Abstract
Diagnosis and management of patients with SLE (Systemic Lupus Eritematosus), autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), involves specific diagnostic tests, such as IFA-AMA, IFA anti-dsDNA and immunoblotting for the detection of autoantibodies for specific autoantigens (mitochondria, dsDNA, M2, LKM-1, LC-1, SLA/LP). We established specific correlation between the detected autoantibodies and corresponding clinical findings.
The total of 813 serum specimens were probed with IFA-anti-dsDNA, 98 of which tested positive. We also performed dilution analysis to the end point for all the positive specimens. Numerous specimens were tested by IFA, AMA and immunoblotting.
Keywords: SLE, AIH, PBC, IFA, AMA, anti-dsDNA, M-2, LKM-1, LC-1, SLA/LP
INTRODUCTION
Systemic Lupus eritematosus (SLE) is one of characteristic autoimmune diseases in which numerous autoantibodies are produced. Glomerulonephritis and arthritis are often among other clinical manifestations. Immune complexes include autoantibodies and DNA or nucleoprotein antigens. The most frequent type of autoantibodies in SLE patients, are anti-dsDNA. Also, there are autoantibodies against ribonucleoproteins, histones and nucleolus. Autoantibodies against erytrocytes and platelets are characteristic for hemolytic anemia and thrombocytopenia. Autoantibodies against native double stranded (ds) DNA are specific for SLE and thus, Crithidia lucillae anti-dsDNA indirect immunofluorescence test (IFA anti-ds-DNA) is the principal diagnostic test. (1). Hemoflagelate Crithidia lucillae possesses a modified mitochondrion called kinetoplast, which naturally contains numerous molecules of double stranded DNA (ds-DNA) that are not associated with histones. Kinetoplast is a spherical organelle, smaller than nucleus, located near nucleus and basal body. The IFA anti-dsDNA diagnostic test is highly specific and moderately sensitive in the management of diseases such as SLE which is predominant in women (incidence 1: 700, age between 20-60 years, male to female ratio 1:10). The main clinical features include: rashes, arthritis, glomerulonephritis, hemolytic anemia, thrombocytopenia (2, 3). In the suspect cases of liver diseases or autoimmune hepatitis, early and fast detection of the following human autoantibodies of the class IgG, against four different specific antigens, is very important:
-M2 (pyruvate dehidrogenase complex)
-LKM-1 (liver - kidney microsomes - cytochrome P450 II-D6)
-LC -1 (cytosolic liver antigen type -1)
-SLA/LP (soluble liver antigen/ liver -pancreas antigen).
Diagnostic Western blot method offers the possibilities of fast, highly sensitive, highly specific and simple procedure for the detection of autoantibodies against the above mentioned specific antigens, in either serum or plasma, and gives the chance for timely and successful treatment of patients. Specially designed immmunoblot strips contain parallel lines of antigens purified by affinity chromatography. Specific IgG, IgA and IgM antibodies bind to corresponding antigen site. The detection is based on enzyme-labeled conjugate promoting a color reaction. No cross-reactions with other autoantibodies were found (4). During one year, we tested the total of 192 clinical blood specimens for presence of specific autoantibodies against M2, LKM-1, LC-1 and SLA/ LP antigens by Western blot method (Euroimmun). We also analyzed the total of 813 serum specimens by IFA anti-dsDNA and 308 specimens by IFA-AMA. The tests were performed according to the obtained recommendations of clinicians at University of Sarajevo Clinics Centre.
MATERIAL AND METHODS
Serum specimens were collected from patients at different Clinics at University of Sarajevo Clinics Centre.
Immunofluorescence test
We performed IFA anti dsDNA test using BioSystems anti nDNA antibodies test designed for this purpose. The main substrate is flagellate Crithidia lucillae which contains kinetoplast with numerous molecules of double stranded circular DNA.
