Abstract
Pitt–Hopkins syndrome (PTHS) is a neurodevelopmental disorder, classified as an autism spectrum disorder that is caused by the haploinsufficiency of Transcription Factor 4 (TCF4). The most common non-neurological symptoms in PTHS patients are gastrointestinal (GI) disturbances, mainly gastroesophageal reflux and severe constipation (in about 30 and 75% of PTHS patients, respectively). We hypothesized that the recently recognized mouse model of PTHS will exhibit problems with their gut function. We conducted series of in vivo tests on 15- to 19- week old male mice, heterozygous for the TCF4 functional deletion, mimicking the TCF4 haploinsufficiency in PTHS patients, and their wild type littermates. Data collection and initial analysis were performed blindly, that is, the genotyping key was received after the mean values were calculated for each individual animal, and then mean/median of each group was subsequently calculated. Body weight, fecal pellet output, and fluid content were similar between the groups, indicating normal gross growth of PTHS mice and their overall physiological GI motility and intestinal secretion/absorption. There were no significant differences in gut length and gross appearance pointing out that PTHS mice have normal gut in gross anatomical terms. However, the assessment of gut transit indicates that, while whole-gut transit velocity was similar between the groups, the upper GI and distal colon transit velocities were significantly reduced in the PTHS mice. This is the first evidence of specific gut related problems in the PTHS mice. Our study also validates the TCF4 functional knockout mice as an animal model to study PTHS-associated GI disturbances.
Keywords: PTHS mouse model, gut transit, TCF4, E2-2, ITF2
Introduction
Pitt–Hopkins syndrome (PTHS, OMIM #610954) is an autism spectrum disorder that was initially characterized in 1978 as mental retardation, wide mouth and intermittent overbreathing [Pitt & Hopkins, 1978]. Other common clinical features are developmental delay, an absence of speech, autistic behaviors, motor incoordination, eye anomalies, seizures, and gastrointestinal (GI) signs, such as constipation and gastroesophageal reflux [Whalen et al., 2012]; PTHS is yet to be fully characterized, however.
The cause of PTHS was identified in 2007 as de novo heterozygous mutations of the Transcription Factor 4 gene (TCF4, located at 18q21.1, OMIM #602272) [Amiel et al., 2007; Brockschmidt et al., 2007; Zweier et al., 2007], ranging from point mutations to partial and total gene deletions and resulting in the TCF4 haploin-sufficiency [Whalen et al., 2012]. TCF4, also known as E-protein E2-2 or Immunoglobulin Transcription Factor 2 (ITF2), belongs to ubiquitously expressed class I of basic helix-loop-helix factors and is involved in many developmental processes and regulation of cell growth [Massari & Murre, 2000], hence the multitude of disease features. This protein should not be confused with T-Cell Factor 4, also abbreviated as TCF4, which is another transcription factor encoded by the Transcription Factor 7-Like 2 (TCF7L2) gene [van Es et al., 2012]. The breakthrough in PTHS etiology opened vistas for the use of animal models, and TCF4 functional knockout mouse, developed a decade earlier to harbor a deletion of the TCF4 DNA-binding domain [Zhuang, Cheng, & Weintraub, 1996], became readily available and was repurposed as the PTHS mouse model [Sweatt, 2013]. Naturally, most of the recent research has been focused on the neurological deficits. However, constipation is the most common complaint (74–77% of patients) for daily disease management and frequently requires medication [Sweatt, 2013; Whalen et al., 2012]. Similarly, gastroesophageal reflux is a substantial problem as it occurs in 15–42% of patients [Whalen et al., 2012]. In this report, we systematically investigated the PTHS model using established in vivo GI transit tests.
