Abstract
Background
The aims of this study were to examine the expression of miRNA-21 in the serum of elderly patients (>65 years) with acute myocardial infarction (AMI) and to investigate the potential role of serum miRNA-21 as a marker of early cardiac myocyte damage.
Material/Methods
Thirty-eight elderly patients with recent AMI, 27 elderly patients with unstable angina pectoris, and 25 healthy elderly individuals were included in the study. Serum miRNA-21 expression was determined following total RNA extraction and reverse-transcribed into cDNA, followed by reverse transcription-polymerase chain reaction (RT-PCR). Serum creatine kinase MB isoenzyme (CK-MB) and cardiac troponin I (cTnI) levels were analyzed by electrochemiluminescence. Apoptosis of human cardiac myocytes (HCM) was analyzed using fluorescence-activated cell sorting (FACS), and protein expression of caspase-3 was detected using Western blot.
Results
Expression levels of miRNA-21 in the serum of elderly patients with AMI were positively correlated with serum levels of CK-MB (r=0.3683, P=0.0229) and cTnI (r=0.5128, P=0.009). Following tumor necrosis factor (TNF)-α induction, the apoptosis rates of HCM transfected with the miRNA-21 mimic short hairpin RNA (shRNA) were downregulated by 39.1% compared with control HCM cells, and protein expression of c-Jun N-terminal kinases (JNK) and p38 were unchanged (P>0.05); protein expression of p-JNK, p-p38 and caspase-3 were downregulated by 37.1%, 35.8%, and 36.0%, respectively.
Conclusions
Expression of miRNA-21 was upregulated in the serum of elderly patients with AMI, which inhibited TNF-α induced apoptosis in HCM by activating the JNK/p38/caspase-3 signaling pathway.
MeSH Keywords: Apoptosis, Frail Elderly, MicroRNAs
Background
Acute myocardial infarction (AMI) results in myocardial cell necrosis and is usually caused by occlusion of the coronary arteries, resulting in chronic morbidity or death. In developed countries, including Europe and the United States, the annual incidence of new patients with AMI is estimated to be 1,500,000 [1]. Recently, with the continued development of China’s economy and the acceleration of urbanization and the increasingly aging population, the incidence of chronic disease, including ischemic heart disease, is increasing annually [1].
According to published data in the 2015 Report of Cardiovascular Diseases in China, released by National Cardiovascular Disease Center [2], by the end of 2015, the number of myocardial infarctions in China exceeded 2,500,000 while the morbidity and mortality rates of AMI showed an annually increasing trend. At present, the clinical diagnosis of AMI is still dependent on the clinical symptoms, changes in the electrocardiogram (ECG), and the increased levels of serum markers for myocardial necrosis. Recent studies of detection of AMI in recent years, a large number of sensitive and specific biomarkers for early diagnosis and prognosis of AMI have been found, among them is serum detection of miRNA-21 [3].
Studies have shown that miRNA-21 is a widely expressed miRNA regulated by RNA polymerase II and has been found in many tissues, including the heart, small intestine, and ovary [3]. Research studies in oncology have shown that miRNA-21 participates in the regulation of tumor cell proliferation, apoptosis, and migration, and may also be involved in carcinogenesis by inhibition of cell senescence [4–6]. Published studies on cardiovascular disease have shown that increased serum levels of miRNA-21 have been found in patients suffering from cardiovascular disease, including heart failure, aortic stenosis, and myocardial ischemia [7,8]. In an animal model of acute myocardial infarction (AMI), it has been shown that the expression of miRNA-21 was downregulated in the infarcted area (P<0.05) and that overexpression of miRNA-21 could protect myocardial cells from entering apoptosis by targeting apoptosis protein 4, heat shock protein (HSP), and nitric oxide synthase (NOS), thus reducing the size of the myocardial infarction [9]. A further study has shown that increased expression of miRNA-21 could suppress the activation of the PTEN/AKT/FOXO3a signaling pathway, and could TNF-α-induced apoptosis of neonatal rat cardiac myocytes [10].
The aims of this study were to examine the expression of miRNA-21 in the serum of elderly patients with AMI, patients with unstable angina (UA), and healthy elderly people, and to establish a human cardiac myocyte (HCM) cell line that overexpressed miRNA-21 to investigate its potential role as a marker of early cardiac myocyte damage.
