Abstract
Neuronal stimulation is an emerging field in modern medicine to control organ function and reestablish physiological homeostasis during illness. Transdermal nerve stimulation with electroacupuncture is currently endorsed by the WHO, the NIH, and is used by millions of people to control pain and inflammation. Recent advances in electroacupuncture might allow the activation of specific neuronal networks to prevent organ damage in inflammatory and infectious disorders. Experimental studies of nerve stimulation are also providing new information on the functional organization of the nervous system to control inflammation and its clinical implications in infectious and inflammatory disorders. These studies might allow the design of novel non-invasive techniques for nerve stimulation to help control immune and organ functions.
Keywords: Neuromodulation, nerve stimulation, inflammation, infectious diseases
Clinical Implications of Neuromodulation
Neuronal stimulation is an emerging field in modern medicine to control organ functions and reestablish physiological homeostasis during illness. Recent studies indicate that nerve stimulation provides therapeutic benefits in treating inflammation in arthritis, colitis, diabetes, obesity, hemorrhage/resuscitation, pancreatitis, quadriplegia and infectious disorders such as endotoxemia, septic shock and severe sepsis[1–8] (Glossary). However, most of these studies have been performed with surgical isolation of a given nerve for direct electrical stimulation. Given that surgical anesthetics inhibit neuronal signals to decrease pain, they also interfere with neuromodulation; consequently, the need for surgery to achieve direct nerve stimulation precludes repeating the treatment in chronic disorders (e.g. arthritis or colitis)[9]. Thus, recent studies seek alternative non-invasive strategies for nerve stimulation, and show that non-surgical transdermal nerve stimulation with electroacupuncture can control inflammation and prevent organ damage in inflammatory and infectious disorders, as in the case of experimental sepsis [9, 10]. Neuromodulation with acupuncture or electroacupuncture is used by millions of people to control pain, inflammation and reestablish physiological homeostasis during illness (Box 1). Clinical studies show that acupuncture can improve postoperative recovery, osteoarthritis, migraine, joint pain, stroke, post-traumatic stress disorder and drug addiction[11]. The National Institutes of Health estimate that acupuncture has been used by over 15 million Americans [12, 13]. The use of electroacupuncture is now endorsed by the American Pain Society, the National Center for Complementary and Alternative Medicine, the National Institutes of Health and the World Health Organization [14–16].
Box 1. Neuromodulation.
Inflammation is critical for survival, but unregulated inflammation is lethal. One dramatic example is sepsis that accounts for 9% of the overall deaths in the United States annually. Sepsis is characterized by detrimental systemic inflammation that causes multiple organ failure. Neuromodulation encompasses efficient systems selected through evolution to control inflammation. Cytokines and neurotransmitters are produced by neurons to modulate immune cells, and vice versa. This bidirectional communication allows the nervous system to sense inflammation and to activate specific neuronal networks to control immune cells and avoid the detrimental effects of excessive inflammation. Given that these mechanisms are not specific for single cytokines, they can control inflammation in diverse inflammatory and infectious disorders including complex disorders such as sepsis[26].
Acupuncture was developed by traditional Chinese practitioners to control pain [10, 17, 18]. The points of stimulation were selected by empirical assays based on the responses of the patients. All but one of 361 acupoints in humans are located close to neuronal networks[17–19]. As neuronal networks were evolutionarily selected to achieve physiological homeostasis, it is not surprising that neuromodulation emerged as one of the first strategies used in medicine to reestablish homeostasis during illness[17]. However, the efficacy of acupuncture or electroacupuncture is very controversial because it relies on the experience and precision of the practitioner with needle inseretion, and because of a lack of proper mechanistic studies. Moreover, nerves can be stimulated with different techniques including pressure (acupressure), massage (Tui na), heat (moxibustion and fire cupping), sound (sonopuncture) or electricity (electroacupuncture)[17, 18]. Each technique might activate distinct mechanisms of action with different intensity[17, 20]. The most typical examples of this medical hypothesis include the differences between acupuncture and electroacupuncture. While acupuncture induces mechanical stimulation in neuromuscular junctions and causes local release of neuromodulators, electroacupuncture represents a transdermal electrical stimulation of the nerves with voltage-dependent effects[17, 20]. Many clinical reviews conflate these techniques and create confusion regarding their efficacy, mechanisms, and statistical meta-analyses[21]. Furthermore, the efficacy of acupuncture is also controversial because the lack of proper mechanistic studies in this field prevent an explanation of the clinical limitations of these techniques, and on why electroacupuncture may be effective in some diseases, and in some patients, but not in others with similar symptoms. These mechanistic studies will allow the design of more effective techniques for specific disorders and cohorts of patients. There are many preliminary studies based on the systemic administration of a single drug treatment, single denervation (e.g. vagotomy) procedure, or isolated clinical case reports or trials that have not been replicated. Some of the most reliable results are produced in experimental animal models because, in contrast to clinical trials that can be tainted by the placebo effect and the heterogeneity of patients[21–23], experimental studies are performed in well-characterized models of diseases using homogenous groups of animals [24–26]. These recent studies show that electroacupuncture can attenuate inflammation and improve organ function in inflammatory and infectious disorders such as experimental sepsis. Here, we review the recent advances on the neuronal networks and their mechanisms of neuromodulation of the immune system, as well as their clinical implications to control inflammation (Box 2). This article intends to connect the empirical clinical information from acupuncture and electroacupuncture with the recent basic cellular and molecular mechanistic studies of neuromodulation. Both fields will benefit from this connection, bringing rigor to clinical and mechanistic studies on electroacupuncture and neuromodulation, and connecting clinical data to recent mechanistic studies in experimental animal models.
Box 2. Sensory networks and central nervous processing.
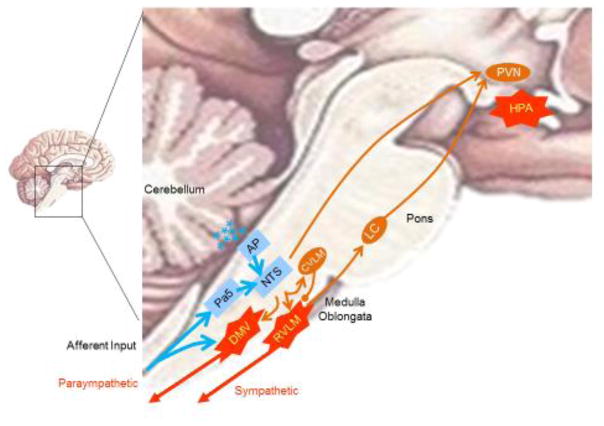
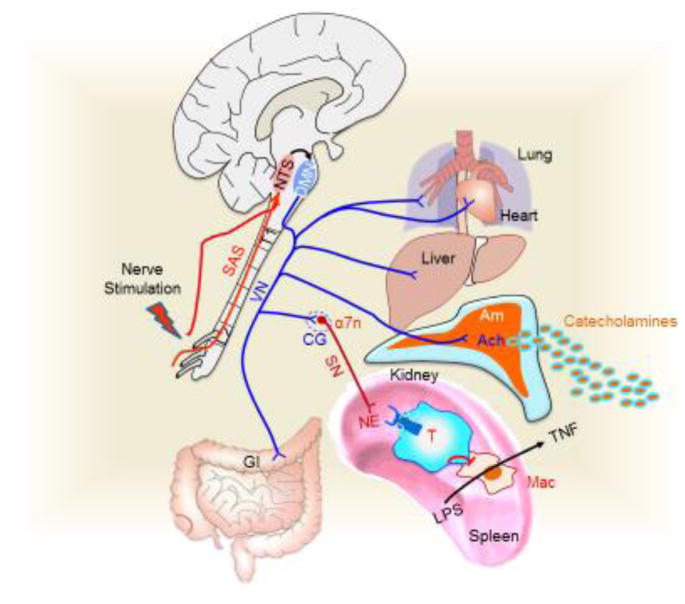
Nerve stimulation activates sensory afferent nerves that transmit the information to the somatosensory centers of the central nervous system (CNS) across species [17, 18] (Fig. 1). A typical somatosensory system has two neuronal connections: the ‘peripheral’ first-order neurons and the ‘central’ second-order neurons[123]. Classically, the first-order neurons innervate around the acupoints and have an axon connecting to the second-order neuron in the central system. The two major populations of sensory neurons are the vagal, originating at the nodose ganglia (NG) and the spinal afferent nerves originated at the dorsal root ganglia (DRG)[123]. The second order neurons terminate in integrative centers of the somatosensory systems including the reticular system, cerebellum, medulla oblongata and thalamus[123].
A typical example of processing sensory signals is the baroreceptor reflex. When the blood pressure falls, baroreceptor firing decreases to restore blood pressure. The nucleus tractus solitarius (NTS) receives sensory signals from the carotid sinus baroreceptors via the glossopharyngeal nerve (CN-IX) and the aortic arch baroreceptors via the vagal (CN-X) branches[124]. Then, the NTS activates efferent pathways inducing heartbeat and blood vessel constriction. Electroacupuncture activates similar networks depending on the acupoint and electrical stimulation. For instance, ST36 stimulation activates the sciatic nerve, which in turn activates the NTS via the paratrigeminal nucleus (Pa5) region of the medulla oblongata[125, 126]. The NTS also integrates endocrine signals through the area postrema (AP), because this region lacks the blood-brain-barrier and therefore it can receive endocrine signals from the peripheral bloodstream[127]. Then, the NTS coordinates efferent pathways either directly or indirectly. Direct innervations from the NTS to the paraventricular nucleus (PVN) and the vagal dorsal motor nucleus (DMN) allow specific activation of the hypothalamic-pituitary-adrenal (HPA) axis and the parasympathetic nervous system, respectively[128]. However, parasympathetic innervations of the heart originate mostly from the nucleus ambiguous. Indirect innervations allow the NTS to coordinate the sympathetic nervous system (via the rostral ventrolateral medulla (RVLM)). The NTS sends excitatory (glutamatergic) fibers to activate the caudal ventrolateral medulla, which sends inhibitory (GABAergic) fibers to inhibit C1 neurons of the RVLM[127].
