Synopsis
Obesity in older adults impacts not only morbidity and mortality, but importantly impacts quality of life and the risk of institutionalization. Weight loss interventions can effectively lead to improved physical function. Diet-alone interventions can detrimentally impact muscle and bone physiology and without interventions to affect these elements, can lead to adverse outcomes. Understanding social and nutritional issues facing older adults is of utmost importance to the primary care provider. The insufficient evidence related to pharmacotherapy is discussed as well as an overview of using physiologic rather than chronologic age is addressed for identifying suitable candidates for bariatric surgery.
Keywords: obesity, older adult, weight loss, physical function, pharmacotherapy, bariatric surgery, review
Epidemiology of Aging & Obesity
By the year 2030 in the United States, over 20% of the population will be over the age of 65 years1 (Figure 1), up from 15% of the population today2. The fastest growing demographic are the ‘oldest old’, individuals aged over 85 years. Much of the demographic shift is due to the emergence of baby boomers, adults born mid-1946 to mid-1964, into older adulthood (aged ≥65 years). Improvements in medical care, chronic disease management and infection control over the past century has also led to increases in life expectancy3,4. Based on recent census data, life expectancy at age 65 is now 82.8 years in males and 85.3 years in females5. The demographic changes observed during the aging process leads to a trajectory of disability6, independent of other influencing comorbidities. For instance, individuals surviving into old age are at risk of functional impairments – inability to transfer, walk, dress, eat, toilet, and bathe7 – which subsequently lead a loss of independence, impairment in quality of life and institutionalization8–10. Individuals are exposed to a longer period of time in which they may develop comorbidities and be at risk for developing incident disability11.
Figure 1. Projected Elderly Population Aged 65 years and older (represented as percentages) in the United States based on the 2010 Census and Future Projections.

Data from United States Census Bureau. 2010 Census Data. Available at: https://www.census.gov/2010census/data/. Accessed Jan 20 2013.
The obesity epidemic is not unique to a middle-age or a pediatric population. The prevalence of obesity in older adults, classified using body mass index (BMI), continues to rise over time. Recent estimates from the National Health and Nutrition Examination Surveys demonstrate that adults over age 60 have obesity rates exceeding 37.5% in males and 39.4% in females12 (Figure 2). These estimates have been replicated and are increasing in other developed countries as well, including the United Kingdom13 and Canada14.
Figure 2. Prevalence of Obesity in ages 60 years and older using National Health and Nutrition Examination Surveys over the past 4 decades based on body mass index. Current estimates indicate that obesity is present in men in 37.5% and 39.4% in females in the population.
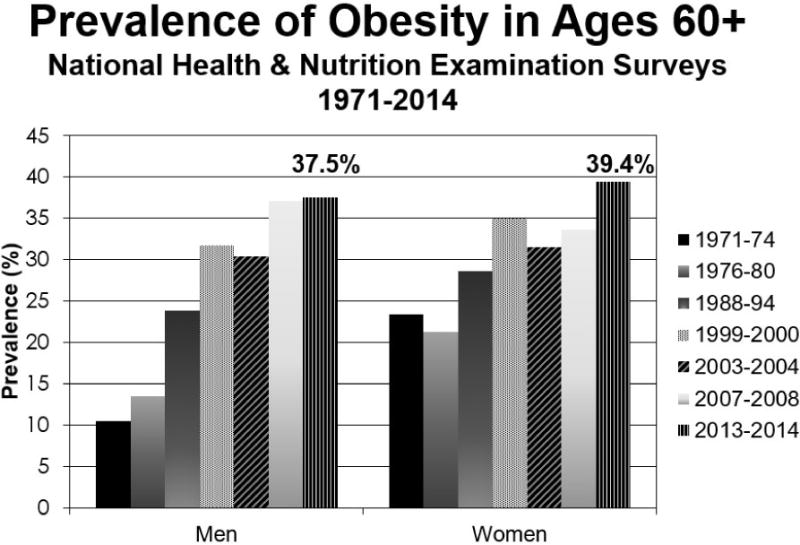
Data from Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315(21):2284–2291.
Defining Obesity in Older Adults
Body composition changes with ageing. Throughout adulthood, a natural increase in body fat develops up to the 8th decade of life, after which there is a reduction15. Redistribution of fat from peripheral and subcutaneous sources to a central location leads to increased waist circumference and waist-hip ratio in older adults. Importantly, there is a natural loss of muscle mass and strength with aging, termed sarcopenia16. Sarcopenia can also be accelerated in other processes including deconditioning, immobility or other acute illnesses17–19. Muscle mass and strength is believed to be vitally important in the preservation of physical function and independence in this population20. Using standard adult classifications for weight status, such as BMI, may thus underestimate adiposity for a given individual.
Many epidemiological and intervention-based studies have used BMI as a surrogate for adiposity. Standard BMI categories are used ubiquitously in clinical practice and are based on the World Health Organization cut-points of underweight (BMI<18.5kg/m2), normal weight (18.5–24.9), overweight (25–29.9), and obese (30+). BMI is easy to use, cheap and can be measured using a simple stadiometer and a weight scale; however, BMI is a poor measure of adiposity in older adults. First, individuals lose height as they age. The Baltimore Longitudinal Study of Aging noted that both males and females lost height with age21 which impacts the BMI’s denominator, possibly leading to an overestimation of its overall value in this population. Second, while BMI is clearly valuable as a population-level measure, it has poor diagnostic accuracy for identifying older adults with obesity22,23,24. Using data from the 1999–2004 National Health and Nutrition Examination Survey, the sensitivity of BMI in accurately identifying adiposity was 32.9% in males and 38.5% in females. Third, as previously described, older adults tend to gain weight in central regions of their body. BMI fails to distinguish between peripheral and visceral obesity, an important consideration in individuals who are classified as having normal weight central obesity25. Based solely on BMI, this category of individuals would often not be a target for obesity therapy because they fall within the normal range (BMI of 18.5–24.9kg/m2). Individuals with central obesity presenting with a normal BMI may also be at risk for adverse cardiometabolic dysfunction including dyslipidemia, coronary disease, hypertension, and early mortality26–28. One large scale epidemiological study using 15,184 adults aged 18–90 years with normal BMI and central adiposity based on waist-to-hip ratio29 found that individuals with normal weight central obesity had a higher risk of total and cardiovascular mortality (HR of 1.87 [1.53,2.29] in males and 1.48 [1.35,1.62] in females). These relationships have been observed in older adults as well, whereby women have higher short-term cardiovascular mortality than males, and males have higher long-term cardiovascular mortality than females27. Irrespective of sex, individuals with normal weight central obesity (waist-hip ratio or waist circumference) are at higher risk of long-term disability28. Identifying and evaluating individuals in clinical practice who otherwise may not be identified as high risk is needed. Such misclassification can be problematic from a population-based management standpoint. Lastly, BMI accounts for both fat-free (muscle) and fat mass, the former of which declines during the aging process. This can further lead to misclassification and potential underestimation of adiposity and risk.
Unfortunately, gold-standard methods for identifying adiposity with accuracy, including magnetic resonance imaging and computer tomography, are non-covered indications in clinical practice unless performed for other reasons. Dual-energy absorptiometry is more routinely available, but assessment of body composition is not covered by Medicare. Bioelectrical impedance can be a crude measure for body composition assessment in older adults and portable devices are available; however, the alterations in fluid balance in older adults30, along with a higher incidence of prosthetic implants31 and implantable cardiovascular devices32 makes this modality less favorable. As such, BMI combined with a marker of central adiposity may provide a cost-effective approach to improved diagnostic accuracy within a clinical practice.
