Abstract
OBJECTIVE
To assess whether the use of a lab test specific for intact human chorionic gonadotropin (hCG) would reduce the number of false-positive pregnancy cases.
METHODS
From 10/21/14 – 01/20/15 and 04/01/15 – 06/02/15, all serum samples sent for pregnancy screening at a large cancer center with a value of ≥5 mIU/mL total β-hCG were frozen and stored, then re-tested using intact hCG reagent. We compared the accuracy of total β-hCG and intact hCG results for the diagnosis of clinically confirmed pregnancy. A negative test was defined as ≤14 mIU/mL, our current institutional cutoff. We also assessed a cutoff of <5 mIU/mL, a historical cutoff to rule out pregnancy.
RESULTS
We performed intact hCG testing on 64 patient samples, of which 34 had originally resulted positive when tested for total β-hCG. These included 21 cases of clinically confirmed pregnancy, and 13 false positive cases. No women were pregnant when their intact hCG concentration was ≤14 mIU/mL, and all pregnancies were detected at and above this concentration. Intact hCG reduced the number of false positive pregnancy tests from 13 to 1, a 92% reduction [95% Confidence Interval (CI) 64–99%], corresponding to a reduction in the false-positive rate from 38% [95% CI 22–56%] to 3% [95% CI 1–15%].
CONCLUSION
The use of intact hCG reagent in cancer patients reduces the rate of false positive pregnancy tests without increasing the rate of false negative tests.
INTRODUCTION
Screening for pregnancy is a standard protocol at our cancer center for all women of potential childbearing age who may be undergoing medical or surgical procedures. However, a number of different epithelial cancers can produce a form of the β-hCG subunit, and lead to false positive pregnancy results.1
Typically, pregnancy testing is done qualitatively on urine or serum, or by quantifying serum β-hCG. Current quantitative assays target the beta subunit and thus do not discriminate between intact-hCG and β-hCG. Therefore, what they measure is often referred to as total β-hCG.2
Since intact hCG predominates in early pregnancy,2,3 we sought to determine whether the use of a reagent specific for intact hCG would reduce the rate of falsely positive pregnancy tests among patients with cancer while preserving screening sensitivity.
MATERIALS AND METHODS
In this diagnostic performance study, we reviewed 3679 serum pregnancy screens over the periods of 10/21/14 – 01/20/15 and 04/01/15 – 06/02/15, of which 64 samples having total β-hCG values ≥5 mIU/mL were frozen and stored. These samples were obtained under the Memorial Sloan Kettering IRB approved protocol 16-1276. Testing was performed on the Tosoh AIA 2000 by immunoenzymometric assay. This method uses an immobilized capture antibody (magnetic bead) specific for the C-terminal peptide (CTP) region of β-hCG (Figure 1). Quantification is derived from a signal produced by a chemical reaction between a substrate and an enzyme attached to a monoclonal antibody. This antibody is specific to the beta subunit folded core antigen of hCG. Both intact hCG and β-hCG are included in the measurement. The capture antibody to the CTP region is what makes the assay specific for hCG, differentiating it from other similar proteins such as LH (luteinizing hormone), FSH (follicle stimulating hormone), and TSH (thyroid stimulated hormone).
Figure 1.
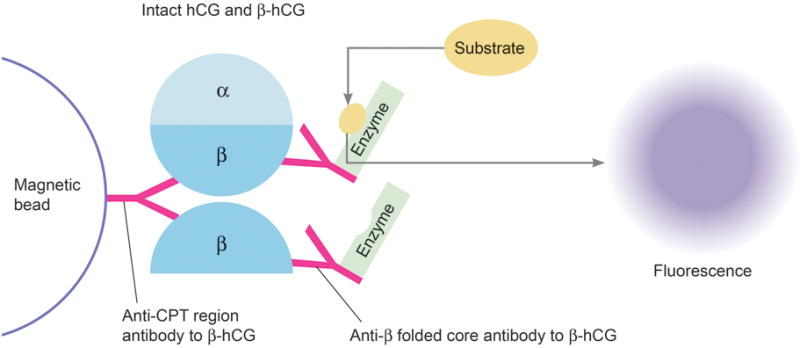
Total β-hCG assay (current method): human chorionic gonadotropin (hCG) and free β-hCG are both bound onto magnetic beads and tagged with antibody labeled enzyme which breaks down substrate into a fluorescence signal that can be measured. Both intact hCG and free β-hCG are measured together.