The RVLM is the primary regulator of the sympathetic nervous system, also sending excitatory (glutamatergic) fibers to the preganglionic neurons of the spinal cord[127]. In addition, the RVLM sends neurons to the locus coeruleus in the pons, the principal source of norepinephrine innervations of the hypothalamic paraventricular nucleus[129]. The NTS also modulates the parasympathetic system via the dorsal motor nucleus. Fibers from the NTS converge with vagal afferent fibers from the nodose ganglion in the vagal dorsal motor nucleus of the medulla oblongata, which generates the preganglionic vagal efferent fibers, whereas the medullar nucleus ambiguous generates the cardiovascular vagal efferent nerves[127]. After processing, these centers coordinate the effector pathways that control physiological homeostasis.
Transdermal stimulation of sensory afferent nerves
Aiming to improve therapeutic efficacy, recent studies have focused on how acupuncture or electroacupuncture activate sensory afferent nerves. Whereas some procedures have not changed much since ancient Chinese medicine, modern techniques of electroacupuncture induce voltage-dependent transdermal nerve stimulation[17, 20]. Most studies analyzing how acupuncture modulates sensory afferent nerves have been performed in models of nociception; standard experimental models of nociception are based on rodent time-of-response to noxious stimuli applied to a tail or paw (tail-flick or paw withdrawal latency period tests) [17, 27]. For instance, acupuncture on ST36 (3 mm below the knee joint) reduces pain nociception to a noxious stimulus, thereby increasing the tail-flick and paw withdrawal ‘latency periods’ or ‘time of response’. Preliminary results suggest that acupuncture on ST36 induces analgesia by activating the sciatic nerve through mechanical stimulation of the connective tissue[28]. In addition, these effects can be prevented by disrupting the connective tissue around the acupoint with collagenase[28]. Conversely, the rotation and surface roughness of acupuncture needles can increase the mechanical stimulation of connective tissue, and thereby can enhance the effects of acupuncture[28–30]. Although these mechanisms are not well-known, preliminary experimental studies in mice suggest that acupuncture induces a mechanical stimulation of fibroblasts (the most common cells of connective tissue) through mechanical tension of the actinomyosin cytoskeleton [29, 31, 32]. Future studies are required to determine the precise mechanisms of activation and whether this type of mechanical stimulation is similar to that of endothelial cells in arteries mediated by the stress-activated protein kinases (SAPK)/Jun amino-terminal kinases (JNK) pathways [33, 34]. The SAPK and JNK pathways can activate mammalian immune and endothelial cells to produce inflammatory factors that can cause antinociception such as TNF and IL-6 [33, 35, 36]. Indeed, preliminary studies indicate that acupuncture induces mechanical stimulation of rat mast cell degranulation, which causes antinociception by releasing TNF and IL-6 [28]. The potential of these factors to prevent nociception has been demonstrated in several studies showing that intraplantar injection of either TNF or IL-6 prevents nociception in rodent paws[35, 36]. It is not well-known how these factors affect sensory nerves, but recent studies suggest that this effect may be mediated by T-lymphocytes because it was prevented by inhibiting T-lymphocytes; conversely, it was restored by transferring allogeneic lymphocytes into immunosuppressed mice[36, 37]. The potential of TNF and IL-6 to affect nociception can also be prevented by opioid inhibitors such as naloxone, neutralizing antibodies, or μ-opioid receptor antagonists[38–41]. Together, these results suggest that acupuncture has the capacity of inducing mast cells (and perhaps other degranulating immune cells) to release inflammatory cytokines. These cytokines can activate T-lymphocytes to produce opioids, which in turn can activate μ-opioid receptors of sensory nerves to prevent nociception [42]. Indeed, these mechanisms do not appear to be specific for TNF and IL-6 only, as preliminary studies in rats show that interferon (IFN)-α can also affect neuronal networks through opioid receptors [42].
Detailed and rigorous mechanistic studies are needed to design more effective and specific techniques for non-invasive neuronal stimulation. A classic mechanistic study on acupuncture in mice shows that acupuncture on ST36 causes antinociception by inducing extracellular adenosine[43]. Specifically, adenosine-1 receptor (A1R) knockout (Adora1tm1Jgsc) mice are refractory to ST36-induced antinociception[43]. A1Rs inhibit adenylyl cyclase, activate potassium channels, block transient calcium channels, and thus, neuronal sensory nerve activity. Accordingly, deoxycoformycin -- a selective A1R-agonist -- can replicate the analgesic effects of acupuncture on ST36 in mice, and thus, might be potentially considered as a novel therapeutic treatment against pain [44].
Electroacupuncture induces antinociception through mechanisms similar to those described for acupuncture[17, 45]. For instance, mechanical stimulation contributes to the effects of electroacupuncture based on the electrical frequency of the stimulation. Mechanical stimulation at high-frequency induces stress fibers in fibroblasts to orient away from the direction of stretch, but the fibers remain randomly oriented when subjected to stretches at low frequencies [46]. Similar to acupuncture, electroacupuncture on GB30 (acupoints innervated by the sciatic and gluteal nerves) induces antinociception through a mechanism prevented by opioid antagonists such as naloxone[47]. However, these mechanisms appear to vary depending on the immune status of the animal. For instance In healthy-uninjured control rats, electroacupuncture on GB30 could prevents nociception via μ and δ opioid receptors at low-frequency (under 10Hz), but via κ receptors, at high-frequency (above 100Hz) [47]. In injured rats (treated with a plantar injection of complete Freund’s adjuvant (CFA)), both low- and high-frequency electroacupuncture on GB30 prevented nociception via m μ- and δ- but not by κ-receptors[47]. Thus, persistent inflammation or pain might diminish the role of κ opioid receptors at high-frequency electroacupuncture. Similar to the effects described in acupuncture, the effects of electroacupuncture are also mediated by the inhibition of adenylyl cyclase and blockage of calcium channels to abrogate pain nociception in sensory nerves[48–50]. Moreover, a sub-threshold dose of morphine (2.5 mg/kg) was shown to enhance electroacupuncture-induced analgesia additively at low-frequency (10 Hz) but synergistically, at high-frequency (100 Hz) in this model [47]. Thus, these mechanistic studies are important for the design of novel candidate non-invasive techniques for nerve stimulation, as well as tor determining the proper combination approach of electroacupuncture with conventional pharmacological treatments.
Neuronal efferent networks of neuromodulation
The central nervous system (CNS) coordinates three effector pathways to control organ function: the hypothalamic-pituitary-adrenal (HPA) axis, the sympathetic, and parasympathetic pathways (Fig. 1 and Box 2). The HPA is a chemically stable humoral pathway that allows lasting systemic signals through the bloodstream. The parasympathetic system is a neuronal pathway that produces acetylcholine, an unstable neurotransmitter with a short life span that produces transient and local effects. The sympathetic system is a hybrid mechanism with both neuronal innervations and humoral responses. It innervates most of the organs with pre-ganglionic cholinergic fibers (producing acetylcholine) or post-ganglionic adrenergic fibers (producing catecholamines). The sympathetic system also induces systemic release of catecholamines from adrenal glands.
Figure 1. Central processing of mammalian sensorial information.
Neuronal stimulation activates the nucleus tractus solitarius (NTS) via the paratrigeminal nucleus (Pa5) of the medulla oblongata. The NTS also integrates endocrine signals (*) through the area postrema (AP), which lacks the blood-brain-barrier. Direct innervations from the NTS to the paraventricular nucleus (PVN) and the vagal dorsal motor nucleus (DMN) allow specific activation of the HPA axis and the parasympathetic nervous system, respectively. However, parasympathetic innervations of the heart originates mostly from nucleus ambiguous. Indirect innervation allows the NTS to coordinate both the HPA axis and the sympathetic nervous system. The RVLM also sends neurons to the locus coeruleus (LC) in the pons, the principal source of norepinephrine innervations of the hypothalamic paraventricular nucleus (PVN). NTS innervates the caudal ventrolateral medulla (CVLM), which in turn can modulates the RVLM.