Established BMI cut-points correspond to adverse disease processes, including mortality33. Multiple population-based cohort studies have examined the relationship between obesity and premature death. In one study, obesity led to an estimated 111,909 excess deaths34. While obesity in mid-life is associated with reduced life-expectancy, the duration of obesity has a considerable effect on long-term mortality35, disability36 and nursing home placement37 as evidenced in numerous epidemiological studies. The relationship between BMI and mortality in populations has been shown to be representative of a “U” shaped curve34; individuals classified as underweight and obese are at the extremes with higher risks of mortality. With age, the curve flattens and shifts to the right, indicating that standard BMI cut-points differ in older adults than in younger populations. A meta-analysis that evaluated 32 studies between 1990–2013 (including 197,940 adults aged 65 and over) also demonstrated a “U” shaped curve38. Yet, the lowest risk of mortality was observed in those with a BMI of ~27.5kg/m2. In fact, the risk of death only increases at a BMI>33.0 (HR 1.08 [95%CI: 1.00,1.15]).
In select older adult populations, not all participants with elevated BMIs should be considered at high risk. An ‘obesity paradox’ exists whereby elevated body weight may be protective, mitigating death in select populations. For instance, a nursing home systematic review evaluated 19,538 subjects with a BMI≥30kg/m2 and found that the risk of death was significantly lower than for the referent (normal BMI) group (HR 0.69 [95% CI:0.60,0.79];p<0.001)39. A number of factors are thought to explain this: first, issues related to the inability of BMI to discern between visceral and subcutaneous fat; second, cardiovascular fitness likely moderates the relationship between obesity and death. Individuals with high levels of cardiovascular fitness irrespective of their obesity classification portend to better outcomes40; third, excess adiposity in high risk populations at risk for frailty, itself a predictor of death, may be protective. Populations such as hemodialysis patients, congestive heart failure, or nursing home residents all tend to lose weight (consisting of both fat and muscle) with aging which promotes wasting, cachexia and mortality41. Fourth, there may be self-selection—individuals with excess adiposity who have survived to old age may have a survival advantage as compared to those who have died earlier in life. As such, practitioners should be made aware of these considerations in select populations, particularly when using BMI as a measure for adiposity in older adults with these specific co-morbidities.
Impact of Obesity on Physical Function/Disability
Overweight and obesity predisposes to disability and decreased physical functioning. Using the Health, Aging and Body Composition data, adults classified as overweight or with obesity using BMI at ages 25, 50 and 70–79 years had a HR 2.38 of incident disability over a 7 year period35. Similar relationships have been observed using either waist circumference or body fat percent, both in males and females. A systematic review by Schaap et al42 demonstrated that adults with a BMI≥30kg/m2 aged 65years and older had a 60% higher risk of incident disability (95%CI:1.43,1.80). A “U” shaped relationship is also observed between BMI and nursing home admission from community-dwelling adults43. Longitudinally, there appears to be a relationship. As the obesity epidemic has emerged in the past few decades, recent evidence suggests that its relationship with disability continues to be problematic, yet may be leveling off44. A recent study evaluated three consecutive time periods using National Health and Nutrition Examination Surveys (1988–1994, 1999–2004, and 2005–2012) and evaluated the association between obesity and disability. The population attributable fraction for obesity having a functional impairment and severe ADL impairment was 23.2% (95%CI: 20.5,25.7) and 24.6% (95%CI: 12.3,35.2), respectively, in individuals aged 60 years and older. Individuals classified as having obesity were still at much higher risk of limitations, yet limited wave-to-wave variability was observed. Other measures of adiposity, including body fat percent and waist circumference have also been associated with impaired physical function and disability and parallel these estimates. A study evaluating the cross-sectional association between obesity using body fat and disability demonstrated a significant odds of disability45,46. While there are challenges in the diagnostic accuracy of obesity, irrespective of the body composition or anthropometric measure used, in community dwelling adults, obesity is associated with a poorer prognosis of physical function.
Obesity is also associated with increased risk of falling in older adults. Over one-third of adults aged 65 years and older fall each year47, making it necessary to screen for fall risk in the primary care setting48,49. Two studies using Health and Retirement Study 1998–200650, and the Behavioral Risk Factor Surveillance System 2014 data51, demonstrated the degree of obesity (class I vs. II vs. III) was associated with a higher risk of falling. Using the Health and Retirement Study data, those with class III obesity (BMI>35kg/m2 have a OR 1.50 [1.21–1.86] of falling, while estimates from the Behavioral Risk Factor Surveillance System are slightly lower (1.23 [1.13,1.35] in females; 1.18 [1.06,1.32] in males]. While fall risk is increased, risk of hip fractures from obesity is lower in this population52. Evaluating individuals who are at risk can prevent falls that may otherwise lead to restriction of social function, fractures and death48,50.
Sarcopenic Obesity – a subset of high-risk individuals
Sarcopenia in individuals with obesity is a subgroup that deserves specific attention. Sarcopenia is derived from the Greek word, “sarcos” meaning flesh and “penia” meaning lack of. Infiltration of fat occurs within muscle tissue that can lead to impairments in muscle physiological parameters53,54. The definition of sarcopenia and obesity (sarcopenic obesity) continues to be fraught with methodological challenges55 and discrepancies in defining sarcopenia (muscle mass vs. muscle strength) and obesity (body fat vs waist circumference vs BMI). Nonetheless, such individuals are at considerably higher risk than those with either of the two conditions independently. While the medical definition has evolved over the past three decades55, based on the 2014 Foundations for the National Institutes of Health Conference20, sarcopenia is simply the loss of muscle mass and function with aging. Specific cut-points have been developed for use. While several earlier studies focused on loss of muscle mass as the key determinant of sarcopenia56, emerging evidence suggests that muscle strength may be a more powerful determinant of incident disability57.
The development of sarcopenia in old age is a natural phenomenon that can be partially mitigated with lifestyle interventions altering the threshold at which disability ensues16,58. Short- and long-term changes of both muscle mass and strength occur. Yet, changes in strength may occur without corresponding changes in muscle mass as observed in an earlier study using Health, Aging, Body Composition data59. Earlier work using the New Mexico Aging study demonstrated that individuals with sarcopenic obesity had a HR 2.63 [95%CI: 1.19–5.85] of developing an impairment of their instrumental activities of daily living over the course of a 8 year period60. This study defined sarcopenic obesity using appendicular skeletal muscle mass and body fat cutpoints61. Data from the InChianti study demonstrated that individuals with low muscle strength and obesity (based on knee extensor strength and BMI) had lower walking speeds than their counterparts over a 6-year period of time62. Much of our own work has revolved around using dual x-ray absorptiometry-defined muscle mass with the FNIH cutpoints with body-fat defined obesity (men>25%, females>35%), suggesting a significant relationship with limitations63 and mortality64. Mortality is less clear as demonstrated using NHANES III data65 (using BIA-defined muscle mass) and NHANES 1999–2004 data (using dual x-ray absorptiometry-defined muscle mass)64. Impaired muscle strength in conjunction with obesity, irrespective of its definition, is associated in crosssectional and longitudinal studies with adverse and negative outcomes, more so than muscle mass42. Thus, the importance of identifying patients with both sarcopenia and obesity is of paramount importance.
Evidence for Weight Loss in Older Adults?
Previous epidemiologic studies have provided conflicting findings on outcomes following weight loss in older adults; however, these studies failed to differentiate between intentional vs. unintentional weight loss and do not account for important confounding variables and reverse causality66,67. A joint consensus statement, published in 2005 by members of The Obesity Society, American Society of Nutrition and The National Association for the Study of Obesity, provided some evidence on managing this disease in older adults,68 and several randomized controlled trials have since been published demonstrating the benefits and the harms of weight loss in older adults69. (Note that we define ‘weight loss’ as intentional in the section below.)