Material and Methods
Study participants
The protocol for this study was approved by the local ethics committee of our hospital. All study participants provided written informed consent. Thirty-eight elderly patients, aged more than 65 years, who had an acute myocardial infarction (AMI), 27 elderly patients with unstable angina (UA), and 25 healthy elderly (HE) people were randomly selected. The blood of patients with AMI was obtained within 12 hours of the onset of symptoms, the blood of patients with UA was obtained immediately after hospital admission, and fasting morning blood samples were obtained from the HE study participants. For each study participant, levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were measured. Study participants were noted to be taking a range of medications, including nitroglycerin, metoprolol. amlodipine besylate, thiazide diuretics, and aspirin.
All study participants were more than 65 years old. Patients with AMI were included if they had been diagnosed with non-ST-elevation myocardial infarction (NSTEMI) according to the diagnostic criteria from the World Health Organization (WHO). Patients with UA had symptoms of angina without evidence of myocardial infarction. All healthy elderly (HE) participants were over 65 years old without a medical history of heart, liver, or kidney disease.
Patients were excluded from the study if they had a history of malignancy, liver, or kidney disease. Patients with AMI of unknown time of onset were excluded were excluded, as were individuals with an unknown clinical history.
Reagents and equipment used
The human cardiac myocyte (HCM) cell line was obtained from American Type Culture Collection (ATCC) (USA). The human creatine kinase-MB (CK-MB) detection kit was obtained from GENTAUR (CA, USA). The cardiac troponin I detection kit (cTnI) and the miRNeasy Mini Kit designed for purification of total RNA were obtained from Qiagen (USA). Reverse transcription-polymerase chain reaction (RT-PCR) kit was obtained from Takara (Japan). The antibodies, including p-p38 antibody (E-1), p38, p-JNK, and caspase-3 mouse monoclonal antibodies, goat anti-mouse secondary antibodies were obtained from Santa Cruz (USA). Applied Biosystems real-time quantitative PCR (Thermo Fisher, USA), flow cytometry MACSQuant® Analyzer 10 for flow cytometry (Miltenyi Biotec, Germany), and the Elecsys 2010 electrochemiluminescence analyzer (Roche, Switzerland) were used.
Extraction and detection of serum miRNA-21
Blood samples from the study participants were centrifuged at 1000 rpm for 10 minutes to obtain the serum. Total RNA in the serum was extracted using the total-RNA extraction kit and reverse-transcribed into cDNA, which then underwent reverse transcription-polymerase chain reaction (RT-PCR). The relative expression of miRNA-21 was calculated by 2-ΔΔCt (ΔΔCt=(CTmiRNA-21-CTU6) for the AMI or UA group – (CTmiRNA-21-CTU6) for the HE group.
Detection of serum CK-MB and cTnI
Serum samples from the study participants underwent CK-MB and cTnI detection using the Elecsys 2010 electrochemiluminescence analyzer. The methods used were according to the manufacturer’s instruction manual.
Culture and treatment of human cardiac myocytes (HCM)
HCM cells were cultured in high glucose DMEM medium that included 10% fetal bovine serum (FBS) in a 37°C incubator with 5% CO2. Cell transfection was performed 24 hours after the HCM cells were inoculated into 6-well plates at the concentration of 1×106 cells/well using Lip2000-mediated transient transfection of the miRNA-21 mimic short hairpin RNA (shRNA). Twenty-four hours after transfection of miRNA-21 mimic or negative control shRNA, the experimental group was treated with tumor necrosis factor (TNF)-α at a concentration of 10 μg/L, while the control group was treated with the same volume of sterile water for another 24 hours before cell collection for further experiments.
Flow cytometry analysis of apoptosis of HCM
The treated HCM cells were collected and fixed in 70% pre-cooled ethanol, prepared by using pre-cooled PBS and absolute ethanol, overnight at 4°C, After washing in PBS, the cells were stained with propidium iodide (PI), analyzed by flow cytometry and cell apoptosis was quantified.
Western blot analysis of protein expression
The treated HCM cells were collected and underwent protein extraction using a bicinchoninic acid assay (BCA) kit. Then, 70μg of protein in each sample underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a wet polyvinylidene difluoride (PVDF) membrane (fixed in methanol). The membrane was blocked with 5% non-fat milk powder in Tris-buffered saline with 0.1% Tween 20 (TBST) for 2 h at room temperature and incubated with primary antibodies including mouse monoclonal antibodies to p38, p-JNK, β-actin, and caspase-3, at 4°C overnight. After incubation with secondary horseradish peroxidase (HRP)-conjugated goat anti-mouse polyclonal antibody, the band density was analyzed by ImageJ software and normalized against β-actin levels.
