Abstract
Background
Implantable cardioverter defibrillators (ICD) decrease mortality in selected patients with advanced heart failure and have been associated with reduced mortality in patients with pulsatile left ventricular assist devices (LVAD). However it is unclear whether that benefit extends to patients with contemporary continuous-flow LVAD (CF-LVAD).
Objectives
To determine if ICD presence provided a mortality benefit during CF-LVAD support.
Methods
Propensity score matching was used to generate a cohort of patients with similar baseline characteristics. The primary outcome was freedom from death during LVAD support. Secondary endpoints included freedom from unexpected death, likelihood of transplantation & recovery, and adverse events.
Results
Among 16,384 eligible patients in the INTERMACS registry, 2,209 patients with an ICD and 2,209 patients without one had similar propensity scores and were included. The presence of an ICD was associated with an increased mortality risk (Hazard Ratio 1.20, 95% Confidence Interval [CI] 1.04–1.39, p=0.013) and an increased risk of unexpected death during device support (HR 1.33, 95% CI 1.03–1.71, p=0.03). Patients with an ICD were more likely to undergo transplantation (HR 1.16, 95% CI 0.99–1.35, p=0.06) and less likely to have LVAD explant for recovery (HR 0.53, 95% CI 0.29–0.98, p=0.04). Patients with an ICD had a higher rate of treated ventricular arrhythmias (Rate ratio [RR] 1.27 95% CI 1.10–1.48, p=0.001) and rehospitalization (RR 1.08, 95% CI 1.04–1.12, p<0.0001), but rates of hemorrhagic stroke were similar (RR 1.01, 95% CI 0.81–1.26, p=0.98).
Conclusions
Among patients with a CF-LVAD, the presence of an ICD was not associated with reduced mortality.
Keywords: Implantable cardioverter-defibrillator, left ventricular assist device, mortality, arrhythmia, transplantation, heart failure
Introduction
Heart failure (HF) affects over five million in the United States, with 250,000 advancing to Stage D. (1) Continuous-flow left ventricular assist devices (CF-LVAD) are now the most common form of durable support for Stage D HF, with more than 2,500 implants annually in the United States and a one-year survival of about 80%. (2) Ventricular arrhythmias (VA) are common in this population as one third of ambulatory patients with advanced HF experience VA. (3) Implantable cardioverter defibrillators (ICD) reduce the risk of mortality in appropriately selected patients and the HRS/ACC/AHA guidelines provide a Class I recommendation for ICD therapy for NYHA Class II and III patients with a left ventricular ejection fraction less than 35%. (4) However ICD therapy is not indicated (Class III recommendation) for NYHA Class IV patients with drug-refractory HF who are not candidates for heart transplantation or patients with less than one year of life expectancy. (4) Most major societal guidelines do not address ICD use in LVAD patients (4,5). The International Society for Heart and Lung Transplantation’s (ISHLT) 2013 guidelines for mechanical circulatory support provide a Class I recommendation to reactivate an ICD following LVAD surgery and a Class IIa recommendation for ICD placement after LVAD for those without one. (6) Two recent studies involving the UNOS registry (7) and a meta-analysis of previously published studies (8) found a 19% and 39% relative risk reduction in death associated with ICD use during device support respectively; however both included patients with the previous generation pulsatile LVAD in addition to current generation CF-LVAD. A propensity score matched analysis limited to CF-LVAD patients implanted with a bridge to transplant strategy in the UNOS registry found that the presence of an ICD was not associated with a survival advantage during device support. (9) However, this study was limited to patients listed for transplantation and lacked a number of covariates of interest (e.g. arrhythmia history and anti-arrhythmic medication use). We therefore sought to use the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) registry to determine if the presence of an ICD was associated with a mortality benefit during CF-LVAD support.
Methods
Patient Selection
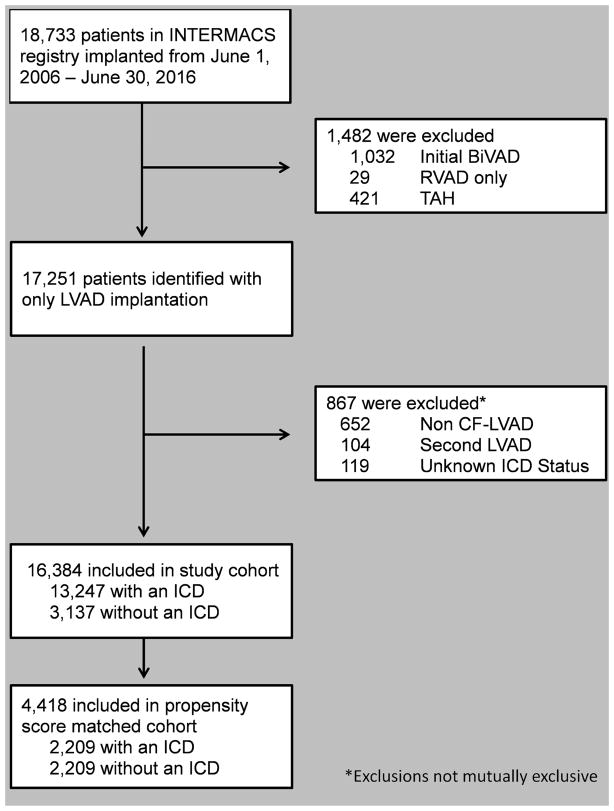
Data for this study was obtained from the INTERMACS registry, funded by the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services under Contract No. HHSN268201100025C. The INTERMACS registry is a prospective national registry of over 19,000 patients supported with Food and Drug Administration (FDA) approved durable mechanical circulatory support devices and has previously been described elsewhere. (10) The INTERMACS Data and Clinical Coordinating Center and each participating institution have received institutional review board/ethics review board approval for either active informed consent or a waiver of consent to enroll participants, link data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act (HIPAA) compliant and INTERMACS has received a Federal Certificate of Confidentiality and other protection for the identities of patients and devices identified within the Registry. Analysis of the INTERMACS registry was performed for all patients who received a CF-LVAD between June 1, 2006 and June 30, 2016. 18,733 adult candidates (age ≥18 years) who received a durable CF-LVAD were identified (Figure 1). Patients who received a biventricular assist device at time of implantation, right ventricular assist device only, or total artificial heart were excluded from the analysis. Similarly, patients with an unknown ICD status, pulsatile device, and those receiving their second LVAD were excluded. Patients were analyzed from the date of LVAD implantation to transplant, death, or device explant for recovery. The primary outcome was freedom from death while on LVAD support. Secondary endpoints included freedom from unexpected death, likelihood of transplantation & recovery, and adverse events including arrhythmia, stroke (hemorrhagic), and infection. Pre-specified subgroup analyses were performed for patients who were suspected to derive the greatest benefit from an ICD.