A healthy lifestyle has been shown to compress the number of years of disability, according to data from the Cardiovascular Health Study 1989–201570. Monitoring multiple lifestyle factors, including physical activity, diet, and BMI, 5,248 community adults aged 65 years and older who were not wheelchair dependent were identified. Activities of Daily Living were assessed and the ratio of the number of years living without any disability to the total number of years lived was ascertained to indicate compression or expansion of the disabling period. Obesity was associated with a decrease of 7.3% (95%CI: 5.4–9.2) as compared to normal weight individuals. The lowest quintile of the Alternative Healthy Eating Index was associated with a 3.7% (95% CI: 1.6–5.9) lower score than the highest quintile. Yet, engaging in physical activity demonstrated that for every 25 blocks walked in a week, 0.5% (95% CI: 0.3–0.8) higher proportion of years was gained disability-free. The article concluded that healthy lifestyles can compress the duration of disability in a person’s remaining lifetime.
A recent qualitative systematic review evaluated six randomized controlled trials from 2005–2015 in adults aged 60 and older (mean age >65 years)69. Obesity was defined as BMI≥30kg/m2 or waist circumference ≥88cm/102cm in females/males respectively. Of 5,741 citations, 19 trials were identified, of which 6 were unique cohorts. Results suggested that a dietary weight reduction program combined with a physical activity program (aerobic and resistance) improved physical performance and quality of life and lowered the risk of reduced muscle mass, strength and bone loss. Weight loss ranged from 0.5 to 10.7kg. A recently published randomized trial with 141 participants, demonstrated that weight loss inclusive of both aerobic and resistance exercises led to improvements in the physical performance test, peak oxygen consumption, and muscle strength, despite only marginal reductions in lean muscle mass71. Additionally, there was no difference in exercise-related adverse events. In a separate review, weight loss in older adults with obesity was associated with reduced risk of death72. The authors identified 15 randomized controlled trials including 17,186 participants with a mean age of 52 years at randomization. The mean BMI was 30–46 kg/m2 with an average follow-up period of 27 months (ranging from 18 months to 12.6 years). The weight loss group experienced a 15% lower all-cause mortality risk (relative risk 0.85 [95% CI:0.73,1.00]). While further evidence is needed to ascertain the impact on long-term mortality, weight loss appears safe and effective in older adults with obesity.
Cautions of Losing Weight in Older Adults
There are important risks that often get overlooked in this population by practitioners. Loss of weight leads not only to loss of fat mass, but also to loss of muscle mass, thereby promoting sarcopenia and its ensuing adverse outcomes73. The general principle that each pound lost equates to 75% fat and 25% muscle has been debated but is generally accepted74. Moreover, loss of weight impacts bone metabolism and turnover promoting osteoporosis75–77.
Although sarcopenia is a natural phenomenon of the aging process, its acceleration with weight loss efforts is of considerable concern. Diet or diet and exercise-induced weight loss induces hormonal changes that negatively impact muscle mass and strength and is exacerbated by moderate caloric restriction. A review of 33 interventions demonstrated significant decreases from baseline in knee extensor strength (−7.5%) and handgrip strength (−4.6%) following diet-induced weight loss with moderate energy restriction76. Failure to engage patients in isokinetic resistance exercises will lead to likely loss of muscle mass and strength and reduce the impact of the gains in function individuals may otherwise attain with weight loss78.
A number of randomized controlled trials have demonstrated that caloric restriction alone leads to loss of bone mineral density (BMD). Villareal’s group demonstrated that a loss of hip BMD was attenuated, in part, when resistance exercise was coupled with a weight loss program, preventing an increase in bone turnover58,71. In another study, a meta-analysis of 32 randomized controlled trials, weight loss had no significant effect on total BMD75. However, the pooled study data suggested that hip and lumbar spine BMD were significantly lower after 4 months, particularly in adults who were classified as having obesity. Lumbar spine BMD was also lower after calorie restriction in interventions longer than 13 months. While these results were in adults age 50 years and older, their sensitivity analysis stratified by age revealed that hip BMD loss was highest in adults age 65 and older. This finding has considerable implications for the older adult who is at risk of falling. Approximately 30% of falls among older adults result in injury, 10% of which are fractures79. Hip fractures are especially devastating in this population and portend considerable morbidity and mortality80.
Generally, obesity in the older adult population is safe and effective and can lead to considerable improvements in cardiometabolic risk and physical function. While there are important known risks associated with weight-loss in this population, they likely can be mitigated with appropriate health promotion interventions. As with all patients in a geriatrics practice, practitioners need to manage the benefits vs. the risks of any interventions, and in select individuals, weight loss should be encouraged.
Obesity in the Primary Care Setting
Obesity prevention efforts should be based in primary care settings, where front-line clinicians have longitudinal relationships to provide brief, motivational interviewing to engage patients in behavioral change. Intensive behavioral counseling can induce clinically meaningful weight loss of between 0.3–6.6kg, but little research is available on primary care practitioners providing this care. A systematic review suggested that different interventionists can deliver counseling, both in person or by telephone in this setting81. An additional review suggested that a multidisciplinary team approach consisting of collaborative care was much more effective82. This review conflicts with a recent two-arm randomized trial of 2,728 patients that demonstrated that a behaviorally informed, very brief (30 second) physician-delivered opportunistic intervention was acceptable and effective to reduce population mean weight (1.43kg [95% CI: 0.89,1.97])83.
In 2011, in the United States, the Centers for Medicare and Medicaid Services began reimbursing obesity counseling (current procedural terminology code G0447) for clinicians in a primary care setting to provide 22 targeted,15-minute intensive behavioral therapy counseling in a continuous 12-month period84. The goal was to achieve a mean weight loss of 3kg in beneficiaries whose BMI was ≥30kg/m2. Practice management barriers85 exist in implementing this benefits, although novel technologies86 may be helpful in addressing these issues. In 2012, the first full year of data, 27,338 (0.1%) of Medicare beneficiaries over age 65 availed of the benefit87. This increased slightly in 2013 to 46,821 (0.17%). The estimated proportion of persons with obesity using the benefit increased from 0.35% to 0.60%, with a mean of 1.99 and 2.16 claims/user. This data suggests its low uptake may not only be due to poor implementation patterns, but other support staff such as health coaches and dieticians should be integrating in delivering this important service. Novel delivery modalities to engage patients may be a strong consideration moving forward that include transition to value-based care models or increased reimbursements.
Medical Evaluation Specific to Older Adults with Obesity
The nutritional needs and caloric intake for healthy older adults is known to decrease with age in both sexes. The caloric difference between early adulthood and older adulthood ranges between 300–500 kCal/day. Much of this is due to age-related phenomena related to basal metabolic rate which drops considerably with age88. The following section will discuss specific concerns that a primary care provider should consider using a ‘geriatric specific’ approach, as compared to a middle aged adult with obesity89.
Communication in clinical settings with older adults requires careful communication strategies that are often overlooked by clinicians. Behavioral techniques must be adapted to accommodate not only the sensory deficits the older adult faces such as hearing and vision, but need to be adapted to changes in cognition and executive function90, and their preference for shared-decision making91. Others have noted of a ‘gap’ between intentions and behavioral change92, which itself can be predicted by measures of executive function93. An inability to implement intentions will lead to poor execution of the desired change94. Older adults may also be better focused on single health behavior change interventions as compared to multiple95, focusing on a specific content. These approaches are less confusing and can be understood much more adequately. On the contrary, though, complex conditions in older adults require multiple strategies to deliver health promotion efforts which can be challenging to the clinician. Engaging individuals in strategies to improve self-efficacy through social support and change can be helpful to provide information to set goals, engage in change, and to promote self-monitoring96. Researchers continue to caution clinicians in applying behavioral change principles to older adults as they may require adaptation from a younger population.