Statistical analysis
Statistical analysis was performed using the SPSS 19.0 statistical program, and cell counting was presented as percentages (mean ± standard deviation). The differences between two groups were compared via an independent t-test, and the differences between three groups were compared by one-way ANOVA. Pearson correlation analysis was used to study the relationship between miRNA-21 and CK-MB/cTnI. P<0.05 was considered statistically significant.
Results
Data on the elderly patients with acute myocardial infarction (AMI), unstable angina (AU) and healthy elderly (HE) individuals
The details of the elderly individuals included in this study, including the 38 cases of elderly patients with acute myocardial infarction (AMI), 27 cases with unstable angina (UA), and 25 healthy elderly (HE) individuals are shown in Table 1. There was no significant difference between the three groups in age, gender, smoking, blood pressure, or plasma levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), or low-density lipoprotein cholesterol (LDL-C) (P>0.05).
Table 1.
Details of the elderly individuals included in this study, including the 38 cases of elderly patients with acute myocardial infarction (AMI), 27 cases with unstable angina (UA), and 25 healthy elderly (HE) individuals.
| Observation index | AMI group | UA group | HE group | P |
|---|---|---|---|---|
| Number (cases) | 38 | 27 | 25 | – |
| Age (years) | 68.3±7.2 | 69.1±5.4 | 68.9±6.6 | 0.139 |
| Male (n/%) | 19/50.0 | 13/48.1 | 13/52.0 | 0.721 |
| Smoking (n/%) | 12/31.6 | 8/29.6 | 8/32.0 | 0.964 |
| Hypertension (n/%) | 26/68.4 | 18/66.7 | 13/52.0 | 0.543 |
| TC (mmol/L) | 4.18±0.89 | 3.89±1.02 | 3.78±0.82 | 0.106 |
| TG (mmol/L) | 1.76±0.96 | 1.64±1.13 | 1.42±0.69 | 0.148 |
| HDL-C (mmol/L) | 1.12±1.23 | 1.11±0.86 | 1.23±0.54 | 0.264 |
| LDL-C (mmol/L) | 2.69±0.85 | 2.38±0.94 | 2.33±0.67 | 0.119 |
Relationship between miRNA-21 expression and CK-MB/cTnI content in the serum of elderly patients with AMI
As shown in Figure 1, the relative expression of miRNA-21 in the serum of elderly patients with UA patients was (4.3±1.7), which was significantly lower than that of elderly patients with AMI (8.6±2.8) and significantly greater than that of healthy elderly people (2.8±1.4). Pearson correlation analysis of the relative expression level of miRNA-21 and the CK-MB/cTnI content in the serum of elderly patients with AMI showed that there was a positive correlation between the relative expression level of miRNA-21 and CK-MB (r=0.3683, P=0.0229) or cTnl (r=0.5128, P=0.009) in the serum of elderly patients with AMI, as shown in Figures 2 and 3.
Figure 1.
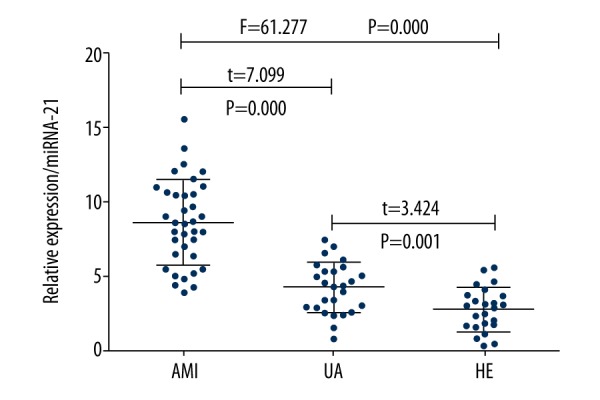
Relative expression of miRNA-21 in different study groups.
Figure 2.