The hypothalamic-pituitary-adrenal (HPA) axis
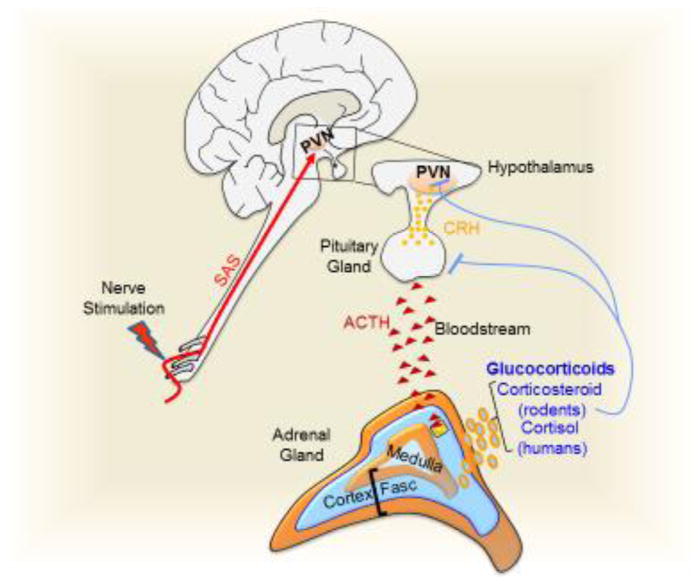
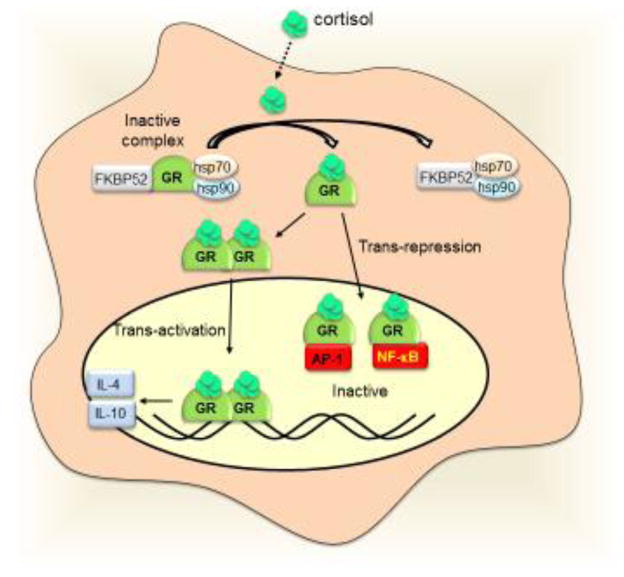
The HPA axis is based on the systemic release of glucocorticoids from the adrenal glands to the bloodstream (Fig. 2a). A typical example of its stimulation is that acupuncture on GB30 inhibits CFA-induced paw edema in mice through a mechanism prevented by adrenalectomy and glucocorticoid inhibitors [51–53]. Afferent sensory networks activate the paraventricular nucleus of the hypothalamus to secrete vasopressin and corticotrophin releasing hormone[53]. Both compounds activate the anterior lobe of the pituitary gland to release adrenocorticotrophic hormone (ACTH) into the bloodstream (Supplementary Table S1). ACTH then activates the zona fasciculate of the cortex of the adrenal glands to release glucocorticoids; mainly corticosterone in rodents and hydrocortisone (cortisol) in humans[51, 54]. Glucocorticoids have a stable half-life span of 8–10 hours, and their effects are broadly classified into: metabolic (inducing glucogenic enzymes in the liver to increase blood glucose) and immune (to reduce inflammation) mechanisms. At the cellular level, mammalian glucocorticoid receptors (GRs) are expressed in almost all cells and regulate development, metabolism and inflammation[55]. Dormant GRs reside in cytosolic complexes with multiple proteins such as FKBP52 (FK506-binding protein 52) and heat shock proteins (hsp70 and hsp90)[56, 57]. Glucocorticoids diffuse through cell membranes and bind to the intracellular receptors releasing them from dormant complexes (Fig. 2b). Active GRs can inhibit inflammation through two mechanisms: transactivation of anti-inflammatory genes, and transrepression of inflammatory genes. During transactivation, activated GRs homodimerize, translocate into the nucleus, and bind to specific DNA elements to induce the expression of anti-inflammatory genes coding for proteins such as IL-4 and IL-10[56, 57]. During transrepression, activated GRs bind to other transcriptional factors such as NF-κB to inhibit their potential to induce inflammatory cytokines[56, 58]. Thus, HPA stimulation can be a successful strategy to induce the production and systemic distribution of glucocorticoids in order to modulate metabolic and immune responses.
Figure 2. The hypothalamic-pituitary-adrenal (HPA) axis.
(a) Electroacupuncture on GB30 induces sciatic sensorial afferent signals (SAS) activating the paraventricular nucleus (PVN) to secrete corticotrophin releasing hormone (CRH). CRH activates the anterior lobe of the pituitary gland to release adrenocorticotrophic hormone (ACTH) into the blood stream. ACTH activates the zona fasciculata (Fasc) of the adrenal glands to produce glucocorticoids, which induce a negative feedback inhibiting the PVN and the pituitary gland. (b) At the cellular level, the inactive glucocorticoid receptors (GRs) reside in the cytosol complex with other proteins including the heat shock proteins (hsp70 and hsp90), and FKBP52. The glucocorticoids diffuse into the cells and release the receptor from the inactive complex. The GRs homodimerize and transactivate anti-inflammatory cytokines like IL4 and IL10. During transrepression, GRs bind to transcription factors such as the AP-1 and NF-κB, and prevent them from inducing inflammatory factors.
The sympathetic system
The sympathetic neuronal network runs through the spinal cord and innervates most of the viscera inducing both local neurogenic or systemic release of catecholamines from adrenal glands (Fig. 3a). The typical mechanism of sympathetic modulation is the activation of the adrenal glands to produce systemic release of catecholamines[42, 59, 60]. Pre-ganglionic sympathetic branches innervate the adrenal medulla and activate chromaffin cells to release catecholamines into the bloodstream. The systemic release of catecholamines can cause adverse effects such as systemic metabolic lipolysis and immunosuppression[42, 59, 60]. Thus, more recent studies have focused on the potential to induce local release of neurogenic catecholamines through sympathetic innervations in different tissues.
Figure 3. The sympathetic nervous system (SNS).
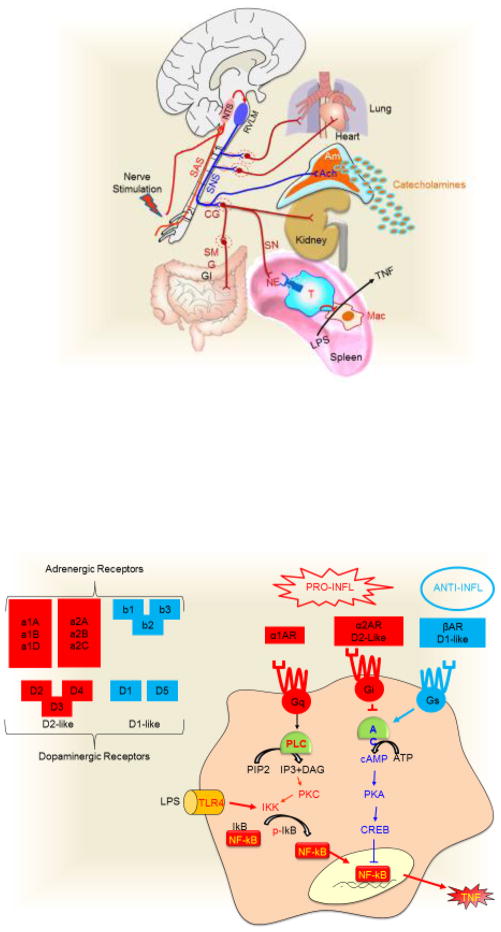
(a) In mammals, neuronal stimulations converge in the activation of the sensorial afferent signal (SAS) that activates the nucleus tractus solitarius (NTS). The NTS activates directly or indirectly the rostral ventrolateral medulla (RVLM), which is the primary regulator of the sympathetic nervous system (SNS). The SNS runs through the spinal cord and innervates most of the viscera including the lung, heart, kidneys and gastrointestinal tract (GI) through post-ganglionic innervations after the activation of the specific ganglia. Neuronal post-ganglionic innervations induces neurogenic norepinephrine (NE). Splenic norepinephrine inhibits the production of inflammatory cytokines in splenic macrophages by activating beta-2 adrenergic receptor (β2AR) of T ‘modulatory’ lymphocytes. The SNS can also induce a systemic production of catecholamines by activating the chromaffin cells via pre-ganglionic innervations releasing acetylcholine (ACh) in the adrenal medulla (Am). (b) At the cellular level, the receptors are classified in three major groups. α1-adrenoceptors (α1AR) signals through Gq, which activates phospholipase C (PLC) to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) to diacyl glycerol (DAG) and inositol trisphosphate (IP3). Both IP3 and DAG activate PKC to phosphorylate the IκB kinase (IKK) and induce phosphorylated (p-) IκB. α2-adrenoceptors (α2AR) and dopaminergic D2-like receptors coupled to Gi protein, which inhibits the adenylate cyclase (AC) and prevents the formation of cAMP. β-adrenoceptors (βAR) and dopaminergic D1-like receptors coupled to Gs protein, which activates AC to induce cAMP and activates PKA and CREB. The anti-inflammatory potential of this pathway relies on the potential of CREB to inhibit the NF-κB pathway.