The detection kit contains:
-12 well slides coated with Crithidia lucillae
-OBS buffer 10 x
-nDNA positive control (human serum containing anti-native DNA antibodies)
-Negative control-Human serum and sodium azide
-IgG FITC (IgG antibodies conjugated with fluorescin isothiocyanate, Evans Blue dye and sodium azide)
-Mounting medium (glycerol, sodium phosphate, sodium chloride, sodium azide)
Before the commencement of test, the reagents and serum specimens are maintained at room temperature for at least half an hour. PBS buffer is diluted with distilled water to final 1x concentration. Serum specimens are diluted with PBS buffer. One drop (25μ!) of diluted serum, specimens and controls, are added into slide wells. After the incubation and rinsing procedures, IgG FITC-EVANS is added and finally, several drops of mounting medium. The slides are examined by fluorescence microscope (495 nm excitation filter and 525 nm emission filter). In order to obtain the best results the slides are analyzed immediately. Positive serum specimens are titrated to the end point dilution defined as the highest dilution giving positive result. Finding is positive only in the case of kinetoplast fluorescent illumination. Nuclei, basal body or flagellum illumination is not positive finding for anti-dsDNA (5, 6, 7).
Immunoblotting
Serum specimens obtained from patients suspected of liver diseases and autoimmune hepatitis, were tested by Euroimmun western blot method according to the manufacturer’s instructions. The serum samples are diluted with sample buffer in the ratio 1:100 (15μ! of serum and 1,5 ml sample buffer). Immunoblot test strips coated with M2, LKM-1, LC-1 and SLA/LP antigens, are incubated in special channels in 1,5 ml of sample buffer in pretreatment of 5 minutes, 30 minutes in 1,5 ml of diluted serum specimens, 30 minutes in 1,5 ml diluted enzyme conjugate (alkaline phosphatase - labeled anti human IgG), and finally for 10 minutes in 1,4 ml substrate solution. Incubation steps are performed at room temperature on rocking shaker. The immunoblot strips are washed 3x5 minutes with diluted wash buffer. Strips are than air-dried and the results evaluated based on signal intensity (negative, borderline, positive and strong positive). Only the strips with strong color reaction in the control band are considered valid (8, 9, 10, 11, 12).
RESULTS
Serum specimens were collected from patients at different Clinics at University of Sarajevo Clinics Centre. The serum specimens were tested by IFA anti dsDNA, IFA-AMA and LKM immunobloting strips for the presence of specific autoantibodies:
-autoantibodies against dsDNA
-antimitochondrial autoantibodies (AMA)
-against M2- pyruvate-dehydrogenase complex
-against LKM-1- liver-kidney microsomes
-against Cytochrome p450IID6
-against LC-1- cytosolic liver antigen type 1
-against SLA/LP - soluble liver antigen/liver pancreas antigen
The results obtained by immunofluorescent and immunoblotting analyses are summarized in Tables 1, 2 and 3 and illustrated by corresponding figures (1, 2, 3 and 4) and diagrams (1, 2, 3 and 4).
TABLE 1.
IFA testing results of serum specimens
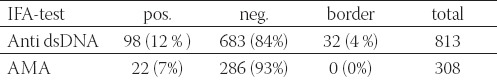
TABLE 2.
AMA Female/Male ratio (positive findings and total analyzed specimens)
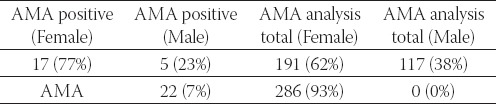
TABLE 3.
The origin of serum specimens analyzed for IFA and im-munoblot - overview per Clinics
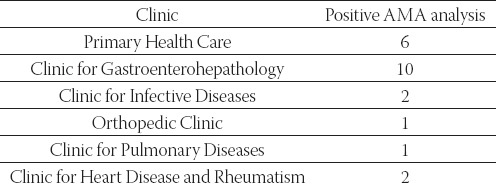
FIGURE 1.