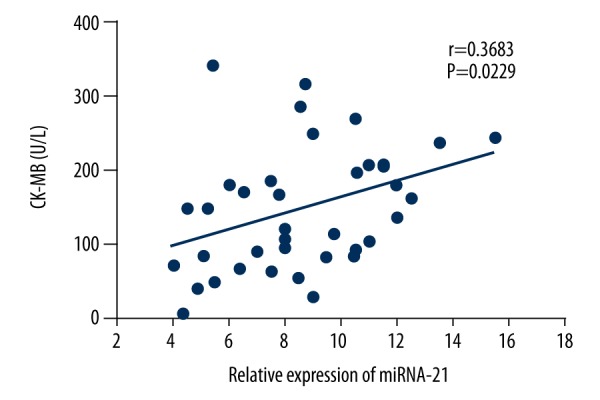
Correlation of the relative expression of miRNA-21 and CK-MB in the serum of elderly patients with acute myocardial infarction (AMI).
Figure 3.
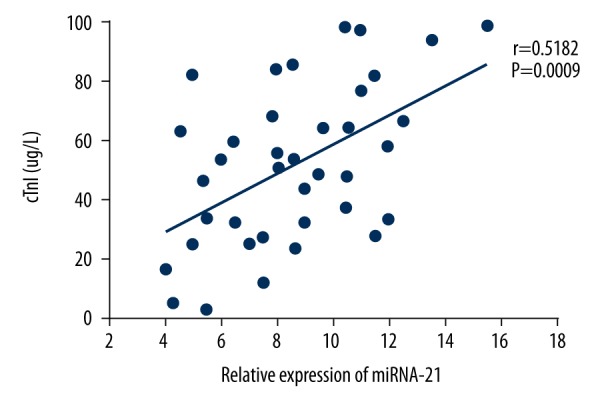
Correlation of the relative expression of miRNA-21 and cTnl in the serum of elderly patients with acute myocardial infarction (AMI).
Effect of miRNA-21 expression on the rate of apoptosis in cultured human cardiac myocytes (HCM)
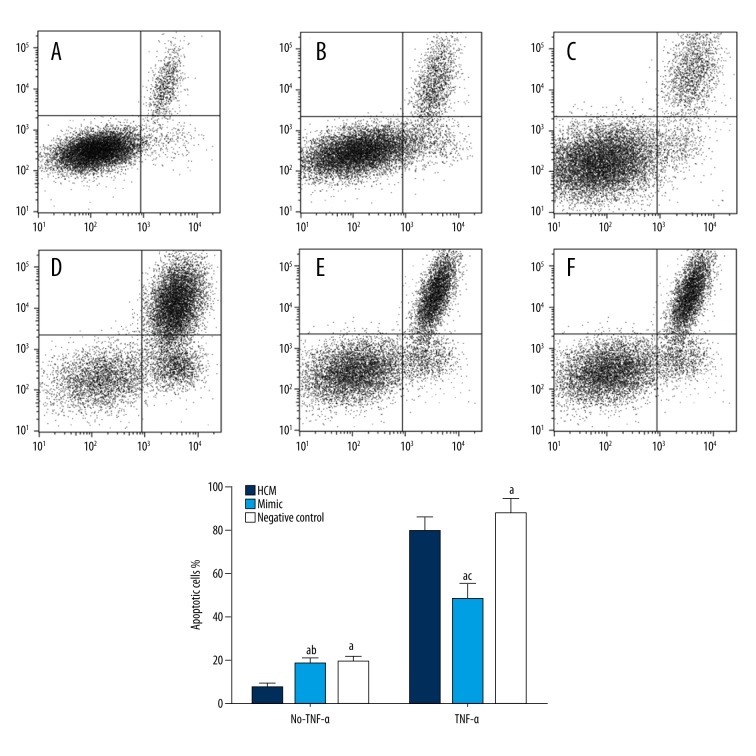
The apoptosis rate of HCM transfected with miRNA-21 mimic, or negative control shRNA was (18.0%±5.4) (Figure 4B) and (18.8%±4.2) (Figure 4C), respectively. There was no significant difference (P>0.05) between the two groups, but the apoptosis rate was significantly greater than that of normal HCM cells (7.16%±2.5) (Figure 4A–4C) (P<0.05). After 24h of TNF-α treatment, the apoptosis rate of normal HCM cells was increased (79.2%±10.2) (Figure 4D), which was significantly greater than that of the mimic group (48.2%±10.7) (Figure 4E) (P<0.05) and significantly lower than that of the negative control group (87.3%±911.6) (Figure 4F) (P<0.05).