Figure 1.
Study population
Propensity Score Matching
The ICD and non-ICD cohorts significantly differed in baseline characteristics (Table 1). In order to create more comparable groups of patients, propensity score matching was performed based on covariates (selected a priori) available in the INTERMACS registry. The propensity score was calculated using a non-parsimonious multivariable logistic regression model including clinical (etiology of heart failure, duration of heart failure, device strategy at implantation, type of device, INTERMACS profile at implantation, recurrent ventricular tachyarrhythmias [frequent shocks from ICD or requirement for external defibrillator, usually more than twice weekly], NYHA Class, intravenous inotrope use, IABP use, ECMO use, ventilator use, amiodarone use, beta blocker use, BMI, MELD-XI [surrogate for RV and hepatic dysfunction], severe diabetes, GFR, serum sodium, serum albumin, history of smoking, peripheral vascular disease, pulmonary hypertension, pulmonary disease, ability to work, previous cardiac surgery) and demographic characteristics (age, sex, ethnicity, implanting center volume). Notable baseline covariates with excessive missing data precluding inclusion in the propensity score were right ventricular function and hemodynamic data. Patients were matched 1:1 using a greedy matching algorithm (nearest match without replacement) based on the propensity score of each patient. A caliper width of 20% of the standard deviation of the logit of the propensity score was used (eliminating 99% of the bias due to measured confounding variables). (11) An absolute standardized difference of less than 10% was considered to represent relative balance. (12)
Table 1.
Patient characteristics of the entire cohort and propensity score matched cohort.
| Entire Cohort | Propensity Score Matched Cohort | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| ICD (n=13,247) | No ICD (n=3,137) | ASD | ICD (n=2,209) | No ICD (n=2,209) | ASD | |
| Male (%) | 79.7 | 74.2 | 27.0% | 74.8 | 76.0 | 2.7% |
|
| ||||||
| Age Group (%) | 34.0% | 4.4% | ||||
| 19–29 years | 3.2 | 9.7 | 8.1 | 7.4 | ||
| 30–39 years | 6.7 | 9.4 | 9.4 | 9.0 | ||
| 40–49 years | 14.0 | 15.5 | 14.1 | 14.9 | ||
| 50–59 years | 27.5 | 28.4 | 27.7 | 27.2 | ||
| 60–69 years | 34.2 | 27.8 | 29.4 | 30.0 | ||
| 70–79 years | 13.8 | 8.7 | 10.5 | 10.8 | ||
| 80+ years | 0.6 | 0.5 | 0.8 | 0.7 | ||
|
| ||||||
| Ethnicity (%) | 27.0% | 2.7% | ||||
| Hispanic | 5.8 | 7.6 | 8.4 | 7.5 | ||
| Non-Hispanic | 94.2 | 92.4 | 91.6 | 92.5 | ||
|
| ||||||
| Etiology (%) | 16.9% | 5.1% | ||||
| Ischemic | 44.3 | 52.4 | 50.7 | 48.6 | ||
| Non-ischemic | 54.3 | 45.9 | 47.3 | 49.7 | ||
| Congenital | 0.4 | 0.5 | 0.4 | 0.4 | ||
| Restrictive | 1.0 | 1.2 | 1.6 | 1.3 | ||
|
| ||||||
| Duration of Heart Failure (%) | 114.0% | 8.9% | ||||
| <1 month | 1.0 | 23.8 | 5.3 | 5.3 | ||
| 1 month-1 year | 6.4 | 27.3 | 28.6 | 26.8 | ||
| 1–2 years | 6.6 | 7.0 | 11.5 | 9.4 | ||
| >2 years | 86.0 | 41.9 | 54.6 | 58.4 | ||
|
| ||||||
| Strategy at Implantation (%) | 33.0% | 2.8% | ||||
| Bridge to Transplant | 28.6 | 20.2 | 23.2 | 23.2 | ||
| Destination Therapy | 42.4 | 37.1 | 39.9 | 40.1 | ||
| Bridge to Candidacy | 28.7 | 40.4 | 36.0 | 35.5 | ||
| Other | 0.3 | 2.3 | 0.9 | 1.2 | ||
|
| ||||||
| Type of Device (%) | 2.0% | 2.0% | ||||
| Axial Flow | 83.8 | 82.9 | 83.8 | 83.2 | ||
| Centrifugal Flow | 16.2 | 17.1 | 16.2 | 16.8 | ||
|
| ||||||
| INTERMACS Profile (%) | 57.9% | 5.9% | ||||
| 1 | 10.9 | 33.0 | 21.9 | 21.3 | ||
| 2 | 37.2 | 32.7 | 37.8 | 36.5 | ||
| 3 | 33.0 | 24.5 | 29.2 | 29.5 | ||
| 4 | 14.6 | 7.4 | 8.3 | 9.8 | ||
| 5–7 | 4.3 | 2.4 | 2.8 | 2.9 | ||
|
| ||||||
| Recurrent VA (%) | 28.5 | 21.5 | 55.0% | 21.7 | 22.0 | 1.5% |
|
| ||||||
| NYHA Class IV (%) | 79.1 | 85.7 | 18.0% | 83.9 | 83.2 | 3.7% |
|
| ||||||
| IV Inotrope Use (%) | 80.9 | 82.6 | 21.0% | 84.1 | 82.8 | 3.6% |
|
| ||||||
| IABP (%) | 27.6 | 41.9 | 30.0% | 37.3 | 35.9 | 3.0% |
|
| ||||||
| ECMO (%) | 1.8 | 11.0 | 39.0% | 5.3 | 5.2 | 0.4% |
|
| ||||||
| Ventilator Use (%) | 7.1 | 26.6 | 54.0% | 17.5 | 16.1 | 3.8% |
|
| ||||||
| Amiodarone Use (%) | 16.0% | 2.4% | ||||
| Current | 42.8 | 35.3 | 35.7 | 36.2 | ||
| Within the last year | 5.8 | 5.2 | 4.7 | 5.2 | ||
| No | 51.4 | 59.5 | 59.6 | 58.6 | ||
|
| ||||||
| Beta-blocker Use (%) | 46.7% | 5.0% | ||||
| Current | 57.4 | 39.1 | 43.6 | 45.5 | ||
| Within the last year | 24.7 | 23.5 | 26.5 | 26.8 | ||
| No | 17.9 | 37.4 | 29.9 | 27.7 | ||