While primary care providers care for older adults, the lack of specific geriatric training can be problematic in delivering behavioral change interventions. First, motivational interviewing, a core tenant in eliciting change and in the Medicare Obesity Benefit is heavily influenced by the contextual aspects of delivery and by the clinician97 – internists may approach elements differently than geriatricians. Second, geropsychological principles are often not integrated in routine interventions98. This includes social participation which is strongly related to better health and can lead to forming new goals in one’s life99. This allows for a reframing of the discussion to engaging in change. Third, goals in seniors are different, in part due to multimorbidity100, but also due to the changing perceptions on aging and health101–104. Fourth, aging individuals have limited lifespans and, hence, patients focus more on the present day than the future. These elements lead to significant challenges in a busy primary care setting.
A thorough medical evaluation is needed in older patients that wish to improve their health and physical function through weight loss. The primary care clinician (or obesity medicine specialist) should identify whether there are any recent changes in health status (medical or economic). Recent hospitalizations and changes in functional status (e.g. joint replacement leading to physical inactivity) that may lead to weight gain105. Other standard questioning on weight history, previous strategies and alcohol use parallel methods used in the general population. Notably, we highlight two main concerns specific to older adults including medications and social support.
The number of medications prescribed increases with age and with the number of chronic conditions. Polypharmacy is a significant risk in older adults as it is associated with increased risk of cognitive impairment, urinary incontinence, falls and declines in physical function106. The American Geriatric Society has developed the Beers Criteria107, which clearly identifies potentially inappropriate medications in older adults and assists healthcare providers in improving medication safety for this population. Its purpose is to inform clinical-decision-making concerning the prescribing of medications for older adults in order to improve safety and quality of care. There are a number of medications that are considered ‘obesogenic’ that should be re-evaluated as part of the evaluation. As older adults have a higher incidence of diabetes108, depression109, pain110 and hypertension111, medications treating these chronic diseases may increase the risk of a person gaining weight. Additionally, a number of these medications are listed on the Beer’s criteria. The primary care practitioner, in concert with a clinical pharmacist on the multidisciplinary team can assist in streamlining not only the number of medications, but also the class of medications that promote weight gain. Table 1 briefly highlights some of the commons medications that predispose to weight gain.
Table 1.
Commonly Prescribed Medications in Older Adults Predisposing to Weight Gain
| Disease/Class of Med | Examples |
|---|---|
| Diabetes | Insulin, TDZ*, sulfonylureas* |
| Depression | Tricyclics*, SSRIs* |
| Antipsychotics | 1st + 2nd generation* |
| Neuropathic | gabapentin |
| Antihistamines | Diphenhydramine* |
| Hypertension | B-blockers |
Medications that are listed in the 2015 Beers Criteria107
Socioeconomic and ethnic disparities are two specific social determinants of health that increasingly are being recognized as important predictors in obesity management and adverse health. An elder on a fixed income (often a social security income) and must make choices between their food consumption and other care needs. Food insecurity is defined as the limited or uncertain availability of nutritional adequate and safe foods, or the limited or uncertain ability to acquire acceptable foods in socially acceptable ways112. Food insecure older adults individuals have poorer dietary intake, nutritional status and health status than food secure older adults113. Simple questions that should be asked include:
Where is your food coming from?
Who purchases your food?
Do you have to pay for your medical bills and scrimp on food?
Such questions are helpful in that it leads to information on affordability of food. A true multidisciplinary team led by the physician that embodies a dietician, social worker and care manager can be helpful in intervening in this population. A dietician is of utmost importance in that they are able to perform, not only the usual functions of evaluation and counseling, but can assist this population in engage in substituting choices that they can afford.
Most established trials have evaluated calorie-restricted diets ranging from 500–750 kCal/day under the guidance of a registered dietician69. In older adults, there is ample evidence that diets such as the Dietary Approaches to Stop Hypertension114 or the Mediterranean diet115 have demonstrated improvements in metabolic parameters, weight loss, long-term disability, mortality and cognition. Very low energy or protein sparing diets should be avoided in older adults due to risks of dramatic fluid and electrolyte shifts and proportionally can lead to augmented muscle mass loss. Villareal, in his studies, proposed a loss of ~10% of baseline weight at six months58,71. Supplemental vitamin D of ~800–1000units and 1200mg of calcium should be considered. The latter can be from dietary or supplement sources, although we emphasize the consumption from dietary calcium if at all possible considering the recent controversies. Protein intake should also be augmented in older adults. Recommended dietary allowance is 1.0g/kg/day, yet since older adults produce less protein than younger persons, this should be revised to 1.0–1.2g/kg/day116. Also, a larger amount of protein is required to produce an equal response116. Notably, a recent Cochrane review demonstrated that protein supplements did not lead to improved outcomes117. Early pilot studies during weight loss interventions suggest improved short performance physical battery measures. The MeasureUP118 in 67 subjects demonstrated, at 6-months, a −8.7±7.4kg and −7.5±6.2kg weight loss in the protein and control groups, respectively. The authors observed improvements in physical function based on the short physical performance battery of +2.4±1.7units and +0.9±1.7 units, respectively (p=0.02). Future studies should determine the amount of protein intake, the type of protein (meat vs. plant-based) and whether advised supplements for the treatment of sarcopenia (whey, leucine carnitine) can augment physical function and further mitigate sarcopenia.
Primary care providers have little training in counseling on physical activity119. They can, however, provide targeted information on exercise prescriptions. Such prescriptions should be individually tailored based on the individual’s functional status and capacity. The American College of Sports Medicine suggest at least 150 minutes of low to moderate intensity aerobic activity in all patients, including older adults120. Seniors who may not have sufficient cardiovascular fitness may subdivide this time period into smaller increments to not only build their endurance but assist in ongoing behavioral change. The practitioner and their team can encourage patients to slowly increase their capacity to do so. During weight loss efforts, resistance exercises are of paramount importance for the prevention of sarcopenia. All individuals should engage in flexibility, balance, and strengthening activities with resistance bands or weights that can be free or be attached to wrists or legs. In those with financial difficulties, we advise individuals to utilize common household items (i.e. soup cans or jugs of milk). The weekly goals are 2–3 days, with at least 48 hours rest between sessions. Each of these sessions should last between 30–40minutes, rotating muscle groups and exercises, with 10–12 repetitions for each sessions. Fatigue normally occurs between 8–12 repetitions and a bit of fatigue is advisable, but it is important to prevent injuries by starting low and going slow, starting with 1–2 pounds or with the lowest resistance color band. Physical therapists can assess a person’s 1-repetition maximum and advise advancement. We advocate that current multidisciplinary physical activity programs should be based on the Life Study121, a multi-center randomized controlled study that demonstrated reduced disability over a mean 2.6 year follow-up in 818 patients. Compared to a self-instructed exercise program, participants in this structured program demonstrated a 28% reduction in incident major mobility disability (95% CI:0.57,0.91). These materials are freely available on <https://www.thelifestudy.org>. Additionally, the National Institute on Aging has a booklet of exercises that can be accessed and downloaded at <https://www.nia.nih.gov/health/publication/exercise-physical-activity/introduction>, free of charge for clinicians and patients to engage at home.