Patients being monitored for trophoblastic cancer were excluded as the pregnancy screen was inappropriately ordered for them. Trophoblastic tumors also produce intact hCG which cannot be differentiated from the hCG produced during a pregnancy. Repeat tests on the same patient were also excluded. The remaining samples were retested using a quantitative assay (Tosoh) specific for intact hCG on the Tosoh AIA 2000. In this assay the immobilized capture antibody is different, being specific for the alpha subunit portion of hCG (Figure 2). Quantification is again through a signal from a substrate and an enzyme labeled monoclonal antibody. In this case, the enzyme labeled antibody is specific to the CTP region of the beta subunit in order to maintain specificity.
Figure 2.
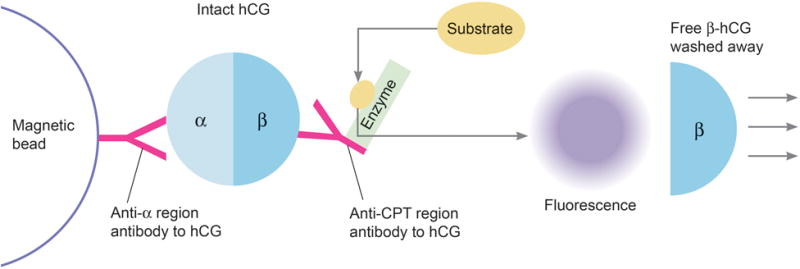
Intact human chorionic gonadotropin (hCG) assay (proposed method): Only intact hCG are bound onto magnetic beads and tagged with antibody labeled enzyme which breaks down substrate into a fluorescence signal that can be measured. Free β-hCG is washed away and not quantified.
Patient specimens with total β-hCG >14 mIU/mL, our institutional cutoff, were considered compatible with pregnancy. This upper limit of 14 mIU/mL as normal is used because the cutoff of <5 mIU/mL, a historically accepted reference value, resulted in a 5.0% false positive rate in our patient population. The higher threshold allowed a 0.4% false positive rate without reducing sensitivity.
Total β-hCG and intact hCG results were compared with each other, and then to clinical follow up as the gold standard of pregnancy. Pregnancy status was confirmed by the authors through the review of clinical notes on follow up visits; in all cases we either verified pregnancy or determined that the test was a false positive. Pregnancy was assumed to be ruled out in patients with total β-hCG <5 mIU/mL. Samples from patients whom both total β-hCG and intact hCG were ≥5 mIU/mL and were not pregnant as per follow up clinical notes were tested quantitatively for FSH to determine whether hCG was coming from the pituitary gland. Samples with FSH >45 mIU/mL were considered to be compatible with hCG of pituitary origin.4
RESULTS
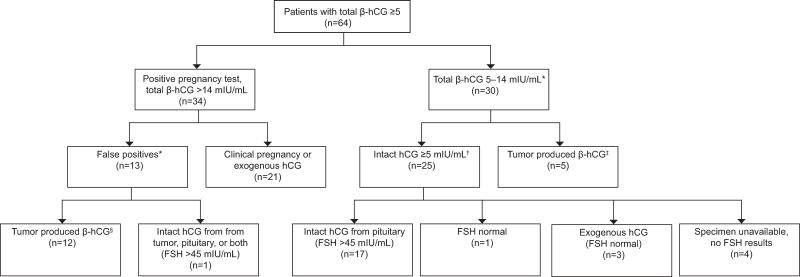
Of the 3,679 samples that were tested for total β-hCG, 64 had levels ≥5 mIU/mL. Of these, 34 had total β-hCG levels >14 mIU/mL, and the remaining 30 ranged from 5–14 mIU/mL. All 64 samples were analyzed for intact hCG (Summarized in Figure 3).