Figure 4.
Fluorescence-activated cell sorting (FACS) analysis of the apoptosis rate of different groups of human cardiac myocytes (HCM). A–C represents the apoptosis rate of the HCM group, mimic group, and negative control group without TNF-α treatment, respectively. D–F shows the apoptosis rate of the TNF-α treated HCM group, mimic group and the negative control group, respectively. (1) Human cardiac myocyte (HCM) group: HCM cells without any treatment; (2) Mimic group: HCM cells transfected with miRNA-21 mimic shRNA; (3) Negative control group: HCM cells transfected with negative control shRNA; (4) a Shows that there was a statistically significant difference compared with HCM group (P<0.05); (5) b Shows that there was no significant difference compared with the negative control group (P>0.05); (6) c Shows that there was a significant difference compared with the mimic group (P<0.05).
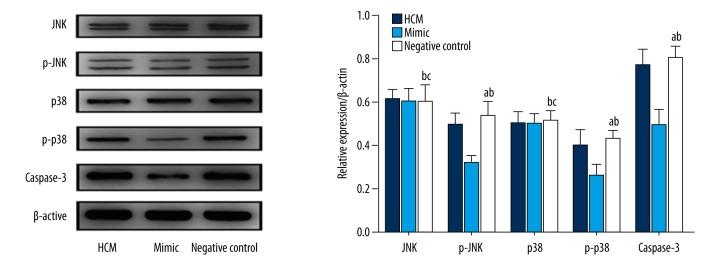
Effect of miRNA-21 expression on the mitogen-activated protein kinase (MAPK) signaling pathway proteins and caspase-3
Western blots were performed to detect the expression levels of mitogen-activated protein kinase (MAPK) signaling pathway proteins and caspase-3 protein in the three study groups. The results showed that, compared with the normal HCM group, the mimic group with miRNA-21 overexpression exhibited unchanged Jun N-terminal kinase (JNK) and p38 protein expression (P>0.05), while the expression of p-JNK, p-p38 and caspase-3 proteins were downregulated by 37.1%, 35.8%, and 36.0%, respectively. The expression levels of JNK, p38, p-JNK, p-p38 and caspase-3 proteins were not significantly different from those in the normal HCM group (P>0.05) (Figure 5).
Figure 5.
Effect of miRNA-21 expression on the expression of MAPK signaling pathway proteins and caspase-3 protein. (1) HCM group: HCM cells without any treatment; (2) Mimic group: HCM cells transfected with miRNA-21 mimic shRNA; (3) Negative control group: HCM cells transfected with negative control shRNA; (4) a Shows that there was a statistically significant difference compared with mimic group (P<0.05). (5) b Shows that there was no significant difference compared with HCM group (P>0.05). (6) c Shows that there was no significant difference compared with mimic group (P>0.05).
Discussion
In this study, we found that the expression of miRNA-21 in the serum of elderly patients with acute myocardial infarction (AMI) was significantly greater than in elderly patients with unstable angina (UA), and healthy elderly (HE) people (P<0.05). This study also showed that the level of expression of serum miRNA-21 in elderly patients with AMI was positively correlated with serum CK-MB (r=0.3683, P=0.0229) and cTnI (r=0.5128, P=0.009). CK-MB is one of the isoforms of CK, derived from the myocardium and is an important clinical diagnostic indicator in patients with AMI [11]. Also, cTnI is one of the three most important subunits of cardiac troponin, which has been widely used in the diagnosis and differential diagnosis of myocardial infarction and angina pectoris due to its high specificity and sensitivity [11]. The findings of this study support the possibility that increased expression of miRNA-21 in the serum of elderly patients with AMI may be related to the apoptosis of myocardial cells, and could be a potential diagnostic marker for AMI.
The human miRNA-21 gene is located on chromosome 17q23.2 and is constantly and highly expressed in vascular smooth muscle cells, cardiac myocytes, and cardiac fibroblasts [7,8]. The aberrant expression of miRNA-21 is involved in the development of cardiovascular disease [7,8]. TNF-α promotes T-cell function in inflammation, and previous studies have shown that TNF-α was highly expressed in the progression of cardiovascular disease such as heart failure, ischemic myocardial injury, AMI, and acute angina pectoris [12–14].