Catecholamines have a short half-life span of 1–4 minutes leading to local effects, such as hepatic glycogenolysis, cardiac contraction, or bronchial relaxation. Sympathetic stimulation can induce either local or systemic catecholamines depending on the electrical frequency. Specifically, high-frequency electroacupuncture on ST36 inhibits carrageenan-induced paw inflammation through pre-ganglionic innervations of the adrenal glands in rodents [61, 62]. By contrast, low-frequency electroacupuncture on ST36 in rodents inhibits inflammation in experimental models of paw swelling, lung leukocyte migration, surgical trauma, sepsis or arthritis, through local sympathetic post-ganglionic innervations, independent of the adrenal glands [63–66]. Thus, high-frequency electroacupuncture appears to activate pre-ganglionic innervations of the adrenal medulla to induce systemic catecholamines, whereas low-frequency electroacupuncture appears to activate specific sympathetic post-ganglionic innervations to induce local release of neurogenic norepinephrine[61]. Recent studies in rodents suggest that local sympathetic regulation of the immune system can provide clinical advantages for treating inflammatory disorders such as arthritis by preventing systemic immunosuppression and susceptibility to secondary infections [67, 68]. It has been postulated that many local effects of sympathetic innervations appear to be mediated by a direct interaction of the post-ganglionic nerves with macrophages. For instance, sympathetic fibers of the gut muscularis in mice release norepinephrine, which binds to β2 adrenergic receptors on muscularis macrophages to activate a tissue-protective phenotype [69] and to regulate gastrointestinal motility [70]. Recent studies also indicate that sympathetic neuron–associated macrophages contribute to obesity by importing and metabolizing norepinephrine in mice[71]. By contrast, sympathetic splenic nerves can control inflammation in experimental sepsis by activating T lymphocytes and not by interacting directly with macrophages [72, 73]. The splenic nerves release norepinephrine, which can activate T lymphocytes via β2 adrenergic receptors to produce acetylcholine [72–75]. Acetylcholine and other cholinergic agonists such as nicotine, bind alpha7 acetylcholine receptors (α7nAChRs) on murine splenic macrophages to inhibit the production of inflammatory factors such as TNF [74, 75]. Similar rodent studies have reported that sympathetic stimulation can also prevent lymphocyte apoptosis induced by surgical trauma by inhibiting the expression of pro-apoptotic proteins such as Fas [64, 65, 76]. Given that TNF induces apoptosis, future studies are needed to determine whether this is a direct effect of the splenic nerve on lymphocytes or a consequence of inhibiting TNF production in macrophages [10, 77, 78].
Other work indicates that splenic nerve activation, with either IFN-α or electrical splanchnic stimulation in mice, can also regulate other immune cells such as inhibiting natural killer cells[42, 79]. Together, these results suggest that sympathetic stimulation can induce local release of neurogenic norepinephrine, which may provide clinical advantages to inducing local control of inflammation, thus avoiding collateral effects in non-targeted tissues. Future studies are required to determine the specific neuronal networks and organ innervations activated by each acupoint of stimulation.
Catecholamines control almost all cellular functions depending on their receptors. Adrenergic (norepinephrine or epinephrine) or dopaminergic receptors (which are G protein-coupled seven-transmembrane domain receptors) are expressed in most mammalian cells (Fig. 3b). These receptors are classified according to their functions into three major groups: i) α1-adrenoceptors coupled to Gq proteins, which are G protein complexes that activate phospholipase C to cleave phosphatidylinositol 4,5-bisphosphate into diacylglycerol (DAG) and inositol trisphosphate (IP3, induces intracellular release of Ca2+ from the endoplasmic reticulum)[80, 81]. IP3 and DAG both act as second messengers that activate protein kinase C[82], which subsequently activates IκB kinase (IKK) to phosphorylate the inhibitory IκB protein leading to NF-κB activation[80, 81], an important signaling pathway inducing inflammatory cytokines such as TNF. ii) α2-adrenoceptors and D2-like dopaminergic (D2, D3, D4) receptors are coupled to Gi proteins, which inhibit adenylate cyclase and prevent the formation of cyclic adenosine monophosphate (cAMP). iii) Conversely, β-adrenoceptors and D1-like dopaminergic (D1, D5) receptors are coupled to Gs proteins, which activate adenylate cyclase and increase the production of cAMP, leading to the activation of protein kinase A and cAMP response element-binding protein (CREB)[83]. The anti-inflammatory effects of this pathway rely on the potential of protein kinase A and CREB to inhibit NF-κB by modulating common transcriptional co-factors, i.e. the CREB binding protein[84]. These receptors have different affinity for catecholamines; norepinephrine has a higher affinity for α1- and β3-adrenoceptors, whereas epinephrine has a higher affinity for β2- and α2-adrenoceptors[85]. Moreover, tissue-specific receptor expression allows epinephrine to induce different cellular responses such as constricting minute blood vessels (via α-receptors), yet dilating blood vessels in skeletal muscles and liver (via β-receptors)[86]. The study of these mechanisms have allowed the design of selective beta-adrenergic blockers for hypertension, arrhythmia, and to protect the heart from a second heart attack. Similar studies are still warranted to determine the precise molecular mechanisms induced by sympathetic stimulation in inflammatory disorders.
The parasympathetic system
The vagus nerve is the principal parasympathetic nerve connecting the CNS with the viscera in mammals (Fig. 4a). Vagal stimulation modulates multiple physiological functions from digestion to inflammation[87, 88]. Either auricular ST (Stomach), SI (Small intestine) or somatic (ST36) acupuncture in humans increases gastrointestinal motility via vagal stimulation[89]. Similarly, electroacupuncture on PC6 and ST36 have been reported to increase gastric motility in patients with gastroparesis via vagal stimulation [90]. Our laboratory group reported that electroacupuncture on ST36 reduces serum levels of inflammatory cytokines and prevents organ damage in mice with polymicrobial peritonitis induced by cecal ligation and puncture [26, 91]. These effects were mediated by the vagus nerve and therefore prevented by surgical vagotomy. Electroacupuncture on ST36 has also been found to prevent burn-induced inflammation and lung tissue injury via vagal stimulation [92]. Vagal stimulation is also achieved by stimulating other acupoints, Indeed, a report indicated that electroacupuncture on GV20 and GV14 could improve cerebral blood perfusion and reduce brain damage, apoptosis, oxidative stress and inflammation via vagal stimulation in mice with experimental ischemic stroke[93].
Figure 4. The parasympathetic nervous system.
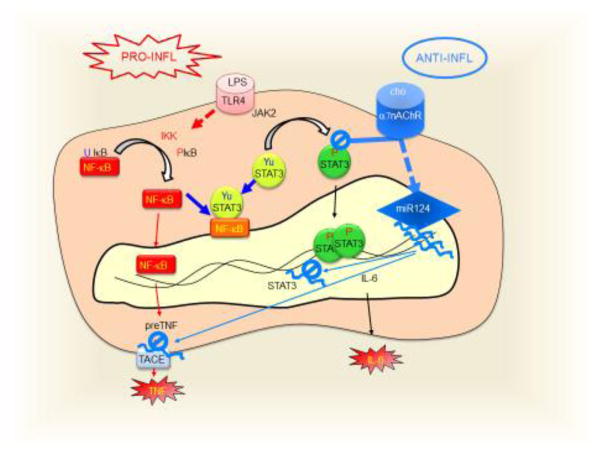
(a) In mammals, sensorial afferent signal (SAS) activates the NTS, which modulates the vagal dorsal motor nucleus (DMN). The vagus nerve (VN) is the principal parasympathetic nerve innervating most of the viscera including the heart, lung, liver, adrenal glands, and the gastrointestinal tract (GI). The vagus nerve inhibits the production of inflammatory cytokines in the spleen by activating the sympathetic adrenergic splenic nerve (SN) via the α7nAChRs at the celiac ganglion (CG). Norepinephrine from the splenic nerve activates T ‘modulatory’ lymphocytes to inhibit cytokine production in splenic macrophages. Furthermore, efferent vagal fibers induce the systemic release of catecholamines from the adrenal medulla (Am). (b) At the cellular level, choline (cho), a selective agonist of α7-nicotinic acetylcholine receptors (α7nAChRs), inhibits LPS-induced STAT3 tyrosine phosphorylation (P-STAT3) preventing STAT3 transcriptional activation of IL6. LPS also induces the phosphorylation of IκB leading to the release of NF-κB, which can interact with tyrosine unphosphorylated STAT3 (Yu-STAT3) to produce a mutual transregulation. α7nAChRs also induce the production of Micro noncoding RNA-124 (miR124) which inhibits the translation of the STAT3 and TACE (TNF-converting enzyme) mRNAs.
The vagus nerve is a cholinergic nerve producing acetylcholine, a neurotransmitter with a short half-life span inducing local effects, but too chemically unstable to produce systemic effects. Acetylcholine has been mostly studied as a neurotransmitter but its effects on immune cells are not well-known [2]. Acetylcholine and other cholinergic agonists such as nicotine inhibit TNF production in murine macrophages by inhibiting the NF-κB pathway via alpha-7 nicotinic cholinergic receptors (α7nAChRs)[2, 72–74, 94, 95] (Fig. 4b). Experimental, epidemiological, and clinical trials show that nicotine inhibits inflammation in multiple disorders including ulcerative colitis, arthritis, sepsis, schizophrenia, and Alzheimer’s disease[96–99]. However, nicotine has many detrimental side effects limiting its clinical use[100, 101]. Thus, current studies focus on specific α7nAChR-agonists to treat inflammation and prevent the side effects of nicotine [1]. LPS activates TLR4 receptors, which activate both the NF-κB and JAK2-STAT3 pathways to induce TNF and IL-6, respectively [102–105]. α7nAChR-agonists can inhibit LPS-induced activation of NF-κB and also STAT3 tyrosine phosphorylation by JAK2, without affecting its serine phosphorylation [102–105]. Thus, α7nAChR-agonists can induce tyrosine unphosphorylated STAT3 (Yu-STAT3), which could bind to NF-κB, leading to a reciprocal trans-repression similar to the one described for glucocorticoids[106]. Recent rodent studies also reported that α7nAChR-agonists could induce the expression of noncoding micro-RNA-124 (miR-124), which inhibits the translation of TNF-converting enzyme (TACE), a protease required for the processing of the membrane-anchored precursor pre-TNF[107, 108]. Collectively, these data suggest that similar to the design of alpha- and beta-adrenergic blockers, selective α7nAChR-agonists may provide pharmacological advantages for treating inflammation. Future tests should also aim to determine how well these findings can be translated to humans.