Figure 3.
Breakdown of the 64 out of 3,679 patient samples with total β-hCG ≥5 mIU/mL. *Pregnancy in these patients were ruled out by chart review. †Results for intact human chorionic gonadotropin (hCG) and total β-hCG were similarly elevated ≥5 mIU/mL in this group. ‡These samples had significantly lower levels of intact hCG, sometimes below detectable range, than total β-hCG consistent with tumor produced beta hCG. §These samples had intact hCG ≤14, thereby reducing the number of false positives from 13 to 1. FSH, follicle-stimulating hormone.
Of the 34 patients with total β-hCG >14 mIU/mL, twelve had intact hCG below 14 mIU/mL attributable to tumor-derived β-hCG (Table 1). These twelve were confirmed as not pregnant on clinical follow up. There was an additional non-pregnant patient with elevated intact hCG possibly attributable to pituitary hCG or from the patient’s urothelial carcinoma which has been shown to produce intact hCG on rare occasion.5–8 Intact hCG, total beta-hCG, and FSH on this sample were 235, 283, and 165 mIU/mL, respectively. Normally, FSH is ≤ 32 in premenopausal patients. The remaining 21 of the 34 patients with total β-hCG >14 mIU/mL cases were true positives. No pregnancies were missed by intact hCG quantification. Overall, there were 13 falsely positive cases based on total β-hCG of which 12 (92% [95% CI 64–99%]) would be eliminated using intact hCG analysis when 14 mIU/mL is used as the cutoff. The false positive rate was reduced from 38% [95% CI 22–56%] to 3% [95% CI 1–15%]. In our facility this would calculate to about 29 patients a year in whom a false positive pregnancy test would be avoided by pregnancy screening with intact hCG.
Table 1.
List of Cancers Producing β-hCG in Study
| Patients Cancer Type | Total hCG (mIU/mL) | Intact hCG (mIU/mL) |
|---|---|---|
| Anaplastic Astrocytoma | 17.7 | <0.5 |
| Breast Mixed Ductal/Lobular Carcinoma | 13.5 | 0.7 |
| Cervical Adenocarcinoma | 11.1 | <0.5 |
| Cervical Squamous Cell Carcinoma | 40.3 | <0.5 |
| Cholangiocarcinoma | 12.5 | <0.5 |
| Cholangiocarcinoma | 11.4 | <0.5 |
| Colonic Adenocarcinoma | 25.4 | 1.2 |
| Colonic Adenocarcinoma | 757.5 | 2.7 |
| Colonic Adenocarcinoma | 102.8 | <0.5 |
| Gastric Adenocarcinoma | 37.3 | 5.5 |
| Lung Squamous Cell Carcinoma | 7.6 | <0.5 |
| Pancreatic Adenocarcinoma | 77.4 | 0.6 |
| Pancreatic Adenocarcinoma | 35.4 | 2.7 |
| Pancreatic Adenocarcinoma | 77.3 | <0.5 |
| Pancreatic Adenocarcinoma | 16.2 | <0.5 |
| Sacral Giant Cell Tumor | 48.5 | <0.5 |
| Thyroid Medullary Carcinoma | 124.9 | <0.5 |
A variety of non-trophoblastic tumors (N=17) were shown to produce β-hCG with comparatively smaller to immeasurable amounts of intact hCG.
Of the 30 patients between ≥5 and ≤14 mIU/mL total β-hCG, five had low amounts of intact hCG with higher total β-hCG consistent with tumor origin (Table 1). The remaining 25 had similar values for both intact hCG and total β-hCG. FSH was elevated above 45 mIU/mL in 17 of them, consistent with pituitary origin (Table 2). Of the remaining eight, three had history of exogenous hCG (FSH = 9, 2, and 2 mIU/mL), four samples were not available for FSH quantification, and one had an FSH of 13 mIU/mL (total β-hCG and intact hCG at about 6 mIU/mL). An additional 63 patients negative for pregnancy screening using total β-hCG quantification were retested for intact hCG quantification, all of which remained negative.