|
| ||||||
| BMI | 28.9±7.1 | 27.7±6.7 | 16.8% | 27.7±6.8 | 27.8±6.9 | 1.0% |
|
| ||||||
| MELD-Xi | 14.7±4.5 | 14.2±5.0 | 11.0% | 14.5±4.7 | 14.5±4.8 | <0.1% |
|
| ||||||
| Severe Diabetes (%) | 4.0 | 4.6 | 1.0% | 4.5 | 4.2 | 2.5% |
|
| ||||||
| GFR (mL/min/1.73m2) (%) | 30.2% | 5.1% | ||||
| > 60 | 44.4 | 55.0 | 53.9 | 52.2 | ||
| 30–60 | 46.6 | 33.4 | 34.6 | 37.0 | ||
| < 30 | 6.7 | 6.4 | 6.9 | 6.6 | ||
| Requiring dialysis | 2.3 | 5.2 | 4.6 | 4.2 | ||
|
| ||||||
| Sodium (mmol/L) | 134.8±4.7 | 135.2±5.0 | 8.0% | 134.9±4.9 | 134.8±4.8 | 2.4% |
|
| ||||||
| Albumin (g/dL) | 3.5±0.6 | 3.2±0.7 | 8.0% | 3.3±0.7 | 3.3±0.6 | 2.4% |
|
| ||||||
| History of Smoking (%) | 6.2 | 10.9 | 21.0% | 9.5 | 9.2 | 3.6% |
|
| ||||||
| Peripheral Vascular Disease (%) | 2.8 | 2.6 | 21.0% | 2.6 | 2.9 | 3.6% |
|
| ||||||
| Pulmonary HTN (%) | 11.1 | 6.8 | 21.0% | 8.3 | 8.6 | 3.6% |
|
| ||||||
| Pulmonary Disease (%) | 3.8 | 3.4 | 21.0% | 3.8 | 3.9 | 3.6% |
|
| ||||||
| Unable to Work (%) | 52.1 | 38.2 | 28.3% | 43.9 | 45.0 | 2.0% |
|
| ||||||
| Previous Cardiac Operation (%) | 64.7 | 70.0 | 18.0% | 67.8 | 68.1 | 2.0% |
|
| ||||||
| Implanting Center Volume (%) | 3.2% | 3.1% | ||||
| 1–10 | 8.1 | 7.6 | 7.1 | 7.6 | ||
| 11–20 | 16.5 | 16.6 | 16.3 | 16.7 | ||
| 21–30 | 18.4 | 17.6 | 17.9 | 17.0 | ||
| 31–50 | 33.6 | 34.7 | 34.7 | 34.6 | ||
| >50 | 23.4 | 23.5 | 24.0 | 24.1 | ||
ASD=Absolute standardized difference, BMI=Body mass index, ECMO=Extracorporeal Membrane Oxygenation, GFR=Glomerular filtration rate, HTN=Hypertension, IABP=Intra-aortic balloon pump, INTERMACS=Interagency Registry for Mechanically Assisted Circulatory Support, MELD-XI=Model for End-stage Liver Disease score excluding INR, NYHA=New York Heart Association, VA=Ventricular arrhythmia
Statistical Analysis
Demographic and clinical variables were expressed as mean (± standard deviation) for continuous variables and count (with percentage) for categorical variables. Missing data were assumed to be missing completely at random and handled with multiple imputation using a Markov chain Monte Carlo method to generate ten imputations. Absolute standardized differences were estimated for all the baseline covariates (between those with and without an ICD) before and after matching to assess group balance. ICD group comparisons were made with McNemar’s test and the Wilcoxon rank-sum test where appropriate. Kaplan-Meier survival analysis and Cox proportional-hazards regression (stratifying on the matched pairs) were performed to determine if survival differed by ICD status. Competing risk analysis of transplantation, death, and recovery were estimated using cause specific hazard regression. Subgroup analyses were performed sub-setting the entire cohort based on pre-specified groups (implant strategy, age [60 years], etiology of HF, duration of HF, INTERMACS profile, recurrent VT, amiodarone use, beta blocker use, type of device, and duration of LVAD support) and then performing Cox proportional-hazards regression, adjusting for the group propensity score. The rates of adverse events were compared with rate ratios. Sensitivity analyses were performed to determine whether there was a consistency of the findings using alternative methods. These included using the entire cohort through stratification into quintiles by propensity score (13), adjusted Cox proportional-hazards regression (using the propensity score and ICD status only to avoid collinearity) (14), and both combined, which has been suggested to be superior to matching alone for estimating the treatment effect. (15) A two-tailed p-value of less than 0.05 was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina). Figures were created using SAS version 9.4 (SAS Institute, Inc., Cary, North Carolina) and R version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 16,384 patients met study entry criteria, of which 13,247 (80.9%) had an ICD and 3,137 (19.1%) did not. The baseline characteristics for the study cohort differed in most baseline characteristics as demonstrated by an absolute standardized difference (ASD) of greater than 10% (Table 1). The greatest differences (ASD>40%) were in duration of HF, INTERMACS profile, recurrent VAs, ventilator use, and beta blocker use. Propensity score matching generated a cohort of 4,418 patients; 2,209 patients with and 2,209 patients without an ICD. The ASD was less than 10% for all baseline characteristics, indicating suitable matching (Table 1).