Neuro-immunomodulation and functional organization of the nervous system
Neuronal immunomodulation represents a collection of mechanisms selected through evolution to check the immune system and prevent deleterious inflammation[109, 110]. These systems can control various inflammatory factors, which may bring clinical advantages in treating complex pathologies, including infectious disorders such as sepsis. Data indicate that either auricular electrostimulation[111], acupuncture[112, 113] or electroacupuncture[114] on ST36 can attenuate serum concentrations of inflammatory cytokines TNF, IL-1β and IL-6, as well as improve kidney and lung injury in septic rodents. These mechanisms are not specific to sepsis, and electroacupuncture on ST36 can also prevent intestinal injury in ischemic and hemorrhagic rodents [115]. Mechanistically, these effects were reported to occur via the vagus nerve as they were prevented by surgical vagotomy [2, 116]. Our studies showed that electroacupuncture on ST36 decreased serum TNF, MCP1, IL-6, and INFγ, and improved mouse survival in polymicrobial peritonitis [26]. These effects were mediated by the vagus nerve as they were prevented by surgical vagotomy. However, these mechanisms appeared to be independent of neuronal modulation of the spleen because ST36 electrostimulation still inhibited systemic inflammation in splenectomized animals [2]. By contrast, electroacupuncture on ST36 activated the production of DOPA decarboxylase in adrenal glands leading to systemic release of dopamine, shown to inhibit the production of inflammatory factors in macrophages [26]. These results were recently confirmed by other investigators showing that acupuncture on ST36, though not electrostimulation, also inhibited serum TNF levels in endotoxemic mice [117]. Similar studies on other acupoints appear to suggest that different neuronal networks can contribute to control inflammation in experimental sepsis. Thus, electroacupuncture on LI4 also reduces serum TNF, IL-6 and IL-1β in septic mice without inducing glucocorticoids[116]. These effects were attributed to both the sympathetic and parasympathetic systems, because the effects were prevented by parasympathetic nicotinic antagonists as well as by chemical sympathectomy and administration of sympathetic beta-blockers such as propranolol [116]. Although specific mechanistic studies are required to delineate these neuronal networks, these studies are valuable by suggesting the clinical potential of different neuronal networks and different levels of functional organization of the nervous system to control inflammation.
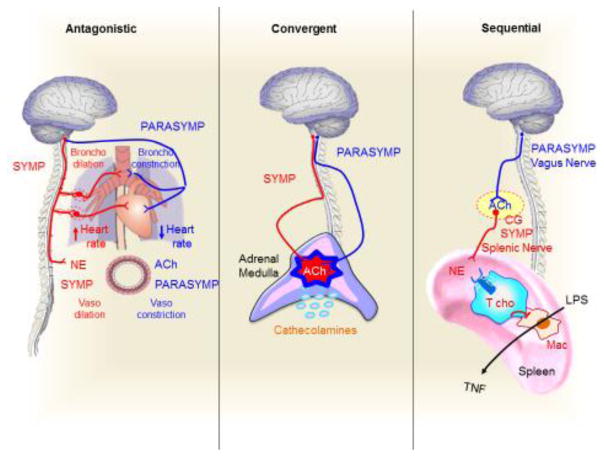
Taken together, emerging studies on neuronal stimulation are suggesting three models of functional organization of the nervous system to induce physiological homeostasis[118]. Classically, the sympathetic and parasympathetic systems are described as ‘antagonistic’ mechanisms opposing one another to balance physiological homeostasis (Fig. 5). A characteristic example is the baroreceptor reflex system (Box 2). Arterial baroreceptors are stretch receptors stimulated by distension of the arterial wall to control blood pressure. If blood pressure falls, baroreceptor firing rate decreases, and the CNS activates the sympathetic system to produce norepinephrine and increase heart rate and blood pressure. Conversely, when blood pressure rises, the baroreceptor reflex activates the parasympathetic nervous system to release acetylcholine and decrease heart rate and blood pressure. The sympathetic and parasympathetic nervous systems produce antagonistic signals with norepinephrine and acetylcholine to balance both heart rate and blood pressure. Although acetylcholine triggers vasodilation, there is no evidence for a parasympathetic innervation of blood vessels. As previously mentioned, recent work from our laboratory suggested a second level of organization where the sympathetic and parasympathetic nervous systems might ‘converge’ and induce an additive production of acetylcholine in the adrenal medulla[26] (Fig. 5). In this scenario, electroacupuncture on ST36 has been shown to inhibit systemic inflammation in experimental murine sepsis by inducing the production of catecholamines from the adrenal medulla[26]. In contrast to the lack of vagal innervations in many organs, there is strong evidence showing both vagal and sympathetic preganglionic innervations in adrenal glands. For instance, early work traced nerve fibers from the posterior vagus trunk in the subdiaphragmatic region of the left adrenal gland in mice. In addition, retrograde tracers injected at the center of the adrenal medulla have demonstrated staining of the ipsi- and contralateral vagal sensory (nodose) ganglia, as well as the ipsi- and contralateral dorsal motor nucleus of the vagus nerve, with the labeling extending from the cranial to the caudal limits of the nucleus[119]. These results suggest that there are direct afferent (sensory) and efferent (motor) vagal innervations in the adrenal glands. We posit that both parasympathetic vagal innervations and cholinergic preganglionic sympathetic neurons converge in the production of acetylcholine in the adrenal medulla[120]. Moreover, ST36 stimulation has been shown to induce catecholamines and inhibit serum TNF in α7nAChR-knockout mice suggesting that acetylcholine-induced production of catecholamines might be independent of α7nAChRs [26]. These results concur with recent studies indicating that α6β4nAChRs modulates exocytosis, and therefore, these receptors may regulate catecholamine release in human chromaffin cells[121]. Lastly, recent findings from different laboratories suggest a third level of functional organization through a ‘sequential’ connection between the parasympathetic and sympathetic systems to inhibit splenic TNF production in septic mice. In this case, the parasympathetic and sympathetic systems are connected as a sequential part of the same mechanism. The parasympathetic vagus nerve (which does not innervate the spleen) activates the sympathetic splenic nerve to release norepinephrine, which inhibits splenic TNF production by activating T cholinergic lymphocytes [72, 73, 95, 122]. The vagus nerve can activate the splenic nerve by inducing acetylcholine in the mesenteric ganglia [95]. Collectively, such studies on neuromodulation are beginning to suggest new models of functional organization of the nervous system to control inflammation and immunity. The models of functional organization of the nervous system might help design novel candidate therapeutic strategies to co-stimulate different neuronal networks and achieve the most effective control of inflammation.
Figure 5. A model of Functional organization of the mammalian autonomic nervous system.
Classically, sympathetic (Symp) and parasympathetic (Parasym) systems are described as “antagonistic” systems with opposite signals to maintain the physiological homeostasis. A characteristic example is the baroreceptor reflex, where the sympathetic and parasympathetic systems secrete norepinephrine (NE) or acetylcholine (ACh) to increase and decrease heart rate and blood pressure, respectively. Although acetylcholine produces vasodilation, there is no evidence for parasympathetic innervation of blood vessels. Recent studies on electroacupuncture reveal a second model of “convergent” functional organization of the nervous system. In this model, both the preganglionic sympathetic and parasympathetic vagus nerves converge in the release of acetylcholine (ACh) in the adrenal medulla to activate the chromaffin cells to secrete catecholamines. A third model of organization is the ‘sequential’ connection between the parasympathetic vagus nerve (VN) that activates the sympathetic splenic nerve (SN). The parasympathetic vagus nerve (VN) inhibits TNF production in splenic macrophages (Mac) by activating the sympathetic splenic nerve (SN) at the celiac ganglion (CG). The splenic nerve releases norepinephrine (NE) that activates T cholinergic (Tcho) lymphocytes via β2-adrenoceptors (β2AR)[72, 73].