Table 2.
Follicle-Stimulating Hormone Levels >45 mIU/mL in Patients With Total Human Chorionic Gonadotropin (hCG) and Intact hCG ≥5, and ≤14 mIU/mL Consistent With hCG of Pituitary Origin
| Patient Age (years) | Intact hCG (mIU/mL) | Total β-hCG (mIU/mL) | FSH (mIU/mL) |
|---|---|---|---|
| 31 | 6.5 | 6.2 | 299.87 |
| 50 | 13.8 | 13.6 | 154.4 |
| 18 | 5.5 | 5.5 | 152.72 |
| 50 | 5.3 | 8.3 | 151.96 |
| 30 | 5.6 | 6.3 | 133.2 |
| 41 | 5.2 | 5.1 | 125.56 |
| 46 | 6.9 | 6.7 | 122.19 |
| 50 | 5.3 | 5.2 | 117.97 |
| 56 | 5.4 | 5.3 | 105.87 |
| 49 | 5.8 | 6.1 | 100.95 |
| 43 | 6.7 | 6.7 | 100.62 |
| 53 | 7 | 7.1 | 94.72 |
| 48 | 11.1 | 11 | 93.47 |
| 54 | 5.7 | 7.6 | 77.33 |
| 49 | 6 | 5.9 | 67.31 |
| 50 | 6 | 6.2 | 56.94 |
| 49 | 7.2 | 6.8 | 45.39 |
Note that FSH and hCG levels do not correlate proportionally.
DISCUSSION
We found that the use of intact hCG reagent in cancer patients with total hCG ≥5 mIU/mL reduces false positive pregnancy tests. This reduction is important, because in our experience as a cancer center, it is most distressing to patients when a pregnancy test unexpectedly comes back positive. In response, a clinician may need to order other studies, send the patient for an obstetric ultrasound evaluation, or delay procedures or treatments for a few days then retest total β-hCG to assess its trend.9 Such instances can cause stress to patients due to delays in critical treatment and unnecessary concern regarding an unintended pregnancy during cancer therapy. Furthermore, there are resource and financial implications to the institution if procedures are canceled and rescheduled. A false positive pregnancy test is also dangerous as it may prompt incorrect therapy which could cause direct harm to the patient. Instances of this include postponement of critical surgeries, needless chemotherapy, inappropriate hysterectomy, cancellation of renal transplants, and expensive follow-up testing.2,10–13 For these reasons it is important to limit the number of false positives.
A limitation of the study was that it was of a small cohort. Although all pregnancies detected over the period of five months were also adequately detected by the intact hCG method, the number was low (n=12). A larger cohort of pregnant patients would have to be tested with the intact reagent to better characterize actual assay sensitivity and false negative rate. The possibility of decreased sensitivity, however, is low since the monoclonal antibodies in the reagent are specifically designed to capture and label normal intact hCG, and this assay is already FDA approved for use in the detection of pregnancy. Also, due to constraints in available intact hCG reagent, only cases ≥5mIU/mL were tested. We were also unable to assess the new test in non-cancer patients as they were not available in our patient population.
In summary, we have shown that by using the intact hCG reagent for pregnancy testing, false positive results among cancer patients are decreased by more than 90%. Clinically, this reduces the amount of unwarranted follow-up testing and prevents improper medical care such as surgery or chemotherapy.
Acknowledgments
The authors thank Barbara Burch, Administrative Operations Manager, for presenting the idea of the project and providing guidance in areas of laboratory and hospital operations; Lindsay Boyce, Research Informationist, for assistance in a literature review; Caryn Tursky, Illustrator, for producing figures 1 and 2; and Katherine Panageas, Associate Attending Biostatistician, for assistance in statistical calculations and interpretations.
Funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Presented at the 2015 AACC Annual Meeting (July 28) in Atlanta, GA.
Contributor Information
Samuel I McCash, Memorial Sloan Kettering Cancer Center, Department of Laboratory Medicine.
Deborah J. Goldfrank, Memorial Sloan Kettering Cancer Center, Department of Surgery.