Materials and Methods
Animals
We used adult (15- to 19-week old) male mice, heterozygous for the deletion of the DNA-binding domain of the Transcription Factor 4 (B6;129-TCF4tm1Zhu/J; stock number 013598, Jackson Laboratory, Bar Harbor, ME) [Zhuang et al., 1996], an animal model for PTHS [Sweatt, 2013], and their wild type (wt) littermates. Mice were Helicobacter spp. free and maintained in a temperature (24–25°C)-controlled environment on a 12-hr light (7 am on):12-hour dark (7 pm off) cycle, with ad libitum access to food and water. All experiments assessing GI functions described below started at zeitgeber (ZG) + 1 hr (8 am). At that juncture, mice were individually housed for an hour in cages containing only a single sheet of adsorbent paper at the cage bottom, and food and water were removed. All procedures were performed in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Pellet Output and Fluid Content
This assay was done as previously described [McClain et al., 2014]. Fecal pellets were collected at ZG + 2 hr (1 hr after individually housing the animals), counted, and weighed (wet weight). The pellets were then incubated for a day at 60°C and weighed again (dry weight). Fluid content was calculated as follows:
| (1) |
Upper Gastrointestinal Transit
The test was modified from another study [De Winter et al., 1997] and executed at ZG + 2.5 hr to ZG + 5 hr. Mice were gavaged with 0.2 mL of a solution containing 5% (w/v) Evans blue (Cat. No. E2129, Sigma-Aldrich Co., St Louis, MO) and 5% (w/v) dextrose (Cat no. DX0145, EMD Millipore, Billerica, MA) dissolved in ultrapure water [Milli-Q® Synthesis system (Millipore Corporation, Billerica, MA)]. Fifteen minutes later, the animals were euthanized and the pyloric region of the stomach with the whole intestines were removed. We then measured the distance from the pylorus to the dye front (dye distance). We also measured the length of the whole intestine, as the pylorus-anus distance, and the lengths of small and large intestines, as the pylorus-ileocecal junction distance and the cecocolic junction-anus distance, respectively. We calculated upper GI transit velocity (mm/min) as the dye distance (mm) over 15 min.
Colon Bead Assay
Distal colon transit was assessed using glass beads (2 mm in diameter) as previously described [McClain et al., 2014], and executed at ZG + 2.5 hr to ZG + 5 hr. In short, mice were briefly anesthetized and single bead was inserted through the anus and pushed 2 cm orally using a 20 gauge stainless steel feeding needle with a silicon tip (1.9 mm in diameter) (Cat. No. 9921, Cadence Inc., Staunton, VA). The needle was then withdrawn and the bead expulsion latency was obtained. We calculated distal colon transit velocity (mm/min) as 20 mm over the bead expulsion latency (min).
Whole-Gut Transit
Whole intestinal transit was determined as previously described [McClain et al., 2014] and executed at ZG + 2.5 hr to ZG + 3.5 hr. Briefly, mice were gavaged with 0.2 mL of a solution containing 6% (w/v) Carmine (Cat. No. C1022, Sigma-Aldrich Co., St Louis, MO) and 0.5% (w/v) methyl cellulose (Cat. No. M0262, Sigma-Aldrich Co.) dissolved in ultrapure water and were left undisturbed in individual cages with food and water ad libitum. After 2 hr, pellet coloration was checked regularly every 20 min. Time elapsed from gavage until the detection of the first red pellet was obtained. We calculated whole GI transit velocity (mm/min) as the whole intestine length (mm) over the red pellet excretion latency (min).
Data Collection and Analysis
To reduce individual variability [Wyman, Heaton, Manning, & Wicks, 1978], every animal was assayed 2–3 times for all the tests except the upper GI transit (terminal procedure). All tests were performed blindly, that is, experimenter was not aware of the mouse genotype, until all data were collected, pruned (data from all distressed animals were disregarded) and mean values calculated for each individual animal. At this juncture the genotyping key was revealed and the individual mouse values were arranged into wt and PTHS groups and mean/median of each group was subsequently calculated.
Statistics
We used the GB-Stat 6.5 software (Dynamic Microsystems Inc., Silver Spring, MD) for statistical analysis. The number of subjects required for individual set of experiments was estimated using power analysis (set at 80% and α = 0.05). Student’s t-test was used for statistical comparisons if both data sets passed Shapiro–Wilk test for normality; Mann–Whitney U-test was performed otherwise. As all normally distributed variables were also homoscedastic, as tested by Levene’s test for variance homogeneity, we used pooled variances version of the Student’s t-test. For all statistical comparisons P < 0.05 was regarded as statistically significant.