Concluding Remarks
Neuronal stimulation is a promising emerging field in modern medicine to control organ function and reestablish physiological homeostasis during illness. Multiple recent studies show the potential of nerve stimulation to control inflammation and improve organ function in multiple experimental disorders; from arthritis and colitis, to diabetes and sepsis. Conversely, multiple clinical studies show that neuromodulation with acupuncture or electroacupuncture can control pain and inflammation in multiple disorders such as postoperative recovery, osteoarthritis, migraine, stroke, post-traumatic stress disorder and drug addiction. Electroacupuncture is now endorsed by the American Pain Society, the National Center for Complementary and Alternative Medicine, the National Institutes of Health and the World Health Organization, and used by millions of people to control pain and inflammation. However, as discussed, the efficacy of these techniques remains controversial. For instance, it is not clear why electroacupuncture on ST36 can control inflammation and improve organ function in experimental sepsis, but not in septic patients with adrenal insufficiency. These studies have allowed the design of selective dopaminergic agonists for treating inflammation and renal function in experimental sepsis[9]. Investigating the molecular mechanisms of neuromodulation may allow the design of novel pharmacological treatments. In line with this, the study of the hypothalamic-pituitary-adrenal has allowed the design of glucocorticoids for treating inflammation, while the study of sympathetic regulation in the baroreflex system has allowed the design of selective beta-adrenergic blockers for treating hypertension and arrhythmia. Accordingly, studies on vagal neuromodulation seem to support the design of specific α7nAChR-agonists to control inflammation in infectious disorders such as sepsis. Mechanistic studies on acupuncture and electroacupuncture are also providing critical information on the functional organization of the nervous system to control the immune system and inflammation. These models of functional organization may help the design of novel therapeutic strategies where co-stimulating different neuronal networks are intended to achieve the most effective control of inflammation. As such, mechanistic studies of transdermal stimulation of sensory afferent nerves are important in the development of new non-invasive techniques for nerve stimulation. Consequently, although many unanswered questions evidently remain (see outstanding questions and Box 3), nerve stimulation is emerging as a promising medical area that may provide clinical advantages in the putative treatment of inflammatory disorders such as arthritis or sepsis, aiming to prevent systemic immunosuppression and susceptibility to secondary infections, respectively.
Outstanding Questions.
Why have clinical trials provided controversial results regarding the efficacy of acupuncture and electroacupuncture?
There are three major factors tainting the results of these clinical trials. The placebo effect, the heterogeneous practices of nerve stimulation and the lack of mechanistic explanations. In contrast to clinical trials that are tainted by the placebo effect and the heterogeneity of patients, experimental studies with well-characterized models of diseases in homogenous groups of animals are needed to define the therapeutic efficacy of these mechanisms. These mechanisms are essential to understand why acupuncture or electroacupuncture may provide clinical advantages in specific diseases and cohorts of patients but not in others with similar diagnoses. Still, neuromodulation with acupuncture or electroacupuncture is endorsed by the WHO and the NIH and it is used by millions of people to control pain, inflammation and to reestablish physiological homeostasis during illness.
Can neuromodulation provide any clinical advantages for different ailments?
Recent studies indicate that neuromodulation with nerve stimulation may provide significant clinical advantages to control pain, inflammation and organ function without inducing side effects. For instance, nerve stimulation can activate specific networks to induce local control of inflammation in inflammatory disorders such as rheumatoid arthritis without inducing general immunosuppression and affecting other regions and organs. In order to reach this point, many additional questions, robust mechanistic insight, and further validation in humans remains to be addressed.
Box 3. Clinician’s Corner.
Vagal stimulation was approved by the FDA for treating refractory epilepsy and depression[129]. Right cervical VNS is effective for treating heart failure in preclinical studies and a phase II clinical trial[130]. Small reports described the use of VNS for bipolar disorder, treatment-resistant anxiety disorders, Alzheimer’s disease, chronic refractory headaches, and obesity, although none of these uses received FDA approval [131]. Vagal stimulating with an implantable electrical generator improved rheumatoid arthritis[132]. Nerve stimulation can provide clinical advantages as compared with conventional interventions, such as small-molecule medications and monoclonal antibodies. Biological anti-rheumatic drug therapies are expensive and increase the risk of systemic immunosuppression, infections, and malignancies. On the other hand, nerve stimulation may provide therapeutic advantages to induce “local” regulation of inflammation and to avoid the “systemic” side-effects of pharmacological anti-inflammatory therapies. Non-invasive transdermal nerve stimulation prevents organ damage in inflammatory and infectious disorders. Acupuncture or electroacupuncture is used worldwide by millions of people in multiple disorders. Current studies focus on how these techniques activate neuronal networks and their mechanisms of neuromodulation. Proper mechanistic studies are critical to determine why acupuncture and electroacupuncture are effective in some patients and diseases but not in other with similar symptoms. For instance, ST36 electroacupuncture can control inflammation in experimental sepsis, but not in septic patients with adrenal insufficiency[26].
Supplementary Material
Trends.
Neuronal stimulation for physiological control is a recent field with major clinical implications for inflammation, infectious diseases, colitis, diabetes, obesity, hemorrhage, pancreatitis, quadriplegia, resuscitation, endotoxemia, septic shock, and sepsis.
Transdermal nerve stimulation with acupuncture or electroacupuncture is currently endorsed by the WHO, the NIH, and is used by millions of people to control pain, inflammation and organ function.
Transdermal nerve stimulation with acupuncture or electroacupuncture can activate neuronal networks via local immune factors and neuronal opioid receptors.
Neuronal sympathetic stimulation can induce local release of neurogenic norepinephrine, which may provide clinical advantages to inducing local control of inflammation avoiding collateral effects in non-targeted tissues.
Neuromodulation studies of the immune system are suggesting new models of functional organization of the nervous system to control inflammation and may have important clinical implications in specific cohorts of patients.
Acknowledgments
The authors thank Drs. P. Morcillo and B. Joseph for their suggestions. LU is supported by the NIH R01-GM084125 and the NSFC #81774429.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4(8):673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 2.Huston JM, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203(7):1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue T, et al. Vagus nerve stimulation mediates protection from kidney ischemia-reperfusion injury through alpha7nAChR+ splenocytes. J Clin Invest. 2016;126(5):1939–52. doi: 10.1172/JCI83658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161(1):133–45. doi: 10.1016/j.cell.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouton CE, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533(7602):247–50. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–21. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 7.Trakhtenberg EF, Goldberg JL. Immunology. Neuroimmune communication. Science. 2011;334(6052):47–8. doi: 10.1126/science.1213099. [DOI] [PubMed] [Google Scholar]
- 8.Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017;20(2):156–166. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- 9.Torres-Rosas R, et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med. 2014;20:291–5. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grech D, et al. Intraoperative Low-frequency Electroacupuncture under General Anesthesia Improves Postoperative Recovery in a Randomized Trial. J Acu Mer Stud. 2016;9(5):234–241. doi: 10.1016/j.jams.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staff, W.H.O. Acupuncture: Review and analysis of reports on controlled clinical trials. World Health Organization; 2002. [Google Scholar]
- 12.Zhang Y, et al. Acupuncture Use among American Adults: What Acupuncture Practitioners Can Learn from National Health Interview Survey 2007? Evidence-based Complementary and Alternative Medicine: eCAM. 2012;2012:710750. doi: 10.1155/2012/710750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke TC, et al. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015;(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 14.Chou R, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–91. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 15.Kong J, et al. Are all placebo effects equal? Placebo pills, sham acupuncture, cue conditioning and their association. PLoS One. 2013;8(7):e67485. doi: 10.1371/journal.pone.0067485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskinazi DP, Jobst KA. National Institutes of Health Office of Alternative Medicine-Food and Drug Administration Workshop on Acupuncture. J Altern Complement Med. 1996;2(1):3–6. doi: 10.1089/acm.1996.2.3. [DOI] [PubMed] [Google Scholar]
- 17.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85(4):355–75. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhou F, Huang D, Ying X. Neuroanatomic Basis of Acupuncture Points. In: Xia Y, et al., editors. Acupuncture therapy for neurological diseases: a neurobiological view. Springer; Berlin Heidelberg: 2010. pp. 32–80. [Google Scholar]