Clinicians can play a paramount role in the monitoring of their older patients undergoing weight loss. While there is no firm evidence pertaining to monitoring parameters, we recommend the following. First, consideration should be given to assess baseline bone density during such efforts. Medicare indications for women are quite broad (ie: post-menopausal) yet those for men may be slightly more challenging to find. The United States Preventive Services Task Force recommendations for osteoporosis screening in men do not provide a firm statement and conclude that the evidence is insufficient to assess the balance of benefits and harms of screening122. Some potential indications in men include: x-ray evidence of possible osteopenia, osteoporosis or vertebral fractures; a person taking steroids; hyperparathyroidism; monitoring if osteoporosis drug therapy is working. Some examples of biomarkers of bone turnover that can be considered in the evaluative process, including osteocalcin, type I procollagen, urine collagen type-1 cross-linked N-telopeptide. These may help direct the impact of weight loss on bone turnover. Baseline and longitudinal monitoring of grip strength is an office procedure that can be easily integrated in any busy practice. The recent approval of an ICD-10 code for sarcopenia may allow for further screening and routine integration in practice. Last, monitoring of vitamin D levels may be helpful. Different societies have different views on monitoring of levels49. Our practice is to assess baseline Vitamin D levels during routine weight loss management. As with any intervention in older adults, risks vs. benefits of monitoring is needed, and further studies should best inform the appropriate indices clinicians should considering during such efforts.
Pharmacotherapy
With the emergence of newer medications effective in weight management, older adults are increasingly asking about the possibility of taking such medications. The AACE/ACE guidelines explicitly state that there is insufficient evidence to recommend weight-loss medications in older adults123. As is the case with most pharmaceutical-based clinical trials, to prove efficacy, older adults were excluded from most trials, biasing outcomes towards younger patients. Unfortunately, we caution extrapolating such results to older adults whose pharmacokinetics and pharmacodynamics properties differ from those in younger, robust populations who often have fewer comorbid conditions and are on fewer medications.
Of the obesity medications available, two trials (phentermine/topiramate and liraglutide) have documented efficacy analyses between older adults and younger counterparts. These trials enrolled 7% (n=254) and 6.9% (n=232) older adults, respectively, of their study subjects123–128. There were no observed differences in efficacy, safety and pharmacokinetics between subgroups. The other commercial medications had insufficient study sample to make any statistical comparisons. Preliminary data suggest that pharmacokinetic data on phentermine/topiramate, liraglutide, lorcaserin or naltrexone/bupropion were no different between younger/older. The propensity to add to an older adult’s polypharmacy should not be overstated as this leads to medication errors and subsequent adverse drug events, increased risk for falls, delirium and costs. Table 2 highlights some of the absolute contraindications to these medications and some of the common side effects and major risks that older adults may face, irrespective of the lack of evidence. Given the need for obesity treatments and the limited data currently available, further research in older adult populations is important.
Table 2.
Relative and Absolute Contraindications to Weight-Loss Medications in Older Adults
| Generic Name | Absolute Contraindications | Side-Effects (>10%) & Major Risks in Older Adults |
|---|---|---|
| Locarserin | — | Renal insufficiency, Tramadol, Heart failure, Serotonin excess, hypoglycemia |
| Phentermine/Topiramate | Glaucoma, MAOI hyperthyroidism | Constipation headache, xerostomia |
| Phentermine | Glaucoma, Heart failure, CAD, Hyperthyroidism, Arrhythmias | Renal insufficiency, Reduced exercise tolerance |
| Orlistat | Malabsorption, cholestasis | Fecal urgency, Flatulence, Steatorrhea |
| Buproprion/naltrexone | HTN, Seizures, Hepatic impairment | More sensitive to CNS effects, Renal insufficiency, Headache, Constipation, N/V |
| Liraglutide | Angioedema, MEN-2, MTC | Constipation, Diarrhea, Hypoglycemia, Palpitations, N/V |
Bariatric Surgery
An effective treatment approved by the NIH in 1991 is bariatric surgery129. This procedure has gained considerable popularity and is increasingly being performed in persons with obesity who are at high risk of medical complications and/or have co-morbidities. In the general population, there is considerable epidemiologic and trial data demonstrating its safety, efficacy, and effectiveness130–135. The extent of the safety and efficacy in older adults continues to be debated in the surgical literature. Many studies have used varying cut-points for older adults (ranging from 50–65 years), and are fraught with considerable methodological problems, including reduced study power, study time period bias, and inconsistent definitions. Additionally, the evolving surgical and medical care of this patient population, and the establishment of high volume Bariatric Surgery Centers of Excellence have led to considerably improved outcomes136,137. A number of systematic reviews have been published discussing the short- and long-term outcomes of bariatric surgery in an older adult population138, and are outside the scope of this particular review.
European guidelines139 have noted that the procedure should be considered in carefully selected patients. We previously developed an approach in older adults that highlights physiologic as opposed to chronologic age140. A laparoscopic approach is favored as compared to an open approach. By applying the principles of a comprehensive geriatric assessment on patients evaluated for surgery, the hope is that those carefully selected individuals will have improved short- and long-term outcomes. Highlighting the importance of future life expectancy, presence of undiagnosed cognitive impairment, medical co-morbidity that could be impacted by the surgical procedure, and important social support mechanisms for the immediate post-operative care are of utmost importance. Previous history of post-operative delirium and impairments in vision and hearing are also important factors in successful recovery. Understanding such limitations could sway a decision to consider surgical intervention or not in a given patient. Being classified as ‘geriatric’ should not preclude evaluation of surgery in those motivated older adults who fulfill many of the above noted elements in the geriatric evaluation.
Conclusions
The epidemic of geriatric obesity will continue to impact the role of a primary care provider with time. The importance of lifespan prevention measures cannot be overstated to delay the onset of disability and impairments in health-related quality of life. Effective lifestyle modifications for weight loss can easily be implemented within a busy primary care setting to engage individuals. Community-based physical activity interventions are easy, cost-effective ways to delay disability and enhance physical function. Future studies should focus on disseminating and implementing practical ways to integrate established evidence-based practices into routine clinical care, without overburdening clinical staff. The use of emerging technologies may be helpful adjuncts. Evaluation of pharmacotherapy in this high-risk population remains a priority and including older, robust adults may be a reasonable first step in evaluating their safety and efficacy. We recommend that bariatric surgery be considered for older adults following a comprehensive geriatric assessment and interdisciplinary team-based approach is helping in evaluating and engaging these patients in this process.
Key Points.
Older adults with obesity will be an emerging demographic that primary care practitioners will need to develop skills in managing.
Intentional weight loss in this population can be successful and safe.
Appropriate understanding of the dangers of weight loss on muscle and bone are required.
Pharmacotherapies that are FDA approved for adults have not been extensively studied in older adult populations.
Bariatric surgery can be considered in selective candidates.
Acknowledgments
Funding
Dr. Batsis’ research reported in this publication was supported in part by the National Institute on Aging of the National Institutes of Health under Award Number K23AG051681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Dartmouth Health Promotion and Disease Prevention Research Center (Cooperative Agreement Number U48DP005018) from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Dr. Batsis has received honoraria from the Royal College of Physicians of Ireland for policy statement review and an honorarium from the Endocrine Society for an educational CME presentation at their Annual Conference.