Overexpression of TNF-α and TNF-α receptors on the cell membranes of vascular endothelial cells, myocardial cells, and cardiac fibroblasts have been shown to induce cell apoptosis and participate in pathophysiological processes of cardiovascular disease [15,16]. Cardiac myocyte apoptosis is an important pathophysiological process in AMI, and inhibition of cardiac myocyte apoptosis has been proposed via the signaling transduction pathway [17,18]. In this study, we found that the apoptosis rate of TNF-α treated human cardiac myocytes (HCM) transfected in vitro with the miRNA-21 mimic shRNA was downregulated by 39.1%, which suggested that overexpression of miRNA-21 had protective effects on TNF-α-induced human cardiac myocyte (HCM) apoptosis in vitro and that miRNA-21 could be an important factor in cardiac myocyte protection during ischemia.
In order to further investigate the protective mechanism of miRNA-21 on TNF-α induced HCM apoptosis, we performed Western blots to detect the protein expression of Jun N-terminal kinase (JNK), p38 and caspase-3 as well as their phosphorylated forms in HCM cells. The results showed that the HCM cells with overexpression of miRNA-21 showed unchanged expression of JNK and p38 proteins (P>0.05) compared with normal HCM cells, while the expression of p-JNK, p-p38 and caspase-3 proteins were significantly inhibited (P<0.05).
The mitogen-activated protein kinase (MAPK) signaling pathway is composed of a group of evolutionarily conserved serine and threonine protein kinases, which can be activated by a series of extracellular stimuli and mediate the signal transduction from the cell membrane to the nucleus [19]. JNK/SAPK and p38 MAPK are two important mammalian signaling pathways. MAPK participates in the regulation of physiological processes such as apoptosis, proliferation, carcinogenesis and migration of tumor cells [19]. Previous studies have shown that the JNK/SAPK pathway and p38 MAPK pathway share similar activators, including TNF-α, heat shock proteins, hydrogen peroxide and ultraviolet light [19]. In this study, we found that overexpression of miRNA-21 did not affect the expression of JNK and p38 in myocardial cells, but significantly inhibited the phosphorylation of these proteins. Since the degree of phosphorylation of JNK and p38 represents their activities, we inferred that miRNA-21 overexpression could inhibit the activation of the JNK/SAPK pathway and p38 MAPK pathway.
Activated caspase-3 can induce the expression of multiple proteins involved in apoptosis [20,21]. In the mitochondrial pathway of apoptosis, cytochrome-C is released from the mitochondrial outer membrane by the stimulation of apoptosis-related signals, cytochrome-C then activates caspase-9 by binding to apaf-1, and activated caspase-9 further activates caspase-3, which in turn activates caspase-6/7/8 and finally leads to cell apoptosis [20]. Somatostatin receptor type 2 (SST2) is an IL-33 receptor that can activate NF-κB, JNK/SAPK, and p38 MAPK pathways by binding to IL-33 in the blood to regulate cytokines including IL-2 and TNF-alpha to mediate inflammatory responses [22]. SST2 binds to IL-33 to mediate the inflammatory response and also to induce the rupture of the atherosclerotic plaque through the ST2/IL-33 pathway [23]. In this study, the expression level of miR-21 was also related to the activation of JNK/SAPK, p38 MAPK signaling pathways, and it is possible that it could also mediate inflammation. Although we may hypothesize that the expression of miR-21 in the blood may be similar to SST2 as a marker for the diagnosis of AMI and other cardiovascular disease, this hypothesis requires further investigation. We acknowledge that this was a small preliminary study with a small study population size using a single cardiac myocyte cell line. Further larger studies are required to evaluate the role of miRNA-21 as a potential serum marker for AMI.