Results
We hypothesized that the PTHS mouse model will exhibit problems with their gut function. As the TCF4 homozygous mutants die perinatally, we conducted series of in vivo tests on mice heterozygous for the TCF4 functional deletion, mimicking TCF4 haploinsufficiency in PTHS patients, and their wt littermates.
PTHS Mice Have Normal Body Weight and Pellet Output
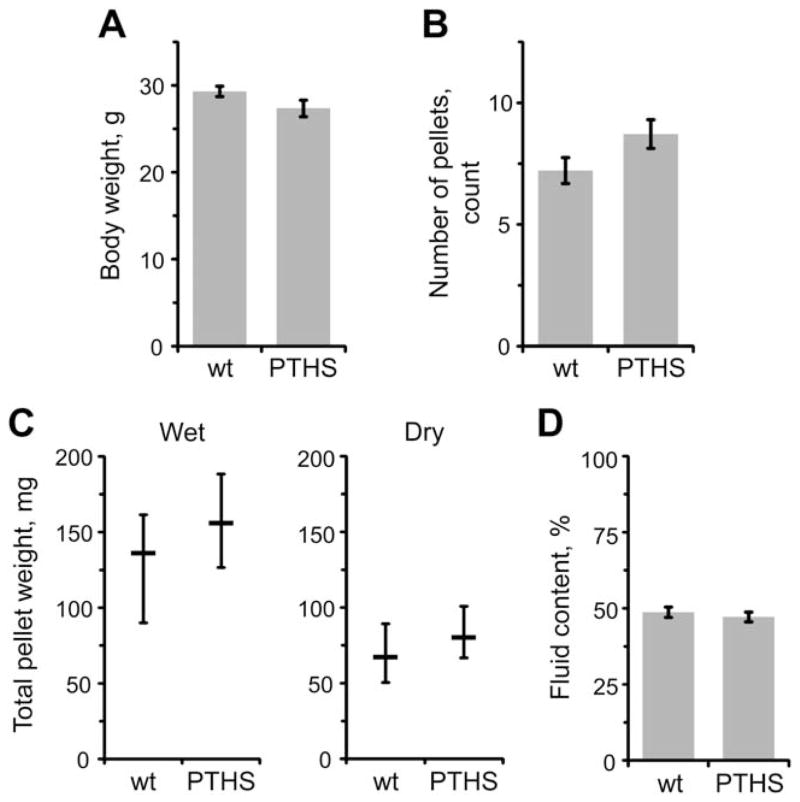
We first obtained the body weights of mice to assess their growth and food intake (Fig. 1A). On average, body weight of the PTHS group was similar to the wt group (P = 0.082, Student’s t-test), indicating normal gross growth of the PTHS mice.
Figure 1.
Pitt–Hopkins syndrome (PTHS) mice have normal body mass and pellet output in vivo. (A) Body weights. Wild type (wt) and PTHS mice have similar body weights. (B–D) Pellet production and composition. PTHS and wt animals do not differ significantly in the total number of pellets (B), pellet wet/dry weight (C, left/right) and pellet fluid content (D). Data are shown as mean ± standard error of mean (SEM) in A, B and D; or as median ± interquartile range (IQR) in C. Sample sizes were the same for all measurements: 18 and 16 mice for wt and PTHS groups, respectively.
Next, we monitored fecal pellet output to evaluate gut propulsive motility. We counted the number of pellets, obtained their wet/dry weight, and calculated the fluid content (Fig. 1B–D). No significant differences were observed in all of these measurements between the groups [P = 0.085, Student’s t-test (pellet count); P > 0.265, Mann–Whitney U-test (wet/dry weight); P = 0.564, Student’s t-test (fluid content)], which pointed out that PTHS mice have overall physiological GI motility and intestinal secretion/abortion. As this is a general test and does not allow to study particular gut transits, we turned to more specific tests assessing just that.