- 19.Napadow V, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861–9. doi: 10.1089/acm.2008.SAR-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagitani F, Uchida S, Hotta H. Afferent nerve fibers and acupuncture. Auton Neurosci. 2010;157(1–2):2–8. doi: 10.1016/j.autneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Colquhoun D, Novella SP. Acupuncture is theatrical placebo. Anesth Analg. 2013;116(6):1360–3. doi: 10.1213/ANE.0b013e31828f2d5e. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Shaanan TL, et al. Activation of the reward system boosts innate and adaptive immunity. Nat Med. 2016;22(8):940–4. doi: 10.1038/nm.4133. [DOI] [PubMed] [Google Scholar]
- 23.Taub A. Thumbs down on acupuncture. Science. 1998;279(5348):159. doi: 10.1126/science.279.5348.155f. [DOI] [PubMed] [Google Scholar]
- 24.Meldrum DR, et al. Acupuncture--help, harm, or placebo? Fertil Steril. 2013;99(7):1821–4. doi: 10.1016/j.fertnstert.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 25.Huang C, et al. Characteristics of electroacupuncture-induced analgesia in mice: variation with strain, frequency, intensity and opioid involvement. Brain Res. 2002;945(1):20–5. doi: 10.1016/s0006-8993(02)02503-9. [DOI] [PubMed] [Google Scholar]
- 26.Torres-Rosas R, et al. Dopamine Mediates the Vagal Modulation of the Immune System by Electroacupuncture. Nat Med. 2014;20(3):291–5. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu IM, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501(7465):52–7. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sa ZY, et al. Relationship between regional mast cell activity and peripheral nerve discharges during manual acupuncture stimulation of “Zusanli” (ST 36) Zhen Ci Yan Jiu. 2013;38(2):118–22. [PubMed] [Google Scholar]
- 29.Langevin HM, et al. Connective tissue fibroblast response to acupuncture: dose-dependent effect of bidirectional needle rotation. J Altern Complement Med. 2007;13(3):355–60. doi: 10.1089/acm.2007.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon S, et al. Coarse needle surface potentiates analgesic effect elicited by acupuncture with twirling manipulation in rats with nociceptive pain. BMC Complement Altern Med. 2017;17(1):1. doi: 10.1186/s12906-016-1505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langevin HM, et al. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: evidence for a mechanotransduction-based mechanism. J Cell Physiol. 2006;207(3):767–74. doi: 10.1002/jcp.20623. [DOI] [PubMed] [Google Scholar]
- 32.Kwon CS, et al. Mononuclear cell metallothionein mRNA levels in human subjects with poor zinc nutrition. Br J Nutr. 2007;97(2):247–54. doi: 10.1017/S0007114507328614. [DOI] [PubMed] [Google Scholar]
- 33.Hsu HJ, et al. Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One. 2010;5(8):e12470. doi: 10.1371/journal.pone.0012470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kito H, et al. Role of mitogen-activated protein kinases in pulmonary endothelial cells exposed to cyclic strain. J Appl Physiol (1985) 2000;89(6):2391–400. doi: 10.1152/jappl.2000.89.6.2391. [DOI] [PubMed] [Google Scholar]
- 35.Taguchi R, Taguchi T, Kitakoji H. Involvement of peripheral opioid receptors in electroacupuncture analgesia for carrageenan-induced hyperalgesia. Brain Res. 2010;1355:97–103. doi: 10.1016/j.brainres.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Czlonkowski A, Stein C, Herz A. Peripheral mechanisms of opioid antinociception in inflammation: involvement of cytokines. Eur J Pharmacol. 1993;242(3):229–35. doi: 10.1016/0014-2999(93)90246-e. [DOI] [PubMed] [Google Scholar]
- 37.Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2–3):119–25. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 38.Mousa SA, et al. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J Neuroimmunol. 2002;126(1–2):5–15. doi: 10.1016/s0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 39.Parkhill AL, Bidlack JM. Reduction of lipopolysaccharide-induced interleukin-6 production by the kappa opioid U50,488 in a mouse monocyte-like cell line. Int Immunopharmacol. 2006;6(6):1013–9. doi: 10.1016/j.intimp.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Gabrilovac J, et al. IFN-gamma up-regulates kappa opioid receptors (KOR) on murine macrophage cell line J774. J Neuroimmunol. 2012;245(1–2):56–65. doi: 10.1016/j.jneuroim.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Labuz D, et al. Peripheral antinociceptive effects of exogenous and immune cell-derived endomorphins in prolonged inflammatory pain. J Neurosci. 2006;26(16):4350–8. doi: 10.1523/JNEUROSCI.4349-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katafuchi T, Take S, Hori T. Roles of sympathetic nervous system in the suppression of cytotoxicity of splenic natural killer cells in the rat. J Physiol. 1993;465:343–57. doi: 10.1113/jphysiol.1993.sp019680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman N, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13(7):883–8. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets--what are the challenges? Nat Rev Drug Discov. 2013;12(4):265–86. doi: 10.1038/nrd3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yen LT, et al. Targeting ASIC3 for Relieving Mice Fibromyalgia Pain: Roles of Electroacupuncture, Opioid, and Adenosine. Sci Rep. 2017;7:46663. doi: 10.1038/srep46663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu HJ, Lee CF, Kaunas R. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS One. 2009;4(3):e4853. doi: 10.1371/journal.pone.0004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang R, et al. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120(2):482–503. doi: 10.1097/ALN.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B, et al. Effects of pertussis toxin on electroacupuncture-produced anti-hyperalgesia in inflamed rats. Brain Res. 2005;1044(1):87–92. doi: 10.1016/j.brainres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Zhang RX, et al. Involvement of opioid receptors in electroacupuncture-produced anti- hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020(1–2):12–7. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 50.Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9(8):1003–8. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- 51.Zhang RX, et al. Electroacupuncture attenuates inflammation in a rat model. J Altern Complement Med. 2005;11(1):135–42. doi: 10.1089/acm.2005.11.135. [DOI] [PubMed] [Google Scholar]
- 52.Li A, et al. Corticosterone mediates electroacupuncture-produced anti-edema in a rat model of inflammation. BMC Complement Altern Med. 2007;7:27. doi: 10.1186/1472-6882-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li A, et al. Electroacupuncture activates corticotrophin-releasing hormone-containing neurons in the paraventricular nucleus of the hypothalammus to alleviate edema in a rat model of inflammation. BMC Complement Altern Med. 2008;8:20. doi: 10.1186/1472-6882-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Hashimi M, et al. Opioids and immune modulation: more questions than answers. Br J Anaesth. 2013;111(1):80–8. doi: 10.1093/bja/aet153. [DOI] [PubMed] [Google Scholar]
- 55.Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 57.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munck A, Naray-Fejes-Toth A. Glucocorticoids and stress: permissive and suppressive actions. Ann N Y Acad Sci. 1994;746:115–30. doi: 10.1111/j.1749-6632.1994.tb39221.x. discussion 131–3. [DOI] [PubMed] [Google Scholar]
- 59.Szelenyi J, et al. Contribution of differently localized alpha 2- and beta-adrenoceptors in the modulation of TNF-alpha and IL-10 production in endotoxemic mice. Ann N Y Acad Sci. 2000;917:145–53. doi: 10.1111/j.1749-6632.2000.tb05378.x. [DOI] [PubMed] [Google Scholar]
- 60.Szelenyi J, Kiss JP, Vizi ES. Differential involvement of sympathetic nervous system and immune system in the modulation of TNF-alpha production by alpha2- and beta-adrenoceptors in mice. J Neuroimmunol. 2000;103(1):34–40. doi: 10.1016/s0165-5728(99)00234-9. [DOI] [PubMed] [Google Scholar]
- 61.Kim HW, et al. Low-frequency electroacupuncture suppresses carrageenan-induced paw inflammation in mice via sympathetic post-ganglionic neurons, while high-frequency EA suppression is mediated by the sympathoadrenal medullary axis. Brain Res Bull. 2008;75(5):698–705. doi: 10.1016/j.brainresbull.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 62.Kim HW, et al. Low-frequency electroacupuncture suppresses zymosan-induced peripheral inflammation via activation of sympathetic post-ganglionic neurons. Brain Res. 2007;1148:69–75. doi: 10.1016/j.brainres.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 63.Kim HW, et al. The anti-inflammatory effects of low- and high-frequency electroacupuncture are mediated by peripheral opioids in a mouse air pouch inflammation model. J Altern Complement Med. 2006;12(1):39–44. doi: 10.1089/acm.2006.12.39. [DOI] [PubMed] [Google Scholar]
- 64.Lenardo M, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, et al. Electroacupuncture suppresses surgical trauma stress-induced lymphocyte apoptosis in rats. Neurosci Lett. 2005;383(1–2):68–72. doi: 10.1016/j.neulet.2005.03.068. [DOI] [PubMed] [Google Scholar]
- 66.Santos-Almeida FM, et al. Carotid sinus nerve electrical stimulation in conscious rats attenuates systemic inflammation via chemoreceptor activation. Sci Rep. 2017;7(1):6265. doi: 10.1038/s41598-017-06703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bassi GS, et al. Baroreflex activation in conscious rats modulates the joint inflammatory response via sympathetic function. Brain Behav Immun. 2015;49:140–7. doi: 10.1016/j.bbi.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bassi GS, et al. Modulation of experimental arthritis by vagal sensory and central brain stimulation. Brain Behav Immun. 2017;64:330–343. doi: 10.1016/j.bbi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabanyi I, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell. 2016;164(3):378–91. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muller PA, et al. Crosstalk between Muscularis Macrophages and Enteric Neurons Regulates Gastrointestinal Motility. Cell. 2014;158(2):300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pirzgalska RM, et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med. 2017 doi: 10.1038/nm.4422. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peña G, et al. Cholinergic regulatory lymphocytes re-establish neuromodulation of innate immune responses in sepsis. J Immunol. 2011;187(2):718–25. doi: 10.4049/jimmunol.1100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vida G, et al. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011;25(12):4476–85. doi: 10.1096/fj.11-191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosas-Ballina M, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, et al. Sympathetic nervous system mediates surgical trauma stress-induced splenocyte apoptosis in rats. Eur J Pharmacol. 2007;565(1–3):76–82. doi: 10.1016/j.ejphar.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 77.Vida G, et al. alpha 7-Cholinergic Receptor Mediates Vagal Induction of Splenic Norepinephrine. Journal of Immunology. 2011;186(7):4340–4346. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, et al. Electroacupuncture downregulates TLR2/4 and pro-inflammatory cytokine expression after surgical trauma stress without adrenal glands involvement. Brain Res Bull. 2009;80(1–2):89–94. doi: 10.1016/j.brainresbull.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Kimura A, Nagai N, Sato A. Somatic afferent regulation of cytotoxic activity of splenic natural killer cells in anesthetized rats. Jpn J Physiol. 1994;44(6):651–64. doi: 10.2170/jjphysiol.44.651. [DOI] [PubMed] [Google Scholar]