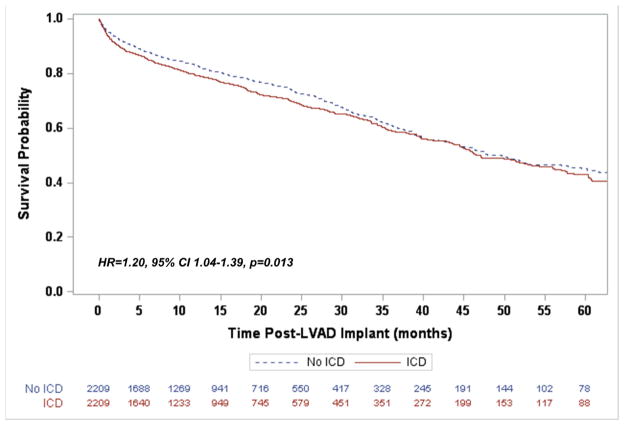
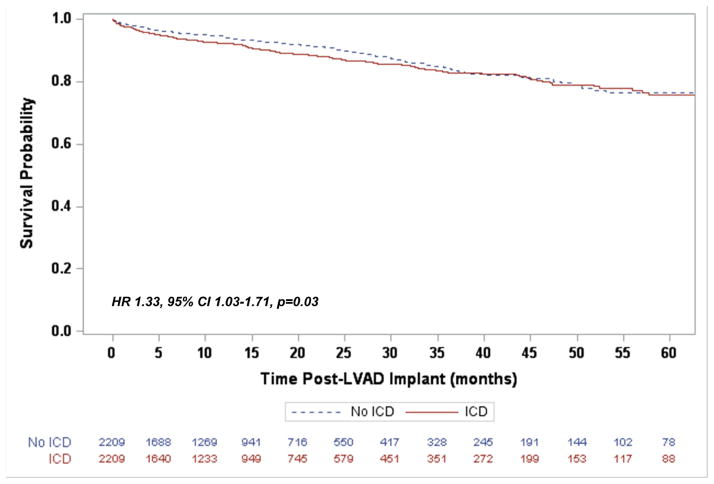
In the matched cohort the median duration of CF-LVAD support was similar for both the ICD and non-ICD groups (12.3 months [IQR 4.7–25.8] vs. 12.5 months [IQR 5.3–24.9], p=0.63). The presence of an ICD was associated with a 20% greater risk of death during LVAD support when compared to those without an ICD (95% Confidence Interval [CI] 1.04–1.39, p=0.013, Figure 2). Recognizing that an ICD mitigates the risk of sudden and unexpected death, a subsequent survival analysis was performed for freedom from unexpected death. This demonstrated that again ICDs were not associated with a decreased risk, but rather an increased risk of unexpected death (Hazard Ratio [HR] 1.33, 95% CI 1.03–1.71, p=0.03, Figure 3). An exploratory analysis limiting the mode of death to only "Circulatory: Sudden Unexplained Death" and "Circulatory: Cardiac Arrhythmia" identified 37 (1.67%) deaths in the ICD group and 46 (2.08%) in the No ICD group. In this group there was a non-significant reduction in risk over time (HR 0.81, 95% CI 0.43–1.53, p=0.52). While cause of death did not differ overall between the two groups (X2 p=0.23), withdrawal of support, neurological dysfunction, and multi-system organ failure account for a majority of the increased mortality in the ICD group (Supplemental Table 1).
Figure 2.
Freedom from death following LVAD implantation for the propensity score matched cohort
Figure 3.