PTHS Mice Show Reduced Gut Transit Velocities
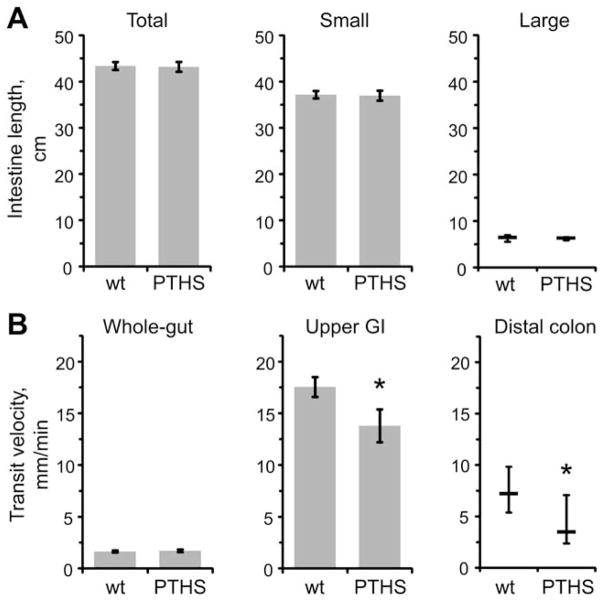
Before assessing gut transits in detail, we obtained the lengths of whole guts, small and large bowel; no significant difference [P > 0.545, Student’s t-test (whole guts and small bowel) or Mann–Whitney U-test (large bowel)] was found between the wt and PTHS mice (Fig. 2A). Also, we did not observe any gross malformations in the intestines. Both findings indicate that PTHS mice have normal gut in gross anatomical terms.
Figure 2.
Particular in vivo gut transit velocities are reduced in PTHS mice. (A) Lengths of intestines. Total (left), small (center) and large (right) intestines were similar in length between the wt and PTHS animals. (B) Gastrointestinal (GI) transit velocities in vivo. Whole-gut transit velocity (left) was similar between the groups. Upper GI (center) and distal colon (right) transit velocities were significantly reduced in the PTHS mice.*P < 0.05, Student’s t-test and Mann–Whitney U-test, respectively. Data are shown as mean ± SEM (left and center graphs) or as median ± IQR (right graphs). 18 wt and 16 PTHS mice were used for all the measurements except the upper GI transit. Three animals were excluded from the analysis of the upper GI transit, 1 in wt and 2 in PTHS groups, due to obvious distress signs after the gavage.
While whole-gut transit velocity was similar between the groups (P = 0.346, Student’s t-test), upper GI and distal colon transit velocities were significantly reduced in the PTHS mice (P = 0.044 and 0.027, using Student’s t-test and Mann–Whitney U-test, respectively) (Fig. 2B). Taken together, these findings indicate specific disturbances in gut function, namely retarded gastric emptying with reduced propulsion activity in the oral part of small bowel and reduced propulsion of the distal colon.
Discussion
We provide the first evidence that PTHS mice have specific gut malfunctions, namely reduced upper GI and distal colonic transit velocities, that resemble symptoms, that is, gastroesophageal reflux and constipation, respectively, of the PTHS patients [Sweatt, 2013]. Therefore, our findings validate this transgenic mouse strain, harboring functional deletion of TCF4 [Zhuang et al., 1996], as an animal model to study PTHS associated GI disturbances. No obvious differences in the animal’s physical appearance or changes in their gross behavior were observed that could distinguish the PTHS mice from their wt littermates, hence making it feasible for the experiments to be performed with the person blinded to the genotype of the mice. However, detailed behavioral and neurological characterization of PTHS animals is needed in the future.
Interestingly, the whole-gut velocity in PTHS mice was unchanged although both the upper GI and distal colon velocities were reduced. One possible explanation could be that the upper GI and distal colon transits, as two fast components (~18 and ~7 mm/min, respectively) of the whole-gut transit (~2 mm/min), make only a minor part (together < 20 min) of the whole-gut transit time (~4.7 hr). Additionally, PTHS mice could have compensatory acceleration of the slower component of the whole-gut transit.