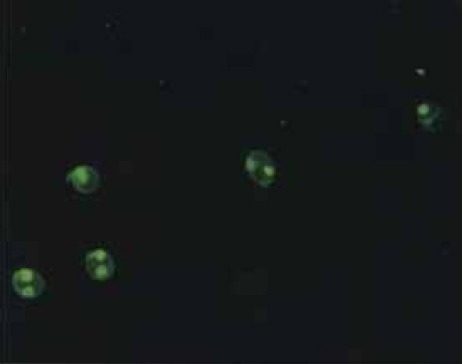
Anti dsDNA (Crithidia lucillae) - positive specimen - kinetoplast labeled with fluorescin
FIGURE 2.
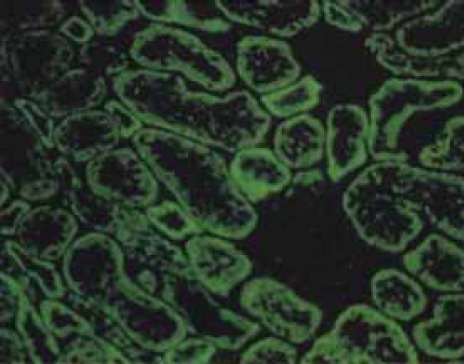
AMA (antimitochondrial antibodies) - rat kidney cells as substrate
FIGURE 3.
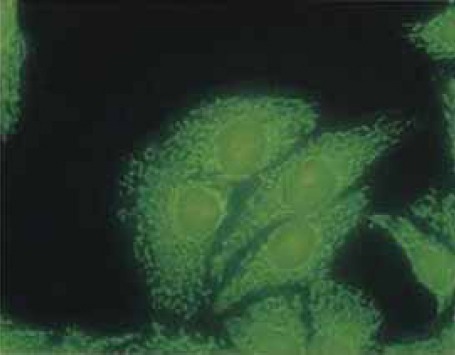
AMA (antimitochondrial antibodies) - HEp2 cells as substrate
DIAGRAM 1.
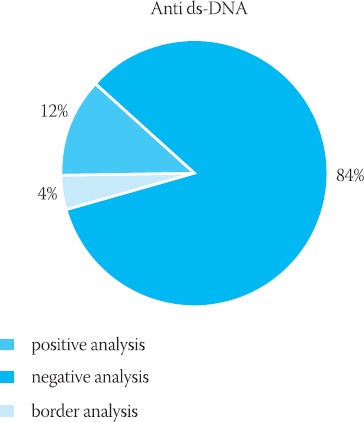
The results obtained by IFA-anti dsDNA testing
DIAGRAM 2.
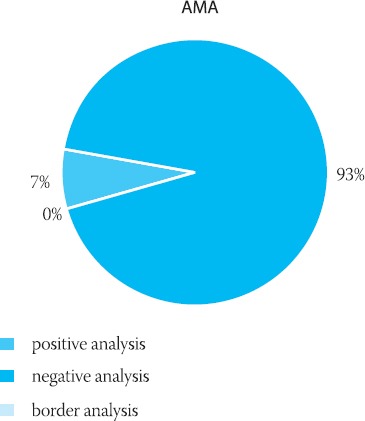
The results obtained by IFA-AMA testing
DIAGRAM 3.
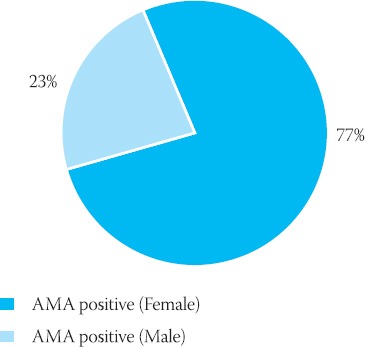
IFA AMA female/male ratio for positive tests
DIAGRAM 4.