Freedom from unexpected death following LVAD implantation for the propensity score matched cohort
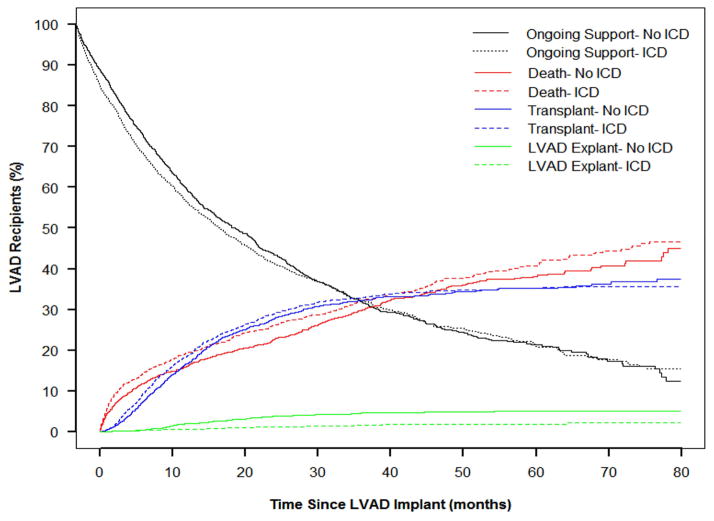
A cause-specific hazard model was created treating death, transplantation, and LVAD explant for recovery as competing events (Figure 4). The likelihood of transplantation was 16% greater in those with an ICD, although this finding only approached statistical significance (95% CI 0.99–1.35, p=0.06). LVAD explant for recovery, however, was less likely to occur in those with an ICD (HR 0.53, 95% CI 0.29–0.98, p=0.04).
Figure 4.
Competing outcomes curves for events terminating device support. Outcome events are mutually exclusive
Adverse Events
There was a greater rate of any arrhythmia in the ICD group during device support (Rate Ratio [RR] 1.11, 95% CI 1.01–1.22, p=0.03, Table 2). This trend was largely attributable to a 27% increased rate of VA requiring defibrillation or cardioversion (95% CI 1.10–1.48, p=0.001). There was no difference in infection, bacteremia, neurologic dysfunction, or hemorrhagic stroke. However, there was an increased rate of rehospitalization in the ICD group (RR 1.08, 95% CI 1.04–1.12, p<0.0001).
Table 2.
Adverse events while on device support.
| ICD | NO ICD | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Patients (n, %) | Events (n) | Annual Rate | Patients (n, %) | Events (n) | Annual Rate | Rate Ratio (95% CI) | p-value | |
| Any Arrhythmia | 570 (24.8) | 921 | 0.28 | 541 (24.5) | 816 | 0.25 | 1.11 (1.01–1.22) | 0.03 |
|
| ||||||||
| Ventricular arrhythmia requiring defibrillation/cardioversion | 274 (12.4) | 406 | 0.12 | 226 (10.2) | 314 | 0.10 | 1.27 (1.10–1.48) | 0.001 |
|
| ||||||||
| Any Infection | 1,046 (47.4) | 2169 | 0.65 | 992 (44.9) | 2100 | 0.64 | 1.02 (0.96–1.08) | 0.57 |
|
| ||||||||
| Bacteremia | 353 (16.0) | 511 | 0.15 | 346 (15.7) | 482 | 0.15 | 1.04 (0.92–1.19) | 0.51 |
|
| ||||||||
| Rehospitalization | 1,602 (72.5) | 6,399 | 1.92 | 1,584 (71.7) | 5,854 | 1.79 | 1.08 (1.04–1.12) | <0.0001 |
|
| ||||||||
| Neurological Dysfunction | 509 (23.0) | 664 | 0.20 | 473 (21.4) | 620 | 0.19 | 1.06 (0.94–1.18) | 0.35 |
|
| ||||||||
| Hemorrhagic Stroke | 161 (7.3) | 172 | 0.05 | 1.57 (7.1) | 168 | 0.05 | 1.01 (0.81–1.26) | 0.98 |
|
| ||||||||
| Device Malfunction and/or Pump Thrombosis | 487 (22.1) | 790 | 0.24 | 497 (22.5) | 777 | 0.24 | 1.00 (0.91–1.11) | 0.99 |
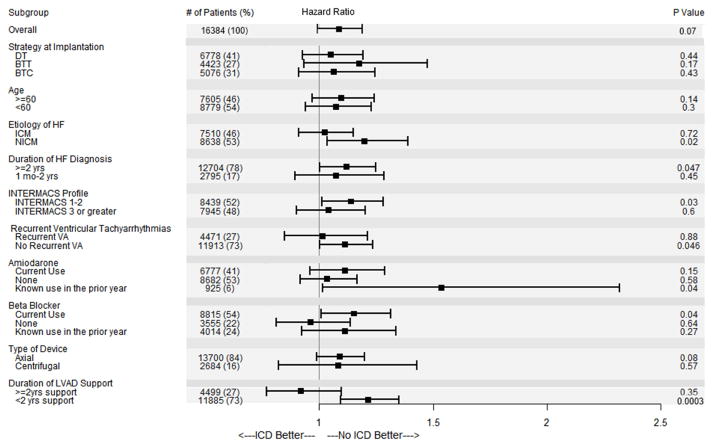
Subgroup Analysis
An exploratory subgroup analysis was performed to investigate for effect modification by a priori selected covariates (Figure 5). Tests for interaction failed to demonstrate heterogeneity of treatment effect among subgroups (p=0.09–0.99). Only two subgroups (LVAD support ≥ 2 years & no beta blocker use) had a point estimate for the HR favoring ICD use, though neither approached significance. Conversely a number of subgroups found an association between improved survival and the No ICD group: those with a NICM, chronic HF, INTERMACS profile 1–2, no recurrent pre-implant VA, current beta blocker use, amiodarone use in the prior year, and device support of less than two years. However, only duration of LVAD support of less than two years remained significant after adjustment for multiple comparisons.
Figure 5.
Risk of death for individual subgroups. Note: Interaction testing was not significant for any subgroup.