Similarly, PTHS mice had slowed propulsion of the distal colon (Fig. 2B, right) but normal fecal output (Fig. 1B, C). This is also not an unusual finding as it has already been shown that fecal output does not have to correlate with the colonic motility [Nakade, Mantyh, Pappas, & Takahashi, 2007]. Furthermore, these findings could indicate that PTHS mice deposit fecal pellets in the distal colon. This hypothesis could be tested in the future using radiopaque markers [Zakhary et al., 1997].
We did not find gross anatomical malformations in the intestines of PTHS mice, so decreased transit velocities are probably due to functional impairment in gut propulsive motility, a phenomenon under the regulation of the enteric nervous system (ENS) [Goyal & Hirano, 1996]. Alternatively, although less likely, gut transit could be slowed due to the overgrowth of the mucosa or other microanatomical defects of the intestinal wall [Forrest, Waite, Martin-Rendon, & Blake, 2013]; this issue could be addressed by histological examination. Basic helix-loop-helix transcription factors play a role in ENS development [Nelms & Labosky, 2010] and TCF4 was already shown to regulate the differentiation and migration of a subset of neuronal progenitors [Flora, Garcia, Thaller, & Zoghbi, 2007], so slowed transits could be caused by the TCF4 haploinsufficiency during the formation of the ENS. Indeed, some PTHS patients have comorbidity for the Hirschsprung disease [Zweier et al., 2007], a developmental disorder characterized by the absence of enteric ganglia along a variable length of the intestine. However, it is important to mention that many PTHS patients with constipation do not have underlying Hirschsprung disease [Whalen et al., 2012], indicating other underlying causes for the GI disturbances. TCF4 is expressed in both glia and neurons of the central nervous system [Fu et al., 2009]. If the same holds in the ENS, specific problems in GI transit could be caused by malfunctioning of enteric neurons and/or glia. Furthermore, TCF4 is involved in the development of the gut immune system [Murphy, 2013; Rescigno & Di Sabatino, 2009], which can ultimately have an effect on the gut motility through its interaction with the ENS [De Winter & De Man, 2010]. The role of TCF4 haploin-sufficiency in the development and function of the enteric nervous and immune systems should be studied in the future.
Additionally, as a global transcription factor, TCF4 is involved in many important cellular processes beside cell differentiation and development, for example, signal transduction and metabolism [Forrest, Waite, Martin-Rendon, & Blake, 2013]. Therefore, GI related problems could originate, at least in part, from the reduced amount of TCF4 in adults. This hypothesis should be studied in the future and, if confirmed, would indicate that some of the PTHS associated GI problems might be treatable with already available drugs that increase global gene transcription (reviewed elsewhere [Sweatt, 2013]).
Acknowledgments
This research was supported by National Institutes of Health Awards HD078678 (VP), MH57014 (JDS) and MH104158 (JDS); the Pitt-Hopkins Research Foundation (AJK and JDS) and Civitan International (VG and AJK). We thank Manoj K. Gottipati and Chapin E. Cavender for comments on a previous version of this manuscript and the lay abstract, respectively. The authors declare no competing financial interests. Specific author contributions: VG and VP conceived and designed the study. AJK and JDS provided the animals. VG performed the experiments and analyzed the data. VG and VP wrote the manuscript.
