Abstract
Aim of this study was to evaluate the biotransformation of simple phenols after ingestion of edible fruits and mixed food. It was analyzed hippuric acid in urine as biomarker of conjugation in the liver cells of glycine with aromatic phenolic acids such benzoic and salicylic acid from ingested food. Measurement of hippuric acid in urine samples of 10 healthy individuals: 5 female and 5 male with a mean age 51,5 years were recruited to participate in this study. Urine samples were collected for 24 hours. The additional meals 300 g of fruits: blueberry, cherry, raspberry, melon, blackberry and mixed food were given immediately before the 24 hr urine sampling. Otherwise, the meals given during 24 hr was a usually food. Biotransformation of phenols in edible fruits, that are together with liver glycins precursors of hippuric acid biosynthesis, was evaluated by direct spectrophotometric measurement of excreted hippuric acid in urine at 410 nm. It was established that the highest quantity of hippuric acid was after ingestion of 300g of bilberry fruits (p< 0,003), and same quantity of cherries (p< 0,003). Concentration of excreted hippuric acid was twice higher after ingestion of these fruits in comparison with hippuric acid concentrations in urine after ingestion of common - mixed food. Quantity of biosynthesised hippuric acid was in direct correlation with the concentrations of its precursors, primarily phenol acids and other simple aromatic acids ingested with food.
Keywords: hippuric acid, fruits, phenolic acid, biotransformation
INTRODUCTION
Fruits and vegetables, black and green tea contain phytochemicals, which are thought to protect consumers against cancer (1, 2), diabetes, cardiovascular diseases (3) and diseases of aging (4). However, the conventional bioanalytical techniques have significantly underestimated the phytochemicals content of these foods. Vitamin C, vitamin E and β-carotene make up only a small portion of the total chemicals in fruits and vegetables. There are a number of other phytochemicals in the foods in the chemical form of phenolics, flavonoids, anthocyanins and etc. Little is known about biotransformation of these compounds in food after food ingestion. Hippuric acid is normally present in human urine as a metabolite of dietary components. Therefore, the analysis of hippuric acid in urine provides an diet and liver diagnostic test (5). In humans the synthesis of hippuric acid takes place almost entirely in the liver. Both ATP and CoA are required for this biosynthesis (Figure 1.): Tracing these phytochemicals at physiologic concentrations has been hindered by a lack of quantitative sensitivity for chemical equivalent tracers that could be used safely in healthy people. Accelerator mass spectrometry is a relatively new technique that provides the necessary sensitivity in attomoles and measurement precision (< 3 %) towards 14C labelled phytochemicals for detailed kinetic studies in humans at dietary levels (6). Oxigen Radical Absorbance Capacity assay (7), HPLC-multichannel ECD, mass spectrometry and also classical methods as spectrophotometry can be use for biotransformation studies (8). The biotransformation of phytochemicals from food is very complicated, for example the biotransformation of quercetin included deglycosylation of the flavanol glycoside, biotransformation (intestinal and hepatic) like methylation, glucuronidation, sulphation, glutathionylation and mixtures of conjugates. It is possible 18 types of metabolites possible and more than 30 when considering isomers. Quercetin metabolites indentfiend in urine are methyl-quercetin with 1 isomers, quercetin-mono-glucuronide with 4 isomers, quercetin-di-glucuronide with 1 isomers, methylquercetin-mono-glucuronide with 2 isomers, sulfatequercetin-glucuronide with 1 isomers and sulfatequercetin-glucoside with 2 isomers. Quercetin is the major flavonol present in vegetables and fruits (e.g. apples, onions, grapes, kale, tea, red wine, etc.), occurs as the glycosides in plants, extensively metabolised.
FIGURE 1.