Sensitivity Analysis
Recognizing that despite adequate matching on measured confounders there may be confounding by unmeasured covariates, falsification-hypothesis analysis was performed to assess for potential confounders to explain the primary outcome. This analysis evaluated the matched cohort for the incidence of LVAD malfunction and/or device thrombosis, which was not anticipated to be impacted by ICD status. This analysis demonstrated no significant difference (RR 1.00, 95% CI 0.91–1.11, p=0.99). Additional sensitivity analyses were performed for the full study cohort of 16,384 using the propensity score to stratify and adjust a Cox proportional hazard model. This analysis was consistent with the matched cohort, failing to demonstrate an association between ICD use and decreased risk of mortality during LVAD support using adjustment (HR 1.09, 95% CI 0.99–1.19, p=0.07), quintile stratification (HR 1.09, 95% CI 1.00–1.19, p=0.05), and stratification and adjustment (HR 1.09, 95% CI 0.99–1.19, p=0.07).
Discussion
CF-LVADs improve survival and quality of life of Stage D HF patients. Within six months of implantation over 80% of patients have NYHA Class I or II symptoms and over 97% have less than NYHA Class IV symptoms. (16) VAs remain common following LVAD implantation and as many as half will experience VAs. (17) Further, increases in transplant waitlist times (18) and increased use of LVADs as DT (nearly 50%) (2) have resulted in longer durations of device support. While ICDs are often present at the time of LVAD surgery (80.9% in this study), clinicians are still left with a decision regarding ICD implantation in one of five LVAD patients. Furthermore, for those with ICDs generator changes may be required after LVAD implantation. Many current societal guidelines (ACC/AHA/HRS & ESC) do not specifically address LVADs, but the ISHLT provides a Class IIa recommendation for ICD implantation in LVAD patients without one. (6) Our study examined the impact of ICDs in patients with CF-LVAD and found that during a median follow-up the presence of an ICD was not associated with improved survival during LVAD support during a median follow-up of 12.4 months.
Prior single center studies have lacked adequate statistical power and other registry analyses have lacked key information to conclusively examine the effect of ICDs in patients with CF-LVAD. The present analysis utilized the largest LVAD registry to attempt to answer this question, and following propensity score matching generated a cohort with nearly as many patients without an ICD (n=2,209) as were enrolled in MADIT, MADIT II, SCD-HeFT (placebo arm), DINAMIT, MUSTT, and DEFINITE combined (n=2,362). (19–24) Analysis of this large cohort with a median duration of follow-up of just over a year failed to detect any evidence that the presence of an ICD improved survival. Interestingly, ICD presence was associated with a statistically significant 20% increase in mortality rates in the propensity score matched cohort. This may be in part the result of propensity score matching, which resulted in an ICD group that had decreased prevalence of certain VA risk factors (−7% prior VA, −31% HF >2 years, −14% beta blocker use, −7% amiodarone use) compared to the complete cohort. This was reflected in the sensitivity analyses of the entire study cohort, which found a non-significant 9% increased risk of death in the ICD group. Furthermore, exploratory analyses were performed looking at subgroups that may be at the highest risk (ischemic cardiomyopathy, prior VA, requirement of anti-arrhythmic medication, longer duration of HF, and prolonged LVAD support) to try to identify a specific group that may derive benefit. Importantly, in no subgroup was the presence of an ICD associated with improved survival, including those with a prior VA (Figure 5).
Post-LVAD arrhythmic events were common in this study as nearly one quarter of patients in both arms experienced significant arrhythmias during device support. Focusing specifically on treated VA, the rate was 27% greater in patients with an ICD. Despite this increase in treated VA, there was not an association of improved survival with ICDs. Importantly, only clinically reported treated VA were captured in the registry, which is the likely cause of a lower prevalence of VA in this cohort compared with prior studies with more specific patient level data (e.g. from routine ICD interrogation). Further, the nature of the dataset precludes the ability to determine whether the rate of VA was actually greater in those with an ICD or if those patients simply had more treated VA because the ICD delivered therapies for VA that might have resolved spontaneously. Additionally, there was an increased rate of rehospitalization in the ICD group, and while speculative, it is possible that ICD discharge was a potential driver of the increased hospital utilization.
ICDs not only function to prevent arrhythmic death, but they also may help prevent syncope and its associated head trauma during cerebral hypoperfusion from the cardiac arrhythmia. The latter is especially of concern for LVAD patients who are routinely on therapeutic anticoagulation and aspirin. Reassuringly there was no difference in the rate of neurological dysfunction and specifically hemorrhagic stroke.
Our findings differ from prior studies reporting an associated survival benefit for patients with an ICD and a pulsatile LVAD (7,8,25,26), but are consistent with a previous analysis of bridge to transplant patients supported by CF-LVAD in the UNOS registry. (9) This difference is mechanistically plausible, as pulsatile pumps may be more dependent of native cardiac activity than continuous flow pumps. As previously mentioned medically-treated HF patients often develop cerebral hypoperfusion and hemodynamic compromise during VA. While cardiac output may be reduced during periods of VA, this reduction may be less likely to cause hemodynamic collapse in the presence of a CF-LVAD; indeed, prolonged VA have been reported (27,28) during which the CF-LVAD maintains systemic perfusion likely through Fontan-like physiology.