ABBREVIATIONS
- BMD
bone mineral density
- BMI
body mass index
- CI
confidence intervals
- FNIH
Foundation for the National Institute on Health
- HR
hazard ratio
- NHANES
National Health and Nutrition and Examination Survey
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Government US. Census Bureau Statistics. 2012 Accessed January 20th, 2013, 2013. [Google Scholar]
- 2.Mather M, Jacobsen LA, Pollard KM. Aging in the United States. Population Reference Bureau; 2015. Available at: http://www.prb.org/pdf16/aging-us-population-bulletin.pdf. [Google Scholar]
- 3.Lubitz J, Cai L, Kramarow E, Lentzner H. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003 Sep 11;349(11):1048–1055. doi: 10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 4.Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. N Engl J Med. 1983 Nov 17;309(20):1218–1224. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 5.Arias E. National Vital Statistics Report. 2015 Sep 22;64(11) [PubMed] [Google Scholar]
- 6.Dunlop DD, Hughes SL, Manheim LM. Disability in activities of daily living: patterns of change and a hierarchy of disability. Am J Public Health. 1997 Mar;87(3):378–383. doi: 10.2105/ajph.87.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA. 1963 Sep 21;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 8.Bish CL, Michels Blanck H, Maynard LM, Serdula MK, Thompson NJ, Kettel Khan L. Health-related quality of life and weight loss among overweight and obese U.S. adults, 2001 to 2002. Obesity (Silver Spring) 2006 Nov;14(11):2042–2053. doi: 10.1038/oby.2006.239. [DOI] [PubMed] [Google Scholar]
- 9.Cai Q, Salmon JW, Rodgers ME. Factors associated with long-stay nursing home admissions among the U.S. elderly population: comparison of logistic regression and the Cox proportional hazards model with policy implications for social work. Soc Work Health Care. 2009;48(2):154–168. doi: 10.1080/00981380802580588. [DOI] [PubMed] [Google Scholar]
- 10.Chambers BA, Guo SS, Siervogel R, Hall G, Chumlea WC. Cumulative effects of cardiovascular disease risk factors on quality of life. J Nutr Health Aging. 2002 May;6(3):179–184. [PubMed] [Google Scholar]
- 11.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007 Aug 07;147(3):156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 12.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016 Jun 07;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker C. Obesity Statistics: A briefing Paper. House of Commons Library. 2017 Jan 20;3336 [Google Scholar]
- 14.Twells LK, Gregory DM, Reddigan J, Midodzi WK. Current and predicted prevalence of obesity in Canada: a trend analysis. CMAJ Open. 2014 Jan;2(1):E18–26. doi: 10.9778/cmajo.20130016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000 May;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 16.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008 Aug-Sep;12(7):427–432. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011 May;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutta C. Significance of sarcopenia in the elderly. J Nutr. 1997 May;127(5 Suppl):992S–993S. doi: 10.1093/jn/127.5.992S. [DOI] [PubMed] [Google Scholar]
- 19.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–323. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- 20.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014 May;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorkin JD, Muller DC, Andres R. Longitudinal change in height of men and women: implications for interpretation of the body mass index: the Baltimore Longitudinal Study of Aging. Am J Epidemiol. 1999 Nov 01;150(9):969–977. doi: 10.1093/oxfordjournals.aje.a010106. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008 Jun;32(6):959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okorodudu DO, Jumean MF, Montori VM, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond) 2010 May;34(5):791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 24.Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond) 2016 May;40(5):761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batsis JA, Zbehlik AJ, Scherer EA, Barre LK, Bartels SJ. Normal Weight with Central Obesity, Physical Activity, and Functional Decline: Data from the Osteoarthritis Initiative. J Am Geriatr Soc. 2015 Aug;63(8):1552–1560. doi: 10.1111/jgs.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010 Mar;31(6):737–746. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F. Normal weight obesity and mortality in United States subjects >/=60 years of age (from the Third National Health and Nutrition Examination Survey) Am J Cardiol. 2013 Nov 15;112(10):1592–1598. doi: 10.1016/j.amjcard.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Lopez-Jimenez F. Normal weight obesity and functional outcomes in older adults. Eur J Intern Med. 2014 Jul;25(6):517–522. doi: 10.1016/j.ejim.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, et al. Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann Intern Med. 2015 Dec 01;163(11):827–835. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlanger LE, Bailey JL, Sands JM. Electrolytes in the aging. Adv Chronic Kidney Dis. 2010 Jul;17(4):308–319. doi: 10.1053/j.ackd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maradit Kremers H, Larson DR, Crowson CS, et al. Prevalence of Total Hip and Knee Replacement in the United States. J Bone Joint Surg Am. 2015 Sep 02;97(17):1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silverman BG, Gross TP, Kaczmarek RG, Hamilton P, Hamburger S. The epidemiology of pacemaker implantation in the United States. Public Health Rep. 1995 Jan-Feb;110(1):42–46. [PMC free article] [PubMed] [Google Scholar]
- 33.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013 Jan 02;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005 Apr 20;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 35.Houston DK, Ding J, Nicklas BJ, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults: the health, aging and body composition study. Am J Epidemiol. 2009 Apr 15;169(8):927–936. doi: 10.1093/aje/kwp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strandberg TE, Sirola J, Pitkala KH, Tilvis RS, Strandberg AY, Stenholm S. Association of midlife obesity and cardiovascular risk with old age frailty: a 26-year follow-up of initially healthy men. Int J Obes (Lond) 2012 Sep;36(9):1153–1157. doi: 10.1038/ijo.2012.83. [DOI] [PubMed] [Google Scholar]
- 37.Elkins JS, Whitmer RA, Sidney S, Sorel M, Yaffe K, Johnston SC. Midlife obesity and long-term risk of nursing home admission. Obesity (Silver Spring) 2006 Aug;14(8):1472–1478. doi: 10.1038/oby.2006.167. [DOI] [PubMed] [Google Scholar]
- 38.Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr. 2014 Apr;99(4):875–890. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 39.Veronese N, Cereda E, Solmi M, et al. Inverse relationship between body mass index and mortality in older nursing home residents: a meta-analysis of 19,538 elderly subjects. Obes Rev. 2015 Nov;16(11):1001–1015. doi: 10.1111/obr.12309. [DOI] [PubMed] [Google Scholar]
- 40.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999 Mar;69(3):373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 41.McAuley PA, Artero EG, Sui X, et al. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012 May;87(5):443–451. doi: 10.1016/j.mayocp.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013 Dec 4;35:51–65. doi: 10.1093/epirev/mxs006. [DOI] [PubMed] [Google Scholar]
- 43.Zizza CA, Herring A, Stevens J, Popkin BM. Obesity affects nursing-care facility admission among whites but not blacks. Obes Res. 2002 Aug;10(8):816–823. doi: 10.1038/oby.2002.110. [DOI] [PubMed] [Google Scholar]
- 44.Chang VW, Alley DE, Dowd JB. Trends in the Relationship of Obesity and Disability, 1988–2012. Am J Epidemiol. 2017 May 09; doi: 10.1093/aje/kwx092. [DOI] [PubMed] [Google Scholar]
- 45.Visser M, Langlois J, Guralnik JM, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998 Sep;68(3):584–590. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 46.Visser M, Harris TB, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998 May;53(3):M214–221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 47.Masud T, Morris RO. Epidemiology of falls. Age Ageing. 2001 Nov;30(Suppl 4):3–7. doi: 10.1093/ageing/30.suppl_4.3. [DOI] [PubMed] [Google Scholar]