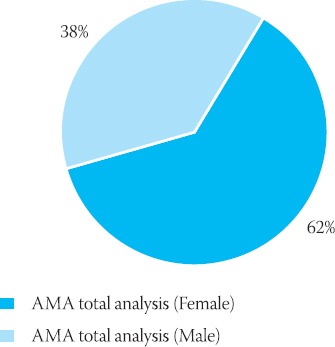
IFA AMA female/male ratio for the total number of analyzed specimens
TABLE 3.
Immunoblot test

Six patients (age from 18 - 67 years) from Clinic for Gastroenterohepathology with positive AMA, were diagnosed with liver disease, as follows:
Patient 1. Dg. Cirrhosis hepatis compensata; Hypertensio portalis
Patient 2. Dg. Cirrhosis hepatis decompensata
Patient 3. Dg. AIH; Cirrhosis hepatis micronodularis inc.
Patient 4. Dg. Cirrhosis hepatis biliaris primaria susp.
Patient 5. Dg. Hepatitis chronica B
Patient 6. Dg. Cirrhosis hepatis decompensata
During the period June 2005 - May 2006, 192 serum specimens were analyzed by Western blot method. Specimens were collected from several Clinics. The following results were obtained: M2 (29 positive samples or 30,5%), LKM-1 (2 positive or 2,1%), LC-1 (13 positive or 20,0%) and SLA/LP (2 positive or 2,1%).
According to our results, diagnosed diseases at corresponding Clinics were: SLE, RA AIH and PBC.
DISCUSSION
Primary biliary cirrhosis (PBC) is a chronic autoimmune disease characterized by the destruction of small intraliver bile ducts. Determination of antimitochondrial antibodies (AMA) is of particular significance for proper diagnosis and PBC management. It is also useful in other cases of liver diseases of unknown etiology. IFA is a gold standard in analyzing suspect cases of AIH, PBC, SLE, Sjogren’s syndrome and rheumatoid arthritis (sensitivity 89% and specificity 98,8%). Also, another test with high sensitivity and specificity (80% and 100%) for the detection of specific PBC autoantigens is ELISA-M2 test. Molecular targets for these autoantibodies are members of 2-oxoacid dehydrogenase complex family of enzymes in the mitochondrial respiratory chain and as well as lipoyl binding domains (E2). In clinical PBC management it is very important to detect these specific autoantigens.
In the suspect cases of liver diseases or autoimmune hepatitis, early and fast detection of following human autoantibodies of the class IgG against four different specific antigens, is very important:
-M2 (pyruvate dehidrogenase complex)
-LKM-1 (liver - kidney microsomes - cytohrome P450 II-D6)
-LC -1 (cytosolic liver antigen type -1)
-SLA/LP (soluble liver antigen/ liver - pancreas antigen).
Diagnostic Western blot method offers the possibilities of fast, highly sensitive, highly specific and simple procedure for the detection of autoantibodies against the above mentioned specific antigens, in either serum or plasma, and gives the chance for timely and successful treatment of patients. Specially designed immmunoblot strips contain parallel lines of antigens purified by affinity chromatography. Specific IgG, IgA and IgM antibodies bind to corresponding antigen site. The detection is based on enzyme-labeled conjugate promoting a color reaction. No cross-reactions with other autoantibodies were found (13, 14, 15). In management of liver autoimmune diseases such as primary biliary cirrhosis (PBC) and autoimmune hepatitis (AIH), it is very important to exclude other causes e.g. viruses, alcohol and drugs. The main criteria for AIH diagnosis are:
-histological findings,
-detection of autoantibodies (ANA, SMA, LKM, SLA/LP),
-hipergammaglobulinaemia,
-negative HB and HCV serology,
-detection of HLA antigens B8, DR or DR4.