It is important to note that data on RV function and PVR, which would impact Fantan-like physiology, were not available for this study. While many of these events may resolve spontaneously, this degree of hemodynamic support allows CF-LVAD patients with a VA that does not resolve (with or without ICD) to present for arrhythmia treatment. (17) Alternatively the lack of mortality benefit from an ICD may be artifact due to confounding by a factor not measured in the INTERMACS registry. While possible, an association between ICD use and improved survival was missing from both the primary analysis and all sensitivity analyses, suggesting this may be less likely. Lastly, it is possible that ICD firing to terminate VA that might have terminated spontaneously is detrimental in patients with a CF-LVAD. ICD discharge has been linked to echocardiographic RV dysfunction (29) and when recurrent, may precipitate RV failure. (30) Unfortunately specific information about ICD discharge was not available in the INTERMACS registry and this could not be examined. It is important to underscore the fact that these data do not support deactivation of ICD therapies after LVAD implant. Furthermore, for patients who have received appropriate shocks whether before or after LVAD-implant, we believe maintenance of ICD therapy is important to prevent the morbidities associated with prolonged VA (most notably RV failure). As such, we advocate for generator changes in patients with prior VA if required after LVAD implantation. However, we do believe these findings may put into question the need for primary prevention ICDs in patients with a CF-LVAD.
Well conducted trials in non-LVAD patients have demonstrated that more permissive ICD programming can be beneficial. The 1902 patient randomized ADVANCE III trial demonstrated that prolonged detection intervals decreased therapies delivered and inappropriate shocks with no difference in mortality or arrhythmic syncope. (31) MADIT-RIT found an increased rate threshold (>200 bpm, after a 2.5-second monitoring delay) and delayed therapy (60 seconds with HR 170–199, 12 seconds HR>200 bpm) were both associated with reductions in inappropriate therapy and all-cause mortality, while not increasing syncope. (32) We propose that these data, in combination with this study, provide an impetus to compare standard ICD therapy to more permissive ICD settings (combination of increased threshold and delayed therapy) among CF-LVAD patients.
Limitations
As a result of the observational nature, this study is limited in its ability to determine causation. The INTERMACS registry that was used is of high-quality, though our analysis was limited to the data collected which might have omitted confounders. ICD interrogation data were not available, resulting in probable underreporting of ICD therapy (anti-tachycardia pacing) and an unknown frequency of inappropriate shocks. Also absent were individual patient ICD settings limiting our ability to comment on ICD programming. The registry also does not specifically include data on de novo ICD implantation after LVAD, creating the possibility of one-way patient crossover and introduction of bias. In this study none of the patients were reported to be hospitalized for ICD implantation and in the UNOS MCS registry fewer than 8% of patients without an ICD at the time of listing went on to receive one (9), suggesting the amount of crossover was unlikely to have been large. The data available also precluded inclusion of right ventricular function and pulmonary vascular resistance in the propensity score, post- implant anti-arrhythmic drug use, and post-LVAD implantation hospitalizations for syncope. While propensity score matching with a caliper width of 20% of standard deviation of the logit of the propensity score eliminates 99% of the bias due to measured confounding variables (11), this technique is unable to account for unmeasured confounders. Lastly, in an effort to completely explore this topic a number of secondary analyses and subgroup analyses were performed. Some of these had significant p-values (p<0.05), however only a few would persist after adjustment for multiple testing (e.g. Bonferroni correction, Hochberg step-up, or Holm step-down method)
Conclusion
The presence of an ICD was not associated with a decrease in mortality among patients with a CF-LVAD.
Supplementary Material
COMPETENCY IN PATIENT CARE.
Nearly 1 in 5 patients who receive a CF-LVAD do not have an ICD. ICDs were not found to be associated with a decrease in mortality in patients in the INTERMACS registry with a CF-LVAD, despite an increase in treated ventricular arrhythmias.
TRANSLATIONAL OUTLOOK.
Prospective randomized studies investigating permissive ICD programming (e.g. increased use of antitachycardia pacing, prolonged detection intervals, and higher detection rates) in CF-LVAD patients are needed.
Acknowledgments
Data collection for this work was supported in whole or in part by the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services under Contract No. HHSN268201100025C. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) or the National Institutes of Health. We would like to thank the INTERMACS investigators, coordinators, and participating institutions for the data they have provided for this registry.
KJC is supported by National Institutes of Health Grant T32 HL007854. ARG is supported by National Institutes of Health Grant KL2TR001874. The data for this study came from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), National Heart Lung and Blood Institute, Contract number HHSN268200548198C, 2010
ABBREVIATIONS
- ESC
European Society of Cardiology
- HF
Heart failure
- HRS/ACC/AHA
Heart Rhythm Society/American College of Cardiology/American Heart Association
- ICD
Implantable Cardioverter Defibrillator
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- ISHLT
International Society for Heart and Lung Transplantation
- LVAD
Left Ventricular Assist Device
- NYHA
New York Heart Association
- RV
Right Ventricle
- VA
Ventricular arrhythmia
Footnotes
Disclosures: YN has received consulting fees from Abbott (Thoratec) and St. Jude (Heartware). ARG has received honoraria from Abiomed. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mancini D, Colombo PC. Left Ventricular Assist DevicesA Rapidly Evolving Alternative to Transplant. Journal of the American College of Cardiology. 2015;65:2542–2555. doi: 10.1016/j.jacc.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Pagani FD, et al. Seventh INTERMACS annual report: 15,000 patients and counting. The Journal of Heart and Lung Transplantation. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Santangeli P, Rame JE, Birati EY, Marchlinski FE. Management of Ventricular Arrhythmias in Patients With Advanced Heart Failure. Journal of the American College of Cardiology. 2017;69:1842–1860. doi: 10.1016/j.jacc.2017.01.047. [DOI] [PubMed] [Google Scholar]