- 48.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003 Jan 02;348(1):42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 49.American Geriatrics Society Workgroup on Vitamin DSfOA. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. J Am Geriatr Soc. 2014 Jan;62(1):147–152. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 50.Himes CL, Reynolds SL. Effect of obesity on falls, injury, and disability. J Am Geriatr Soc. 2012 Jan;60(1):124–129. doi: 10.1111/j.1532-5415.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- 51.Ylitalo KR, Karvonen-Gutierrez CA. Body mass index, falls, and injurious falls among U.S. adults: Findings from the 2014 Behavioral Risk Factor Surveillance System. Prev Med. 2016 Oct;91:217–223. doi: 10.1016/j.ypmed.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 52.Tang X, Liu G, Kang J, et al. Obesity and risk of hip fracture in adults: a meta-analysis of prospective cohort studies. PLoS One. 2013;8(4):e55077. doi: 10.1371/journal.pone.0055077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009 Dec;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002 May;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 55.Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013 Jun;61(6):974–980. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- 56.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010 May;13(3):271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menant JC, Weber F, Lo J, et al. Strength measures are better than muscle mass measures in predicting health-related outcomes in older people: time to abandon the term sarcopenia? Osteoporos Int. 2017 Jan;28(1):59–70. doi: 10.1007/s00198-016-3691-7. [DOI] [PubMed] [Google Scholar]
- 58.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011 Mar 31;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006 Oct;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 60.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004 Dec;12(12):1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 61.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 62.Stenholm S, Alley D, Bandinelli S, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes (Lond) 2009 Jun;33(6):635–644. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res. 2015 Dec;35(12):1031–1039. doi: 10.1016/j.nutres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batsis JA, Mackenzie TA, Emeny RT, Lopez-Jimenez F, Bartels SJ. Low Lean Mass With and Without Obesity, and Mortality: Results From the 1999–2004 National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2017 Feb 16; doi: 10.1093/gerona/glx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014 Sep;68(9):1001–1007. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- 66.Hardy R, Kuh D. Commentary: BMI and mortality in the elderly–a life course perspective. Int J Epidemiol. 2006 Feb;35(1):179–180. doi: 10.1093/ije/dyi302. [DOI] [PubMed] [Google Scholar]
- 67.Richman EL, Stampfer MJ. Weight loss and mortality in the elderly: separating cause and effect. J Intern Med. 2010 Aug;268(2):103–105. doi: 10.1111/j.1365-2796.2010.02227.x. [DOI] [PubMed] [Google Scholar]
- 68.Villareal DT, Apovian CM, Kushner RF, Klein S, American Society for N, Naaso TOS Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005 Nov;82(5):923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 69.Batsis JA, Gill LE, Masutani RK, et al. Weight Loss Interventions in Older Adults with Obesity: A Systematic Review of Randomized Controlled Trials Since 2005. J Am Geriatr Soc. 2017 Feb;65(2):257–268. doi: 10.1111/jgs.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacob ME, Yee LM, Diehr PH, et al. Can a Healthy Lifestyle Compress the Disabled Period in Older Adults? J Am Geriatr Soc. 2016 Oct;64(10):1952–1961. doi: 10.1111/jgs.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or Resistance Exercise, or Both in Dieting Obese Older Adults. N Engl J Med. 2017;376:1943–1955. doi: 10.1056/NEJMoa1616338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One. 2015;10(3):e0121993. doi: 10.1371/journal.pone.0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gill LE, Bartels SJ, Batsis JA. Weight Management in Older Adults. Curr Obes Rep. 2015 Sep;4(3):379–388. doi: 10.1007/s13679-015-0161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014 Apr;15(4):310–321. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soltani S, Hunter GR, Kazemi A, Shab-Bidar S. The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2016 Sep;27(9):2655–2671. doi: 10.1007/s00198-016-3617-4. [DOI] [PubMed] [Google Scholar]
- 76.Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Sainsbury A. Effect of diet-induced weight loss on muscle strength in adults with overweight or obesity – a systematic review and meta-analysis of clinical trials. Obes Rev. 2016 Aug;17(8):647–663. doi: 10.1111/obr.12422. [DOI] [PubMed] [Google Scholar]
- 77.Zibellini J, Seimon RV, Lee CM, et al. Does Diet-Induced Weight Loss Lead to Bone Loss in Overweight or Obese Adults? A Systematic Review and Meta-Analysis of Clinical Trials. J Bone Miner Res. 2015 Dec;30(12):2168–2178. doi: 10.1002/jbmr.2564. [DOI] [PubMed] [Google Scholar]
- 78.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011 Dec;26(12):2851–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995 Nov;43(11):1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 80.Batsis JA, Huddleston JM, Melton LJt, et al. Body mass index and risk of adverse cardiac events in elderly patients with hip fracture: a population-based study. J Am Geriatr Soc. 2009 Mar;57(3):419–426. doi: 10.1111/j.1532-5415.2008.02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014 Nov 05;312(17):1779–1791. doi: 10.1001/jama.2014.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009 Sep;24(9):1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aveyard P, Lewis A, Tearne S, et al. Screening and brief intervention for obesity in primary care: a parallel, two-arm, randomised trial. Lancet. 2016 Nov 19;388(10059):2492–2500. doi: 10.1016/S0140-6736(16)31893-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N) Centers for Medicare & Medicaid Services; 2011. http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253. Accessed March 22nd, 2013. [Google Scholar]
- 85.Batsis JA, Huyck KL, Bartels SJ. Challenges with the Medicare obesity benefit: practical concerns & proposed solutions. J Gen Intern Med. 2015 Jan;30(1):118–122. doi: 10.1007/s11606-014-3031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Batsis JA, Pletcher SN, Stahl JE. Telemedicine and primary care obesity management in rural areas – innovative approach for older adults? BMC Geriatr. 2017 Jan 05;17(1):6. doi: 10.1186/s12877-016-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Batsis JA, Bynum JP. Uptake of the centers for medicare and medicaid obesity benefit: 2012–2013. Obesity (Silver Spring) 2016 Sep;24(9):1983–1988. doi: 10.1002/oby.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging. 2010 Sep 07;5:259–270. doi: 10.2147/cia.s6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation. 2014 Jun 24;129(25 suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Luca CR, Leventer RJ. Developmental trajectories of executive functions across the lifespan. Washington, DC: Taylor & Francis; 2008. [Google Scholar]
- 91.Ende J, Kazis L, Ash A, Moskowitz MA. Measuring patients’ desire for autonomy: decision making and information-seeking preferences among medical patients. J Gen Intern Med. 1989 Jan-Feb;4(1):23–30. doi: 10.1007/BF02596485. [DOI] [PubMed] [Google Scholar]
- 92.Orbell S, Sheeran P. ‘Inclined abstainers’: a problem for predicting health-related behaviour. Br J Soc Psychol. 1998 Jun;37(Pt 2):151–165. doi: 10.1111/j.2044-8309.1998.tb01162.x. [DOI] [PubMed] [Google Scholar]
- 93.Allan JL, Sniehotta FF, Johnston M. The best laid plans: planning skill determines the effectiveness of action plans and implementation intentions. Ann Behav Med. 2013 Aug;46(1):114–120. doi: 10.1007/s12160-013-9483-9. [DOI] [PubMed] [Google Scholar]
- 94.Allan JL, Johnston M, Campbell N. Missed by an inch or a mile? Predicting the size of intention-behaviour gap from measures of executive control. Psychol Health. 2011 Jun;26(6):635–650. doi: 10.1080/08870441003681307. [DOI] [PubMed] [Google Scholar]
- 95.Nigg CR, Long CR. A systematic review of single health behavior change interventions vs. multiple health behavior change interventions among older adults. Transl Behav Med. 2012 Jun;2(2):163–179. doi: 10.1007/s13142-012-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.French DP, Olander EK, Chisholm A, Mc Sharry J. Which behaviour change techniques are most effective at increasing older adults’ self-efficacy and physical activity behaviour? A systematic review. Ann Behav Med. 2014 Oct;48(2):225–234. doi: 10.1007/s12160-014-9593-z. [DOI] [PubMed] [Google Scholar]
- 97.Purath J, Keck A, Fitzgerald CE. Motivational interviewing for older adults in primary care: a systematic review. Geriatr Nurs. 2014 May-Jun;35(3):219–224. doi: 10.1016/j.gerinurse.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 98.Ziegelmann JP, Knoll N. Future Directions in the Study of Health Behavior among Older Adults. Gerontology. 2015;61(5):469–476. doi: 10.1159/000369857. [DOI] [PubMed] [Google Scholar]