Conclusions
The findings of this study showed that the expression of miRNA-21 was upregulated in the serum of elderly patients with AMI, which inhibited TNF-α induced apoptosis in HCM by activating the JNK/p38/caspase-3 signaling pathway that further inhibited TNF-α-induced apoptosis in cardiac myocytes cultured in vitro.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2016;389:197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 2.Sui HCW, Wang W. Interpretation of Report on Cardiovascular Diseases in China (2015) Chinese Journal of Cardiovascular Medicine. 2015;21:259–61. [Google Scholar]
- 3.Meder B, Keller A, Vogel B, et al. MicroRNA signatures in total peripheral blood as novel biomarkers for acute myocardial infarction. Basic Res Cardiol. 2011;106:13–23. doi: 10.1007/s00395-010-0123-2. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Jiang K, Zhao F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol Rep. 2015;33:2829–36. doi: 10.3892/or.2015.3891. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima Y, Iinuma H, Tsukamoto M, et al. Clinical significance of microRNA-21 as a biomarker in each Dukes’ stage of colorectal cancer. Oncol Rep. 2015;33:573–82. doi: 10.3892/or.2014.3614. [DOI] [PubMed] [Google Scholar]
- 6.Han M, Wang F, Gu Y, et al. MicroRNA-21 induces breast cancer cell invasion and migration by suppressing smad7 via EGF and TGF-β pathways. Oncol Rep. 2015;35:73–80. doi: 10.3892/or.2015.4360. [DOI] [PubMed] [Google Scholar]
- 7.Dong S, Ma W, Hao B, et al. microRNA-21 promotes cardiac fibrosis and development of heart failure with preserved left ventricular ejection fraction by upregulating Bcl-2. Int J Clin Exp Pathol. 2014;7:565–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Villar AV, García R, Merino D, et al. Myocardial and circulating levels of microRNA-21 reflect left ventricular fibrosis in aortic stenosis patients. Int J Cardiol. 2013;167:2875–81. doi: 10.1016/j.ijcard.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Dong S, Cheng Y, Yang J, et al. MicroRNA Expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Y, Wang MH. [[MicroRNA-21 protects cardiomyocytes from tumor necrosis factor-alpha-induced apoptosis in vitro via modulating PTEN/AKT/FOXO3a pathway]]. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41:135–42. [in Chinese] [PubMed] [Google Scholar]
- 11.Mythili S, Malathi N. Diagnostic markers of acute myocardial infarction. J Nucl Cardiol. 2015;3:S1. doi: 10.3892/br.2015.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popa C, Netea MG, van Riel PL, et al. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–62. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Satoh M, Ishikawa Y, Itoh T, et al. The expression of TNF-α converting enzyme at the site of ruptured plaques in patients with acute myocardial infarction. Eur J Clin Invest. 2008;38:97–105. doi: 10.1111/j.1365-2362.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 14.Gu Q, Yang XP, Bonde P, et al. Inhibition of TNF-alpha reduces myocardial injury and proinflammatory pathways following ischemia-reperfusion in the dog. J Cardiovasc Pharmacol. 2006;48:320–28. doi: 10.1097/01.fjc.0000250079.46526.38. [DOI] [PubMed] [Google Scholar]
- 15.Schenk B, Fulda S. Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death. Oncogene. 2015;34:5796–806. doi: 10.1038/onc.2015.35. [DOI] [PubMed] [Google Scholar]
- 16.Wagner SA, Satpathy S, Beli P, et al. SPATA2 links CYLD to the TNF-α receptor signaling complex and modulates the receptor signaling outcomes. EMBO J. 2016;35:1868–84. doi: 10.15252/embj.201694300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han SP, Pan Y, Peng YZ, et al. Folbp1 promotes embryonic myocardial cell proliferation and apoptosis through the WNT signal transduction pathway. Int J Mol Med. 2009;23:321–30. doi: 10.3892/ijmm_00000134. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Xie Z, Lin M, et al. Renalase protects the cardiomyocytes of sprague-dawley rats against ischemia and reperfusion injury by reducing myocardial cell necrosis and apoptosis. Kidney Blood Press Res. 2015;40:215–22. doi: 10.1159/000368497. [DOI] [PubMed] [Google Scholar]
- 19.Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: An update. Arch Toxicol. 2015;89:867–82. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 20.Hassan M, Watari H, Abualmaaty A, et al. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2013;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Cheng B, Zhang Y, Wang A, et al. Vitamin C attenuates isoflurane-induced Caspase-3 activation and cognitive impairment. Mol Neurobiol. 2015;52:1580–899. doi: 10.1007/s12035-014-8959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciccone MM, Cortese F, Gesualdo M, et al. A novel cardiac bio-marker: ST2: A review. Molecules. 2013;18:15314–28. doi: 10.3390/molecules181215314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marzullo A, Ambrosi F, Inchingolo M, et al. ST2L transmembrane receptor expression: An immunochemical study on endarterectomy samples. PLoS One. 2016;11(5):e0156315. doi: 10.1371/journal.pone.0156315. [DOI] [PMC free article] [PubMed] [Google Scholar]