- 4.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm AbnormalitiesA Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2008;51:e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 6.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Vakil K, Duval S, Cogswell R, et al. Impact of Implantable Cardioverter-Defibrillators on Waitlist Mortality Among Patients Awaiting Heart Transplantation. JACC: Clinical Electrophysiology. 2017;3:33. doi: 10.1016/j.jacep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Vakil K, Kazmirczak F, Sathnur N, et al. Implantable Cardioverter-Defibrillator Use in Patients With Left Ventricular Assist Devices. JACC: Heart Failure. 2016;4:772. doi: 10.1016/j.jchf.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Clerkin KJ, Topkara VK, Mancini DM, et al. The role of implantable cardioverter defibrillators in patients bridged to transplantation with a continuous-flow left ventricular assist device: A propensity score matched analysis. The Journal of Heart and Lung Transplantation. 2017;36:633–639. doi: 10.1016/j.healun.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS Database for Durable Devices for Circulatory Support: First Annual Report. The Journal of Heart and Lung Transplantation. 2008;27:1065–1072. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical Statistics. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation. 2009;38:1228–1234. [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. Reducing Bias in Observational Studies Using Subclassification on the Propensity Score. Journal of the American Statistical Association. 1984;79:516–524. [Google Scholar]
- 14.Elze MC, Gregson J, Baber U, et al. Comparison of Propensity Score Methods and Covariate Adjustment. Evaluation in 4 Cardiovascular Studies. 2017;69:345–357. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 15.D’Agostino RB. Propensity Scores in Cardiovascular Research. Circulation. 2007;115:2340–2343. doi: 10.1161/CIRCULATIONAHA.105.594952. [DOI] [PubMed] [Google Scholar]
- 16.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous Flow Left Ventricular Assist Device Improves Functional Capacity and Quality of Life of Advanced Heart Failure Patients. Journal of the American College of Cardiology. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Garan AR, Yuzefpolskaya M, Colombo PC, et al. Ventricular Arrhythmias and Implantable Cardioverter-Defibrillator Therapy in Patients With Continuous-Flow Left Ventricular Assist DevicesNeed for Primary Prevention? Journal of the American College of Cardiology. 2013;61:2542–2550. doi: 10.1016/j.jacc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Schulze PC, Kitada S, Clerkin K, Jin Z, Mancini DM. Regional differences in recipient waitlist time and pre- and post-transplant mortality after the 2006 United Network for Organ Sharing policy changes in the donor heart allocation algorithm. JACC Heart failure. 2014;2:166–77. doi: 10.1016/j.jchf.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss AJ, Hall WJ, Cannom DS, et al. Improved Survival with an Implanted Defibrillator in Patients with Coronary Disease at High Risk for Ventricular Arrhythmia. New England Journal of Medicine. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 20.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic Implantation of a Defibrillator in Patients with Myocardial Infarction and Reduced Ejection Fraction. New England Journal of Medicine. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 21.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an Implantable Cardioverter–Defibrillator for Congestive Heart Failure. New England Journal of Medicine. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 22.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic Use of an Implantable Cardioverter–Defibrillator after Acute Myocardial Infarction. New England Journal of Medicine. 2004;351:2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 23.Buxton AE, Lee KL, DiCarlo L, et al. Electrophysiologic Testing to Identify Patients with Coronary Artery Disease Who Are at Risk for Sudden Death. New England Journal of Medicine. 2000;342:1937–1945. doi: 10.1056/NEJM200006293422602. [DOI] [PubMed] [Google Scholar]
- 24.Kadish A, Dyer A, Daubert JP, et al. Prophylactic Defibrillator Implantation in Patients with Nonischemic Dilated Cardiomyopathy. New England Journal of Medicine. 2004;350:2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 25.Refaat MM, Tanaka T, Kormos RL, et al. Survival Benefit of Implantable Cardioverter-Defibrillators in Left Ventricular Assist Device-Supported Heart Failure Patients. Journal of Cardiac Failure. 2012;18:140–145. doi: 10.1016/j.cardfail.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantillon DJ, Tarakji KG, Kumbhani DJ, Smedira NG, Starling RC, Wilkoff BL. Improved survival among ventricular assist device recipients with a concomitant implantable cardioverter-defibrillator. Heart Rhythm. 2010;7:466–471. doi: 10.1016/j.hrthm.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Oz MC, Rose EA, Slater J, Kuiper JJ, Catanese KA, Levin HR. Malignant ventricular arrhythmias are well tolerated in patients receiving long-term left ventricular assist devices. Journal of the American College of Cardiology. 1994;24:1688–1691. doi: 10.1016/0735-1097(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 28.Sims DB, Rosner G, Uriel NIR, GonzÁLez-Costello J, Ehlert FA, Jorde UP. Twelve Hours of Sustained Ventricular Fibrillation Supported by a Continuous-Flow Left Ventricular Assist Device. Pacing and Clinical Electrophysiology. 2012;35:e144–e148. doi: 10.1111/j.1540-8159.2011.03159.x. [DOI] [PubMed] [Google Scholar]
- 29.Malasana G, Daccarett M, Kuppahally S, Wasmund SL, Litwin SE, Hamdan MH. High prevalence of right ventricular dysfunction in ICD patients with shocks: a potential new predictor in risk stratification. Journal of interventional cardiac electrophysiology : an international journal of arrhythmias and pacing. 2011;31:165–9. doi: 10.1007/s10840-010-9536-y. [DOI] [PubMed] [Google Scholar]
- 30.Garan AR, Levin AP, Topkara V, et al. Early post-operative ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1611–6. doi: 10.1016/j.healun.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Gasparini M, Proclemer A, Klersy C, et al. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: The advance iii randomized clinical trial. JAMA. 2013;309:1903–1911. doi: 10.1001/jama.2013.4598. [DOI] [PubMed] [Google Scholar]
- 32.Moss AJ, Schuger C, Beck CA, et al. Reduction in Inappropriate Therapy and Mortality through ICD Programming. New England Journal of Medicine. 2012;367:2275–2283. doi: 10.1056/NEJMoa1211107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.