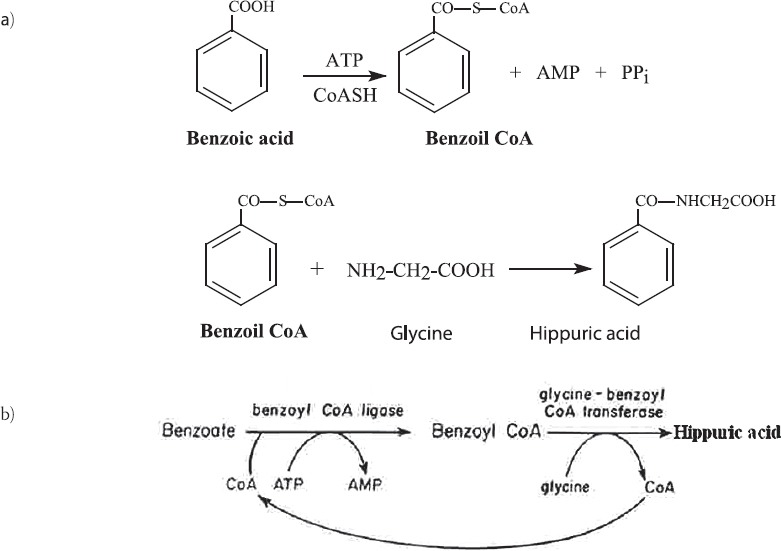
Biosynthesis of hippuric acid
MATERIALS AND METHODS
Subjects: 10 healthy persons, (5 f, 5 m)
Biological samples: urine (24 hr)
Edible portions of fruits (300 g)
Ten healthy subjects (5 f, 5 m) with a mean age 51.5 years were recruited to participate in this study. All study participants were in good health as determined by a medical history questionnaire, physical examination and normal results for clinical laboratory tests. Urine samples were collected for 24 hours. An additional meal (300 g of fruits) was given immediately before the 24 hr urine sampling. Otherwise, the meals given during 24 hr was a usually food.
a) Direct Spectrophotometric determination of hippuric acid in urine
Direct spectrophotometric method was used for analysis of hippuric acid in urine (9). Hippuric acid dissolved in pyridine - water (1: 1) produces a colour when benzen sulphonyl chloride was added. With the method based on this colour reaction urinary hippuric acid follows the Beer-Lambert law up to 1 mg / ml at 410 nm and path length 1 cm.
b) Apparatus and chromatographic conditions (HPLC-UV / VIS)
A Waters Assoc. Model ALC / GPC 202 liqui d chromatograph equipped with a μBondapak C18 prepacked column (30 cm x 4 mm I.D., Waters) at ambient temperature and a UV detector set at 254 nm, was employed for chromatographic analysis (8). The detector was operated at 0,05 absorbance units full scale for most samples. The eluting solvent was 20 % (v / v) methanol in 0,01 M potassium phosphate containing 0,5 % (v / v) acetic acid. The flow-rate was 1,0 ml / min at a pressure of 13789520 Pa. Sample injections were made on-column through a Waters U6K septumless injector with a 10-μ! syringe (Hamilton 701 N).
RESULTS
On the basis of quantitative analysis of excreted hippuric acid in urine samples of ten healthy individuals, five male and five female, it was established that the highest quantity of hippuric acid was after ingestion of 300g of bilberry fruits and cherries.
Statisticaly significantly highest excretion of hippuric acid after ingestion of bilberry fruits (p<0,003) and cherries (p < 0,003) is expressed in Table 1. and Graph 1. Increased excretion of hippuric acid after ingestion of raspberry fruits is also obvious, even it was not statisticaly significantly different in comparison with content of hippuric acid after ingestion of common mixed food.
TABLE 1.
The content of hippuric acid in urine (24 hr)
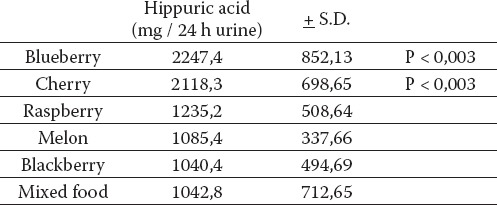
GRAPH 1.
Urinary hippuric acid excretion / 24 hr
However, ingestion of melon fruits and blackberries didn’t cause statisticaly significant differences in hippuric acid content in urine in comparison with same content in urine after ingestion of common mixed food. Excretion of hippuric acid after ingestion of 300 g of different fruits, measured every 2, 6, 10, 15 and 24 hours is showed in Graph 2. High concentration of hippuric acid have been observed in subjects when they ingested prunes and followed blueberries, black grape, mixed foods, strawberries, beets, cranberries, red grape, green tea (Graph 3). The concentration of excreted hippuric acid was twice higher after ingestion of these fruits in comparison with hippuric acid concentrations in urine after ingestion of common - mixed food.
GRAPH 2.