- 80.Lin X, et al. Protein kinase C-theta participates in NF-kappaB activation induced by CD3- CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol. 2000;20(8):2933–40. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Angus DC. Caring for the critically ill patient: challenges and opportunities. JAMA. 2007;298(4):456–8. doi: 10.1001/jama.298.4.456. [DOI] [PubMed] [Google Scholar]
- 82.Mizuno N, Itoh H. Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals. 2009;17(1):42–54. doi: 10.1159/000186689. [DOI] [PubMed] [Google Scholar]
- 83.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine Metabolism: A Contemporary View with Implications for Physiology and Medicine. Pharmacological Reviews. 2004;56(3):331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 84.Parry GC, Mackman N. Role of cyclic AMP response element-binding protein in cyclic AMP inhibition of NF-kappaB-mediated transcription. J Immunol. 1997;159(11):5450–6. [PubMed] [Google Scholar]
- 85.Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- 86.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–48. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 87.Sato A, Sato Y, Suzuki A. Mechanism of the reflex inhibition of micturition contractions of the urinary bladder elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1992;15(3):189–98. doi: 10.1016/0168-0102(92)90004-v. [DOI] [PubMed] [Google Scholar]
- 88.Sato A, et al. Neural mechanisms of the reflex inhibition and excitation of gastric motility elicited by acupuncture-like stimulation in anesthetized rats. Neurosci Res. 1993;18(1):53–62. doi: 10.1016/0168-0102(93)90105-y. [DOI] [PubMed] [Google Scholar]
- 89.Li H, Wang YP. Effect of auricular acupuncture on gastrointestinal motility and its relationship with vagal activity. Acupunct Med. 2013;31(1):57–64. doi: 10.1136/acupmed-2012-010173. [DOI] [PubMed] [Google Scholar]
- 90.Ouyang H, et al. Electroacupuncture accelerates gastric emptying in association with changes in vagal activity. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2002;282(2):G390–G396. doi: 10.1152/ajpgi.00272.2001. [DOI] [PubMed] [Google Scholar]
- 91.Zerari-Mailly F, et al. Trigemino-solitarii-facial pathway in rats. J Comp Neurol. 2005;487(2):176–89. doi: 10.1002/cne.20554. [DOI] [PubMed] [Google Scholar]
- 92.Song XM, et al. The effect of electroacupuncture at ST36 on severe thermal injury-induced remote acute lung injury in rats. Burns. 2015;41(7):1449–58. doi: 10.1016/j.burns.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Chi L, et al. Electroacupuncture brain protection during ischemic stroke: A role for the parasympathetic nervous system. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17697988. p. 271678x17697988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ulloa L. The cholinergic anti-inflammatory pathway meets microRNA. Cell Research. 2013;23(11):1249–1250. doi: 10.1038/cr.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vida G, et al. Alpha7-nicotinic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186(7):4340–6. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pullan RD, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330(12):811–5. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 97.Thomas GA, et al. Transdermal nicotine as maintenance therapy for ulcerative colitis. N Engl J Med. 1995;332(15):988–92. doi: 10.1056/NEJM199504133321503. [DOI] [PubMed] [Google Scholar]
- 98.Papadopoulos NG, et al. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clin Exp Rheumatol. 2005;23(6):861–6. [PubMed] [Google Scholar]
- 99.Corsi-Zuelli F, et al. Neuroimmune Interactions in Schizophrenia: Focus on Vagus Nerve Stimulation and Activation of the Alpha-7 Nicotinic Acetylcholine Receptor. Front Immunol. 2017;8:618. doi: 10.3389/fimmu.2017.00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. J Pharmacol. 2007;151(7):915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matthay MA, Ware LB. Can nicotine treat sepsis? Nat Med. 2004;10(11):1161–2. doi: 10.1038/nm1104-1161. [DOI] [PubMed] [Google Scholar]
- 102.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151(7):915–29. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Jonge WJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6(8):844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 104.Peña G, et al. JAK2 inhibition prevents innate immune responses and rescues animals from sepsis. J Mol Med. 2010;88(8):851–9. doi: 10.1007/s00109-010-0628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pena G, et al. Unphosphorylated STAT3 modulates alpha 7 nicotinic receptor signaling and cytokine production in sepsis. Eur J Immunol. 2010;40(9):2580–9. doi: 10.1002/eji.201040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367(Pt 1):97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun Y, et al. MicroRNA-124 mediates the cholinergic anti-inflammatory action through inhibiting the production of pro-inflammatory cytokines. Cell Res. 2013;23(11):1270–83. doi: 10.1038/cr.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ulloa L. The cholinergic anti-inflammatory pathway meets microRNA. Cell Res. 2013;23(11):1249–50. doi: 10.1038/cr.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5(6):575–81. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 110.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6(4):318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao YX, et al. Effect of electroacupuncture of auricular concha on inflammatory reaction in endotoxaemia rats. Zhen Ci Yan Jiu. 2011;36(3):187–92. [PubMed] [Google Scholar]
- 112.Huang CL, et al. Acupuncture stimulation of ST36 (Zusanli) attenuates acute renal but not hepatic injury in lipopolysaccharide-stimulated rats. Anesth Analg. 2007;104(3):646–54. doi: 10.1213/01.ane.0000255288.68199.eb. [DOI] [PubMed] [Google Scholar]
- 113.Huang CL, et al. Acupuncture stimulation of ST-36 (Zusanli) significantly mitigates acute lung injury in lipopolysaccharide-stimulated rats. Acta Anaesthesiol Scand. 2006;50(6):722–30. doi: 10.1111/j.1399-6576.2006.01029.x. [DOI] [PubMed] [Google Scholar]
- 114.Gu G, et al. Effects of electroacupuncture pretreatment on inflammatory response and acute kidney injury in endotoxaemic rats. J Int Med Res. 2011;39(5):1783–97. doi: 10.1177/147323001103900521. [DOI] [PubMed] [Google Scholar]
- 115.Du MH, et al. Electroacupuncture improves gut barrier dysfunction in prolonged hemorrhagic shock rats through vagus anti-inflammatory mechanism. World J Gastroenterol. 2013;19(36):5988–99. doi: 10.3748/wjg.v19.i36.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song JG, et al. Electroacupuncture improves survival in rats with lethal endotoxemia via the autonomic nervous system. Anesthesiology. 2012;116(2):406–14. doi: 10.1097/ALN.0b013e3182426ebd. [DOI] [PubMed] [Google Scholar]
- 117.Lim HD, et al. Anti-Inflammatory Effects of Acupuncture Stimulation via the Vagus Nerve. PLoS One. 2016;11(3):e0151882. doi: 10.1371/journal.pone.0151882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Montgomery KL, et al. Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system. Sci Transl Med. 2016;8(337):337rv5. doi: 10.1126/scitranslmed.aad7577. [DOI] [PubMed] [Google Scholar]
- 119.Coupland RE, et al. The innervation of the adrenal gland. III. Vagal innervation. J Anat. 1989;163:173–81. [PMC free article] [PubMed] [Google Scholar]
- 120.Mravec B. Possible involvement of the vagus nerve in monitoring plasma catecholamine levels. Neurobiol Learn Mem. 2006;86(3):353–5. doi: 10.1016/j.nlm.2006.06.001. author reply 356–7. [DOI] [PubMed] [Google Scholar]
- 121.Perez-Alvarez A, et al. Native alpha6beta4* nicotinic receptors control exocytosis in human chromaffin cells of the adrenal gland. Faseb J. 2012;26(1):346–54. doi: 10.1096/fj.11-190223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Martelli D, McKinley MJ, McAllen RM. The cholinergic anti-inflammatory pathway: a critical review. Auton Neurosci. 2014;182:65–9. doi: 10.1016/j.autneu.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 123.Pleger B, Villringer A. The human somatosensory system: from perception to decision making. Prog Neurobiol. 2013;103:76–97. doi: 10.1016/j.pneurobio.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 124.Bianchi-da-Silva LM, Menescal-de-Oliveira L, Hoffmann A. Baroreceptor control of heart rate in the awake toad: peripheral autonomic effectors and arterial baroreceptor areas. J Auton Nerv Syst. 2000;80(1–2):31–9. doi: 10.1016/s0165-1838(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 125.Caous CA, de Sousa Buck H, Lindsey CJ. Neuronal connections of the paratrigeminal nucleus: a topographic analysis of neurons projecting to bulbar, pontine and thalamic nuclei related to cardiovascular, respiratory and sensory functions. Auton Neurosci. 2001;94(1–2):14–24. doi: 10.1016/s1566-0702(01)00338-1. [DOI] [PubMed] [Google Scholar]
- 126.Yu YG, et al. Cardiovascular responses to sciatic nerve stimulation are blocked by paratrigeminal nucleus lesion. Auton Neurosci. 2002;98(1–2):70–4. doi: 10.1016/s1566-0702(02)00035-8. [DOI] [PubMed] [Google Scholar]
- 127.Pavlov VA, et al. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med. 2003;9(5–8):125–34. [PMC free article] [PubMed] [Google Scholar]
- 128.Affleck VS, Coote JH, Pyner S. The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience. 2012;219:48–61. doi: 10.1016/j.neuroscience.2012.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Howland RH. Vagus Nerve Stimulation. Current behavioral neuroscience reports. 2014;1(2):64–73. doi: 10.1007/s40473-014-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Ferrari GM, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32(7):847–55. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 131.Howland RH, et al. The emerging use of technology for the treatment of depression and other neuropsychiatric disorders. Ann Clin Psychiatry. 2011;23(1):48–62. [PubMed] [Google Scholar]
- 132.Koopman FA, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113(29):8284–9. doi: 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.