At least four of these criteria must be determined for appropriate diagnosis of AIH. If all criteria are present the diagnosis is definite. Final confirmation is achieved through good response of patients to immunosupresive therapy. Circulating autoantibodies have great significance for AIH diagnosis. Because of unclear correlation between the titre and activity or disease prognosis, the role of these antibodies in the AIH pathogenesis remain unclear. The autoimmune hepatitis is mainly specific for women with incidence of 1,9 cases per 100.000 individuals in Western Europe. Without corresponding timely diagnosis and treatment, AIH often progresses to liver cirrhosis. SLA/LP autoantibodies are highly specific for AIH and have not been described in viral hepatitis. Early, highly specific, sensitive and fast diagnosis provides the patient with a chance for normal life. There are three subtypes of AIH:
-subtype I (presence of ANA and SMA autoantibodies)
-subtype II (autoantibodies against LKM-1 antigen)
-subtype III (SLA/LP autoantibodies)
Autoantibodies against LKM-1 can be found in 1-2% of patients with positive hepatitis C serology. For primary biliary liver cirrhosis autoantibodies against M2 are specific. Clinical overlap between patients with progressive systemic sclerosis and patients with primary biliary liver cirrhosis, in the case of M2 autantibodies finding is possible. Six proteins were identified as M2 antigens with following molecular mass: 74, 55, 45, 36, 51 and 51 kDa (16, 17, 18, 19, 20, 21).
Our IFA-anti dsDNA, AMA, imunobloting analyses of serum specimens, as a part of AIH PBC, SLE diagnostic approach show significant correlation with final clinical diagnosis of these diseases.
CONCLUSION
During one year, we analyzed clinical blood specimens for the presence of specific autoantibodies against M2, LKM-1, LC-1 and SLA/LP antigens by Western blot method (Euroimmun). In combination with other analysis such as histological findings, antinuclear antibodies (ANA), smooth muscle cells antibodies (SMA) and other testing, it is possible to perform fast and timely diagnosis of AIH and PBC.
The results obtained after IFA-AMA and IFA-anti-dsDNA testing has improved diagnostics of AIH, PBC, SLE and RA. Among other diagnostic tools they are important parameters in early diagnostics of these diseases as well as a part of continuing process of monitoring the therapy and its effects.
LIST OF ABBREVIATIONS
AIH - autoimmune hepatitis
AMA - antimitochondrial autoantibodies
ANA - antinuclear antibodies
B8 - antigen
dsDNA - double stranded DNA
HB - hepatitis B negative and serology
HCV - hepatitis C virus detection
HLA - human leukocyte antigen
IFA - immunofluorescence test
IgG FITC - IgG antibodies conjugated with fluorescin isothiocyanate
LC -1 - cytosolic liver antigen type -1
LKM-1 - liver - kidney microsomes - cytohrome P450 II-D6
M2 - pyruvate dehidrogenase complex
PBC - primary biliary cirrhosis
PBS - buffer
SLA/LP - soluble liver antigen/ liver -pancreas Antigen
SLE - Systemic Lupus eritematosus
SMA - Anti-Smooth Muscle
REFERENCES
- 1.Karamehić J, Dizdarević Z. Klinička imunologija, Sistemski lupus eritematodes. Svjetlost-Sarajevo. 2007:179–187. [Google Scholar]
- 2.Aarden L.A, de Groot E.R, Feltkamp T.E.W. Immunology of DNA III Crithidia lucillae, a simple substrate for the determination of anti ds DNA with immunofluorescence technique. Ann. NY Acad. Sci. 1975;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- 3.Konstantinov K.N, Russanova VR. Evidence for absence of histones in the Crithidia lucillae kinetoplast: Study with anti-DNA and monoclonal anti-Hb antibodies. British J Dermatol. 1987;117:451–456. doi: 10.1111/j.1365-2133.1987.tb04924.x. [DOI] [PubMed] [Google Scholar]
