):S117–S122. doi: 10.1002/eat.20458. [DOI] [PubMed] [Google Scholar]
- 11.Eddy KT, Le Grange D, Crosby RD, et al. Diagnostic classification of eating disorders in children and adolescents: how does DSM-IV-TR compare to empirically-derived categories? J Am Acad Child Adolesc Psychiatry. 2010;49:277–87. doi: 10.1016/j.jaac.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker AE, Thomas JJ, Pike KM. Should non-fat-phobic anorexia nervosa be included in DSM-V? Int J Eat Disord. 2009;42:620–35. doi: 10.1002/eat.20727. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Arlington, VA: APA Publishing; 2013. [Google Scholar]
- 14.Eddy KT, Thomas JJ, Hastings E, et al. Prevalence of DSM-5 avoidant/restrictive food intake disorder in a pediatric gastroenterology healthcare network. Int J Eat Disord. 2015;48:464–70. doi: 10.1002/eat.22350. [DOI] [PubMed] [Google Scholar]
- 15.Nicely TA, Lane-Loney S, Masciulli E, Hollenbeak CS, Ornstein RM. Prevalence and characteristics of avoidant/restrictive food intake disorder in a cohort of young patients in day treatment for eating disorders. J Eat Disord. 2014;2:21. doi: 10.1186/s40337-014-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris ML, Robinson A, Obeid N, Harrison M, Spettigue W, Henderson K. Exploring avoidant/restrictive food intake disorder in eating disordered patients: a descriptive study. Int J Eat Disord. 2014;47:495–9. doi: 10.1002/eat.22217. [DOI] [PubMed] [Google Scholar]
- 17.Tanıdır C, Hergüner S. Mirtazapine for choking phobia: report of a pediatric case. J Child Adolesc Psychopharmacol. 2015;25:659–60. doi: 10.1089/cap.2015.0145. [DOI] [PubMed] [Google Scholar]
- 18.Thomas JJ, Eddy KT. Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: children, adolescents, and adults. Cambridge, United Kingdom: Cambridge University Press; (in press) [Google Scholar]
- 19.McNally RJ. Behavioral treatment of a choking phobia. J Behav Ther Exp Psychiatry. 1986;17:185–8. doi: 10.1016/0005-7916(86)90025-x. [DOI] [PubMed] [Google Scholar]
- 20.Nock MK. A multiple-baseline evaluation of the treatment of food phobia in a young boy. J Behav Ther Exp Psychiatry. 2002;33:217–25. doi: 10.1016/s0005-7916(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 21.Lock J, Le Grange D. Treatment manual for anorexia nervosa: a family-based approach. 2. New York: Guilford Publications; 2015. [Google Scholar]
- 22.Marshall J, Hill RJ, Ware RS, Ziviani J, Dodrill P. Multidisciplinary intervention for childhood feeding difficulties. J Pediatr Gastroenterol Nutr. 2015;60:680–7. doi: 10.1097/MPG.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 23.Remington A, Añez E, Croker H, Wardle J, Cooke L. Increasing food acceptance in the home setting: a randomized controlled trial of parent-administered taste exposure with incentives. Am J Clin Nutr. 2012;95:72–7. doi: 10.3945/ajcn.111.024596. [DOI] [PubMed] [Google Scholar]