- 99.Lum TY, Lightfoot E. The effects of volunteering on the physical and mental health of older people. Res Aging. 2005;27:31–55. [Google Scholar]
- 100.Guiding principles for the care of older adults with multimorbidity: an approach for c. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012 Oct;60(10):E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Freund AM. Age-differential motivational consequences of optimization versus compensation focus in younger and older adults. Psychol Aging. 2006 Jun;21(2):240–252. doi: 10.1037/0882-7974.21.2.240. [DOI] [PubMed] [Google Scholar]
- 102.Levy BR, Slade MD, Kasl SV. Longitudinal benefit of positive self-perceptions of aging on functional health. J Gerontol B Psychol Sci Soc Sci. 2002 Sep;57(5):P409–417. doi: 10.1093/geronb/57.5.p409. [DOI] [PubMed] [Google Scholar]
- 103.Lockenhoff CE, Carstensen LL. Socioemotional selectivity theory, aging, and health: the increasingly delicate balance between regulating emotions and making tough choices. J Pers. 2004 Dec;72(6):1395–1424. doi: 10.1111/j.1467-6494.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- 104.Newsom JT, Huguet N, McCarthy MJ, et al. Health behavior change following chronic illness in middle and later life. J Gerontol B Psychol Sci Soc Sci. 2012 May;67(3):279–288. doi: 10.1093/geronb/gbr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keller H, Laporte M, Payette H, et al. Prevalence and predictors of weight change post discharge from hospital: a study of the Canadian Malnutrition Task Force. Eur J Clin Nutr. 2017 Feb 22; doi: 10.1038/ejcn.2016.277. [DOI] [PubMed] [Google Scholar]
- 106.Fulton MM, Allen ER. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract. 2005 Apr;17(4):123–132. doi: 10.1111/j.1041-2972.2005.0020.x. [DOI] [PubMed] [Google Scholar]
- 107.American Geriatrics Society. 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015 Nov;63(11):2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 108.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults: a consensus report. J Am Geriatr Soc. 2012 Dec;60(12):2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soysal P, Veronese N, Thompson T, et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res Rev. 2017 Mar 31;36:78–87. doi: 10.1016/j.arr.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 110.Sibille KT, McBeth J, Smith D, Wilkie R. Allostatic load and pain severity in older adults: Results from the English Longitudinal Study of Ageing. Exp Gerontol. 2017 Feb;88:51–58. doi: 10.1016/j.exger.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mendelson G, Ness J, Aronow WS. Drug treatment of hypertension in older persons in an academic hospital-based geriatrics practice. J Am Geriatr Soc. 1999 May;47(5):597–599. doi: 10.1111/j.1532-5415.1999.tb02575.x. [DOI] [PubMed] [Google Scholar]
- 112.Castillo DC, Ramsey NLM, Yu SSK, Ricks M, Courville AB, Sumner AE. Inconsistent Access to Food and Cardiometabolic Disease: The Effect of Food Insecurity. Curr Cardiovasc Risk Rep. 2012;6(3):245–250. doi: 10.1007/s12170-012-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee JS, Frongillo EA. Nutritional and Health Consequences Are Associated with Food Insecurity among U.S. Elderly Persons. J Nutr. 2001;131:1503–1509. doi: 10.1093/jn/131.5.1503. [DOI] [PubMed] [Google Scholar]
- 114.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997 Apr 17;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 115.Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011 Feb;9(1):1–12. doi: 10.1089/met.2010.0031. [DOI] [PubMed] [Google Scholar]
- 116.Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013 Aug;14(8):542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 117.Colonetti T, Grande AJ, Milton K, et al. Effects of whey protein supplement in the elderly submitted to resistance training: systematic review and meta-analysis. Int J Food Sci Nutr. 2017 May;68(3):257–264. doi: 10.1080/09637486.2016.1232702. [DOI] [PubMed] [Google Scholar]
- 118.Porter Starr KN, Pieper CF, Orenduff MC, et al. Improved Function With Enhanced Protein Intake per Meal: A Pilot Study of Weight Reduction in Frail, Obese Older Adults. J Gerontol A Biol Sci Med Sci. 2016 Oct;71(10):1369–1375. doi: 10.1093/gerona/glv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carroll JK, Antognoli E, Flocke SA. Evaluation of physical activity counseling in primary care using direct observation of the 5As. Ann Fam Med. 2011 Sep-Oct;9(5):416–422. doi: 10.1370/afm.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011 Jul;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 121.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014 Jun 18;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011 Mar 01;154(5):356–364. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 123.Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract. 2016 Jul;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 124.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015 Aug 18;314(7):687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 125.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011 Apr 16;377(9774):1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 126.Perna S, Guido D, Bologna C, et al. Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res. 2016 Dec;28(6):1251–1257. doi: 10.1007/s40520-015-0525-y. [DOI] [PubMed] [Google Scholar]
- 127.Pi-Sunyer X, Astrup A, Fujioka K, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015 Jul 02;373(1):11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 128.Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013 Nov;37(11):1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 129.Gastrointestinal surgery for severe obesity. Am J Clin Nutr; Proceedings of a National Institutes of Health Consensus Development Conference; March 25–27, 1991; Bethesda, MD. Feb, 1992. pp. 487s–619s. [DOI] [PubMed] [Google Scholar]
- 130.Meron Eldar SHH, Kroh M, Chand B, Rogula T, Schauer P, Brethauer S. Bariatric surgery in the elderly – Comparing the 3 major bariatric procedures. Obes Surg. 2011;21(8):1134–1135. [Google Scholar]
- 131.Batsis JA, Miranda WR, Prasad C, et al. Effect of bariatric surgery on cardiometabolic risk in elderly patients: A population-based study. Geriatr Gerontol Int. 2016 May;16(5):618–624. doi: 10.1111/ggi.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flum DR, Kwon S, MacLeod K, et al. The use, safety and cost of bariatric surgery before and after Medicare’s national coverage decision. Ann Surg. 2011 Dec;254(6):860–865. doi: 10.1097/SLA.0b013e31822f2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.O’Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patients aged 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obes Surg. 2010 Sep;20(9):1199–1205. doi: 10.1007/s11695-010-0201-4. [DOI] [PubMed] [Google Scholar]
- 134.Quebbemann B, Engstrom D, Siegfried T, Garner K, Dallal R. Bariatric surgery in patients older than 65 years is safe and effective. Surg Obes Relat Dis. 2005 Jul-Aug;1(4):389–392. doi: 10.1016/j.soard.2005.05.003. discussion 392–383. [DOI] [PubMed] [Google Scholar]
- 135.Ramirez A, Roy M, Hidalgo JE, Szomstein S, Rosenthal RJ. Outcomes of bariatric surgery in patients >70 years old. Surg Obes Relat Dis. 2012 Jul-Aug;8(4):458–462. doi: 10.1016/j.soard.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 136.Ibrahim AM, Ghaferi AA, Thumma JR, Dimick JB. Variation in Outcomes at Bariatric Surgery Centers of Excellence. JAMA Surg. 2017 Apr 26; doi: 10.1001/jamasurg.2017.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Livingston EH. Bariatric surgery outcomes at designated centers of excellence vs nondesignated programs. Arch Surg. 2009 Apr;144(4):319–325. doi: 10.1001/archsurg.2009.23. discussion 325. [DOI] [PubMed] [Google Scholar]
- 138.Giordano S, Victorzon M. Bariatric surgery in elderly patients: a systematic review. Clin Interv Aging. 2015;10:1627–1635. doi: 10.2147/CIA.S70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mathus-Vliegen EM, Obesity Management Task Force of the European Association for the Study of O Prevalence, pathophysiology, health consequences and treatment options of obesity in the elderly: a guideline. Obes Facts. 2012;5(3):460–483. doi: 10.1159/000341193. [DOI] [PubMed] [Google Scholar]
- 140.Batsis JA, Dolkart KM. Evaluation of older Adults with obesity for bariatric surgery: Geriatricians’ perspective. Journal of Clinical Gerontology and Geriatrics. 2015;6(2):45–53. [Google Scholar]