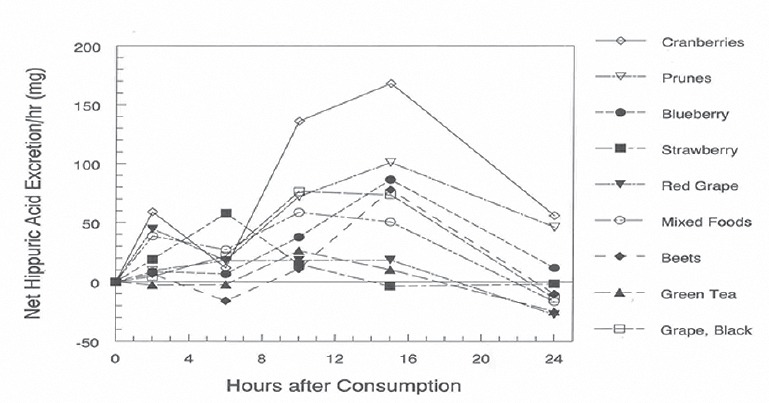
Urinary net hippuric acid excretion / hr (mg)
GRAPH 3.
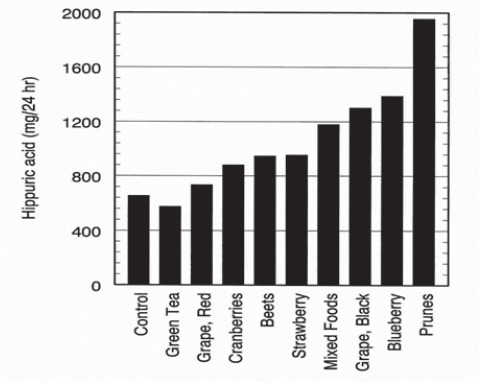
Total hippuric acid excretion (mg / 24 hr)
GRAPH 4.
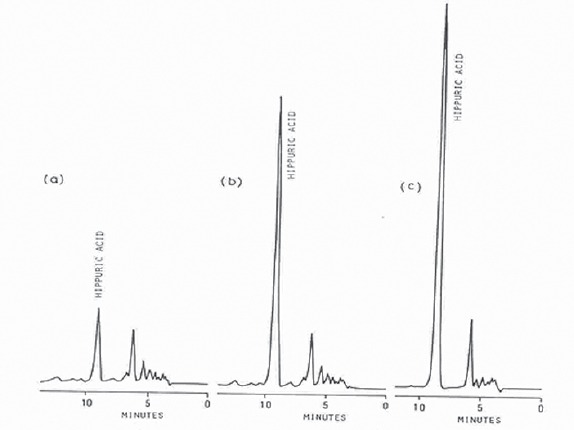
Liquid chromatograms of extracts from (a) normal urine, (b) normal urine containing added hippuric acid, and (c) urine from a person who eat 300 g of edible portion of cranberries.
DISCUSSION
Two common aromatic acids are benzoic acid and cinnamic acid (unsaturated side-chain), which are widely distributed in nature and often occur free and combined in considerable amounts in drugs such as balsams. Truxillic acid, a polymer of cinnamic acid, occurs in coca leaves. Other related acids of fairly common occurrence are those having phenolic or other groupings in addition to a carboxyl group; such are: salicylic acid (o-hydroxybenzoic acid), protocatechuic acid (3,4-dihy- droxybenzoic acid), veratric acid (3,4-dimethoxybenzoic acid), gallic acid (3,4,5-trihydroxybenzoic acid) and 3,4,5- trimethoxybenzoic acid. Similarly, derived from cinnamic acid, one finds p-coumaric acid (p-hydroxycinnamic acid), ferulic acid (hydroxymethoxycinnamic acid), caffeic acid (hydroxycinnamic acid) and 3,4,5-trimethoxy- cinnamic acid. Acids having an alcohol group are quinic acid (tetrahydroxyhexahydrobenzoic acid), which occurs in cinchona bark and in some gymnosperms; shikimic acid, which is found in Japanese star-anise and is an important intermediate metabolite; and mandelic acid, C6H5CHOHCOOH, which occurs in combination in cyanogenetic glycosides such as those of bitter almonds and other species of Prunus (10). Tropic acid and phenyllacetic acid are two aromatic hydroxy acids which occur as esters in tropane alkaloids (q.v.). Aromatic acids and phenols in the fruits and vegetables are for examples: catechol, floroglucinol, salicylic alcohol, salicylic acid, p-anisaldehyd, E-anethol, myristicin, sinapic alcohol, benzoic acid, protocatechuic acid, vanillic acid, galic acid, veratric acid, shikimic acid, homogentizinic acid, p-coumaric acid, caffeic acid, ferulic acid, cinnamic acid, quinic acid, rozmaric acid, litospermic acid and chlorogenic acid (10). Chlorogenic or caffeotannic acid is a condensation product of caffeic acid and quinic acid. It occurs in mate, coffee and nux vomica and is converted into a green compound, which serves for its detection, when an aqueous extract is treated with ammonia and exposed to air. Hippuric acid is normally present in human urine as a metabolite of dietary components and therapeutic components such acetylsalicylic acid. Acetylsalicylic acid is changed to salicilyc acid. This change is a typical reaction of hydrolysis of an active drug to an active metabolite. In the phase II reactions involve conjugation of benzoic and salicylic acid, during which polar groups utilized for linkage to normal body constituents such as carbohydrates, amino acids, glucuronate, glutathione, acetate, alkyl, methyl or, sulfate groups. Conjugation usually but not always results in xenobiotics and drug inactivation (11). Benzyl alcohol as preservative in food and saline solutions is metabolized to benzoic acid and then to benzoilglycine. In neonates, this metabolism is not rapid enough to prevent a sometimes fatal acidosis (11). After exposure to toluene and also administration of acetylsalicylic acid or quercetin, large quantities of hippuric acid are extracted in the urine and quantities of it are correlated to toluene exposure and to quantities of administrated drug or to ingested quercetin in food samples (12). Therefore, the analysis of hippuric acid in urine