References
- Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, et al. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. American Journal of Human Genetics. 2007;80:988–993. doi: 10.1086/515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschmidt A, Todt U, Ryu S, Hoischen A, Landwehr C, Birnbaum S, et al. Severe mental retardation with breathing abnormalities (Pitt-Hopkins syndrome) is caused by haploinsufficiency of the neuronal bHLH transcription factor TCF4. Human Molecular Genetics. 2007;16:1488–1494. doi: 10.1093/hmg/ddm099. [DOI] [PubMed] [Google Scholar]
- De Winter BY, Boeckxstaens GE, De Man JG, Moreels TG, Herman AG, Pelckmans PA. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. British Journal of Pharmacology. 1997;120:464–468. doi: 10.1038/sj.bjp.0700913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Winter BY, De Man JG. Interplay between inflammation, immune system and neuronal pathways: Effect on gastrointestinal motility. World Journal of Gastroenterology. 2010;16:5523–5535. doi: 10.3748/wjg.v16.i44.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora A, Garcia JJ, Thaller C, Zoghbi HY. The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15382–15387. doi: 10.1073/pnas.0707456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest MP, Waite AJ, Martin-Rendon E, Blake DJ. Knockdown of human TCF4 affects multiple signaling pathways involved in cell survival, epithelial to mesenchymal transition and neuronal differentiation. PLoS One. 2013;8:e73169. doi: 10.1371/journal.pone.0073169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Cai J, Clevers H, Fast E, Gray S, Greenberg R, et al. A genome-wide screen for spatially restricted expression patterns identifies transcription factors that regulate glial development. Journal of Neuroscience. 2009;29:11399–11408. doi: 10.1523/JNEUROSCI.0160-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal RK, Hirano I. The enteric nervous system. New England Journal of Medicine. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: Regulators of transcription in eucaryotic organisms. Molecular and Cellular Biology. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain JL, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sevigny J, et al. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology. 2014;146:497–507. e491. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM. Transcriptional control of dendritic cell development. Advances in Immunology. 2013;120:239–267. doi: 10.1016/B978-0-12-417028-5.00009-0. [DOI] [PubMed] [Google Scholar]
- Nakade Y, Mantyh C, Pappas TN, Takahashi T. Fecal pellet output does not always correlate with colonic transit in response to restraint stress and corticotropin-releasing factor in rats. Journal of Gastroenterology. 2007;42:279–282. doi: 10.1007/s00535-006-1947-2. [DOI] [PubMed] [Google Scholar]
- Nelms BL, Labosky PA. Transcriptional control of neural crest development. Chapter 3. San Rafael, CA: Morgan & Claypool Life Sciences; 2010. bHLH Proteins. [PubMed] [Google Scholar]
- Pitt D, Hopkins I. A syndrome of mental retardation, wide mouth and intermittent overbreathing. Australian Paediatric Journal. 1978;14:182–184. doi: 10.1111/jpc.1978.14.3.182. [DOI] [PubMed] [Google Scholar]
- Rescigno M, Di Sabatino A. Dendritic cells in intestinal homeostasis and disease. Journal of Clinical Investigation. 2009;119:2441–2450. doi: 10.1172/JCI39134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Pitt-Hopkins Syndrome: Intellectual disability due to loss of TCF4-regulated gene transcription. Experimental and Molecular Medicine. 2013;45:e21. doi: 10.1038/emm.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, et al. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Molecular and Cellular Biology. 2012;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen S, Heron D, Gaillon T, Moldovan O, Rossi M, Devillard F, et al. Novel comprehensive diagnostic strategy in Pitt-Hopkins syndrome: Clinical score and further delineation of the TCF4 mutational spectrum. Human Mutation. 2012;33:64–72. doi: 10.1002/humu.21639. [DOI] [PubMed] [Google Scholar]
- Wyman JB, Heaton KW, Manning AP, Wicks AC. Variability of colonic function in healthy subjects. Gut. 1978;19:146–150. doi: 10.1136/gut.19.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Y, Cheng P, Weintraub H. B-lymphocyte development is regulated by the combined dosage of three basic helix-loop-helix genes, E2A, E2-2, and HEB. Molecular and Cellular Biology. 1996;16:2898–2905. doi: 10.1128/mcb.16.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier C, Peippo MM, Hoyer J, Sousa S, Bottani A, Clayton-Smith J, et al. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome) American Journal of Human Genetics. 2007;80:994–1001. doi: 10.1086/515583. [DOI] [PMC free article] [PubMed] [Google Scholar]