Melissa S. Pessin, Memorial Sloan Kettering Cancer Center, Department of Laboratory Medicine.
Lakshmi V. Ramanathan, Memorial Sloan Kettering Cancer Center, Department of Laboratory Medicine.
References
- 1.Cole LA. Human chorionic gonadotropin and associated molecules. Expert review of molecular diagnostics. 2009;9(1):51–73. doi: 10.1586/14737159.9.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Cole LA. Human chorionic gonadotropin tests. Expert review of molecular diagnostics. 2009;9(7):721–747. doi: 10.1586/erm.09.51. [DOI] [PubMed] [Google Scholar]
- 3.Cole LA, Khanlian SA, Riley JM, Butler SA. Hyperglycosylated hCG in gestational implantation and in choriocarcinoma and testicular germ cell malignancy tumorigenesis. The Journal of reproductive medicine. 2006;51(11):919–929. [PubMed] [Google Scholar]
- 4.Gronowski AM, Fantz CR, Parvin CA, et al. Use of serum FSH to identify perimenopausal women with pituitary hCG. Clinical chemistry. 2008;54(4):652–656. doi: 10.1373/clinchem.2007.100305. [DOI] [PubMed] [Google Scholar]
- 5.Dirnhofer S, Koessler P, Ensinger C, Feichtinger H, Madersbacher S, Berger P. Production of trophoblastic hormones by transitional cell carcinoma of the bladder: association to tumor stage and grade. Human pathology. 1998;29(4):377–382. doi: 10.1016/s0046-8177(98)90119-8. [DOI] [PubMed] [Google Scholar]
- 6.Iles RK, Chard T. Immunochemical analysis of the human chorionic gonadotrophin-like material secreted by ‘normal’ and neoplastic urothelial cells. Journal of molecular endocrinology. 1989;2(2):107–112. doi: 10.1677/jme.0.0020107. [DOI] [PubMed] [Google Scholar]
- 7.Marcillac I, Cottu P, Theodore C, Terrier-Lacombe MJ, Bellet D, Droz JP. Free hCG-beta subunit as tumour marker in urothelial cancer. Lancet. 1993;341(8856):1354–1355. doi: 10.1016/0140-6736(93)90872-e. [DOI] [PubMed] [Google Scholar]
- 8.Mora J, Gascon N, Tabernero JM, Rodriguez-Espinosa J, Gonzalez-Sastre F. Different hCG assays to measure ectopic hCG secretion in bladder carcinoma patients. British journal of cancer. 1996;74(7):1081–1084. doi: 10.1038/bjc.1996.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pittaway DE, Wentz AC. Evaluation of early pregnancy by serial chorionic gonadotropin determinations: a comparison of methods by receiver operating characteristic curve analysis. Fertility and sterility. 1985;43(4):529–533. doi: 10.1016/s0015-0282(16)48492-x. [DOI] [PubMed] [Google Scholar]
- 10.Cole LA, Khanlian SA, Giddings A, et al. Gestational trophoblastic diseases: 4. Presentation with persistent low positive human chorionic gonadotropin test results. Gynecologic oncology. 2006;102(2):165–172. doi: 10.1016/j.ygyno.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 11.Cole LA, Khanlian SA, Muller CY. Detection of perimenopause or postmenopause human chorionic gonadotropin: an unnecessary source of alarm. Am J Obstet Gynecol. 2008;198(3):275 e271–277. doi: 10.1016/j.ajog.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Cole LA, Khanlian SA, Muller CY. Normal production of human chorionic gonadotropin in perimenopausal and menopausal women and after oophorectomy. Int J Gynecol Cancer. 2009;19(9):1556–1559. doi: 10.1111/igc.0b013e3181a40cf2. [DOI] [PubMed] [Google Scholar]
- 13.Cole LA, Sasaki Y, Muller CY. Normal production of human chorionic gonadotropin in menopause. The New England journal of medicine. 2007;356(11):1184–1186. doi: 10.1056/NEJMc066500. [DOI] [PubMed] [Google Scholar]