provides an exposure test and also an diet test. Hippuric acid is so called because it was first found in the urine of horses. It is benzoilglycine, a compound of benzoic acid and glycine. This is a physiological metabolic reaction that results in the detoxication of benzoic acid and benzoates. The latter occur in vegetables and fruits food or are derived from the oxidation of aromatic substances. About 0,7 g of hippuric acid per day is eliminated on the average diet, but deviations from this are to be expected, depending on the amount of precursors in the diet. Glycine is usually present in sufficient amounts to combine with any quantity of benzoates that are likely to be ingested (13). Cranberries contain 0,05 to 0,09 % benzoic acid, and other fruits and berries contain smaller amounts. Some foods, e.g., catsups, are permitted by law to have 0,1 % sodium benzoate added as a preservative. This is, of course, also converted to hippuric acid (14). The synthetic action of these compounds has been used as a test for liver function but evidently is a test for only this particular liver function, not for all. Nevertheless, subnormal values have been claimed in patients with various types of hepatitis and other liver conditions but not in those with uncomplicated obstruction of the common bile duct; i.e., it may aid in differentiating between hepatic and obstructive jaundice (5, 15). In conclusion biotransformation of phenols in edible fruits, that are together with liver glycins precursors of hippuric acid biosynthesis was evaluated by spectrophotometric measurement of excreted hippuric acid in urine. On the basis of measurement of hippuric acid concentration in urine samples of 10 healthy individuals it was established that the highest quantity of hippuric acid was after ingestion of 300 g of bilberry fruits (p< 0,003) and same quantity of cherries (p< 0,003) (First experiment, spectrophotometric method). Furthermore, as high concentration of hippuric acid have been observed in subjects when they ingested prunes and followed blueberries, black grape, mixed foods, strawberries, beets, cranberries, red grape, green tea (HPLC-UV / VIS method). The concentration of excreted hippuric acid was twice higher after ingestion of these fruits in comparison with hippuric acid concentrations in urine after ingestion of common - mixed food. Quantity of biosynthesised hippuric acid was in direct correlation with the concentrations of its precursors, primarily phenol acids and other simple aromatic acids ingested with food. Increased consumption of fruits and vegetables can increase the plasma, serum and urine antioxidant capacity (16, 17). Increased consumption of fruits and vegetables has been associated with protection against various diseases, including cancer (18, 19, 20) and cerebrovascular diseases (21, 22). It is not know what dietary constituents are responsible for this association, but it is often assumed that antioxidants contribute to the protection (23). Finally, the associations between phenolics intakes, these markers and health outcomes must be studied.
CONCLUSION
-It was established that the highest quantity of hippuric acid was after ingestion of 300g of bilberry fruits (p< 0,003), and same quantity of cherries (p< 0,003).
-Concentration of excreted hippuric acid was twice higher after ingestion of these fruits in comparison with hippuric acid concentrations in urine after ingestion of common - mixed food.
-Quantity of biosynthesised hippuric acid was in direct correlation with the concentrations of its precursors, primarily phenol acids and other simple aromatic acids ingested with food.