- 4.Kamradt T, Mitchison N. Tolerance and autoimmunity. Adv. Immunol. 2001;344 (9):655–664. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 5.Karamehić J, Dizdarević Z, et al. Klinička imunologija, Kliničko laboratorijski imunološki testovi. Svjetlost-Sarajevo. 2007:757–776. [Google Scholar]
- 6.Stingl G, et al. An immunofluorescence procedure for determination of antibodies to native duble strained DNA and circulating DNA-anti-DNA complexs. Clinical immunology and immunopathology. 1976;6:131–146. doi: 10.1016/0090-1229(76)90103-3. [DOI] [PubMed] [Google Scholar]
- 7.Fritzler M.J, Manns M.P. Anti-mitochondrial antibodies. Clin. App. Immunol. Rev. 2002;3:87–113. [Google Scholar]
- 8.Lapierre P, Hajaori O, Hamberg J.C, Alvarez F. Formimin transferase cyclodeaminase is an organ specific autoantigen recognised by sera of patients with autoimmune hepatitis. Gastroenterology. 1999;115:643–649. doi: 10.1016/s0016-5085(99)70186-1. [DOI] [PubMed] [Google Scholar]
- 9.Blake M.S, Johnston K.H, Russel-Jones G.J, Gotschlich E.C. A rapid, sensitive method for detection of alkaline phosphatase conjugated antibody on Western blots. Anal. Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 10.Burnette W.N. Western bloting: Electrophoretic transfer of proteins from sodium dodecyl sulphate-polyacrilamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein. A. Anal. Biochem. 1981;112:195. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- 11.EUROIMMUN Test instructions for liver - profile M2 LKM-1 LC-1 and SLA/LP (IgG) Lubeck Germany: 2003. pp. 1–10. [Google Scholar]
- 12.Subašić Đ. Molekularna biologija-primjena u medicini i transgenetici. Klinički centar Univerziteta u Sarajevu. 2006:63–65. [Google Scholar]
- 13.Weis I, Brunner S, Hennuger J, Herkel J, Kanzler S, Meyer zum B, üschenfelde K.H, Lohse A.W. Anti SLA/LP as autoimmune hepatitis specific autoantibodies: identification of the target antigen. Lancet. 2000;355:1510–1515. doi: 10.1016/s0140-6736(00)02166-8. [DOI] [PubMed] [Google Scholar]
- 14.Obermayer-Strauss P, Strassburg C.P, Manns M.P. Autoimmune hepatitis. J Hepatol. 2000:181–197. doi: 10.1016/s0168-8278(00)80425-0. [DOI] [PubMed] [Google Scholar]
- 15.Robertson E.A, Zweig M.H, van Steirteghem A.C. Evaluating the clinical efficacy of laboratory tests. Am. J. Clin. Pathol. 1983;79:78–86. doi: 10.1093/ajcp/79.1.78. [DOI] [PubMed] [Google Scholar]
- 16.Silverstein A, Bloand B.J. Conceptual framework for evaluation laboratory tests: casefinding in ambulatory patients. Clin. Chem. 1994;40:1621–1627. [PubMed] [Google Scholar]
- 17.Topić E, Primorac D, Janković S. Medicinskobiokemijska dijagnostika u kliničkoj praksi-Bolesti jetre. Medicinska naklada, Zagreb. 2004:40–61. [Google Scholar]
- 18.Andreis I. Imunologija. 2004:176–237. [Google Scholar]
- 19.Abbas A.K, Lichtman A.H. Immunologic Tolerance and Autoimmunity. Second Edition. USA: Elsevier Scientific Publications; 2004. Basic Immunology-functions and disorders of the immune system; pp. 161–176. [Google Scholar]
- 20.Strassburg C.P, Obermayer-Straub P, Manns M.P. Autoimmunity in liver diseases. M. Clin. Rev. Allergy Immunol. 2000;18:127–139. doi: 10.1385/CRIAI:18:2:127. [DOI] [PubMed] [Google Scholar]
- 21.Mackay I.R. Autoimmunity and primary biliary cirrhosis. Bailliéres Clin. Gastroenterol. 2000:519–533. doi: 10.1053/bega.2000.0101. [DOI] [PubMed] [Google Scholar]