REFERENCES
- 1.Greenwald P, Anderson D, Nelson S.A, Taylor P.R. Clinical trials of vitamin and mineral supplements for cancer prevention. Am. J. Clin. Nutr. 2007;85(1):314S–317S. doi: 10.1093/ajcn/85.1.314S. [DOI] [PubMed] [Google Scholar]
- 2.Hou D.X. Potential mechanismis of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 2003;3:149–159. doi: 10.2174/1566524033361555. [DOI] [PubMed] [Google Scholar]
- 3.Nees A.R, Powles J. W. Fruit and vegetables, and cardiovascular disease. A review. Int. J. Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Yu B.P. Aging and oxidative stres: Modulation by dietary restriction. Free. Rad. Biol. Med. 1996;21:651–668. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]
- 5.Štraus B. Funkcija jetre u konjugaciji i detoksikaciji. Zagreb: Medicinska biokemija, Medicinska Naklada; 1992. pp. 888–890. [Google Scholar]
- 6.Ross S.A, Srinivas P.R, Clifford A.J, Lee S.C, Philbert M.A, Hettich R.L. New technologies for nutrition research. J. Nutr. 2004;134(3):681–685. doi: 10.1093/jn/134.3.681. [DOI] [PubMed] [Google Scholar]
- 7.Cao G, Prior R.L. Anthocyanins are detected in human plasma after oral administration of an elderberry extract. Clin. Chem. 1999;45:574–576. [PubMed] [Google Scholar]
- 8.Matsui H, Kasao M, Imamura S. High-performance liquid chromatographic determination of hippuric acid in human urine. J. Chromat. 1978;145:231–236. doi: 10.1016/s0378-4347(00)81343-2. [DOI] [PubMed] [Google Scholar]
- 9.Tomokuni K, Ogata M. Direct colorimetric determination of hippuric acid in urine. Clin. Chem. 1972;18(4):349–351. [PubMed] [Google Scholar]
- 10.Trease G.E, Evans W.C. Pharmacognosy. London: Bailliere Tindall; 1978. Basic metabolic pathways and the origin of secondary metabolites; pp. 255–281. [Google Scholar]
- 11.Theoharides T.C. Essentials of Pharmacology, Little. Boston: Brown Essentials and Company; 1996. General Principles and Pharmacokinetics; pp. 1–33. [Google Scholar]
- 12.Piotrowsky J.K. Exposure tests for organic compounds in industrial toxicology. Washington, D.C: U.S. Government Printing Office; 1977. pp. 48–54. [Google Scholar]
- 13.Griffith W.H. Benzoylated amino acids in the animal organism IV. A method for the investigation of the origin of glycine. J. Biol. Chem. 1929;82:415–427. [Google Scholar]
- 14.Beliveau G.P, Brusilow S.W. Glycine availability limits maxsimum hippurate synthesis in growing rats. J. Nutr. 1987;117:3641. doi: 10.1093/jn/117.1.36. [DOI] [PubMed] [Google Scholar]
- 15.Walle T. Absorption and metabolism of flavonoids. Free Rad. Med. 2004;36(7):829–837. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Cao G, Russell R.M, Lischner N, Prior R.L. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. Nutr. Metabol. 1998:2383–2390. doi: 10.1093/jn/128.12.2383. [DOI] [PubMed] [Google Scholar]
- 17.Cao G, Booth S.L, Sadowski J.A, Prior R.L. Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am. J. Clin. Nutr. 1998;68:1081–1087. doi: 10.1093/ajcn/68.5.1081. [DOI] [PubMed] [Google Scholar]
- 18.Steinmetz K.A, Potter J.D. Vegetables, fruit, and cancer prevention: a review. J. Am. Diet. Assoc. 1996;96:1027–1039. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 19.Kohlmeier L, Simonsen N, Mottus K. Dietary modifiers of carcinogenesis. Environ. Health. Perspect. 1995;103:177–184. doi: 10.1289/ehp.95103s8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin J.S, Brodwick M. Diet, aging, and cancer. Clin. Geriatr. Med. 1995;11:577–589. [PubMed] [Google Scholar]
- 21.Rimm E.B, Ascherio A, Giovannucci E, Spiegelman D, Stampfer M.J, Willet W.C. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996;275:447–451. doi: 10.1001/jama.1996.03530300031036. [DOI] [PubMed] [Google Scholar]
- 22.Gillman M.W, Cupples L.A, Gagnon D, et al. Protective effect of fruits and vegetables on development of stroke in men. JAMA. 1995;273:1113–1117. doi: 10.1001/jama.1995.03520380049034. [DOI] [PubMed] [Google Scholar]
- 23.Gey K.F. The antioxidant hypothesis of cardiovascular disease: epidemiology and mechanisms. Biochem. Soc. Trans. 1990;18:1041–1045. doi: 10.1042/bst0181041. [DOI] [PubMed] [Google Scholar]


