Abstract
Background
Prospective studies to determine associated risk factors and related outcomes for pulmonary fungal infection (PFI) after pediatric lung transplant (PLT) are lacking.
Methods
NIH-sponsored Clinical Trials in Organ Transplantation in Children enrolled PLT candidates, collecting data prospectively for 2 years post-transplant. Demographics, signs/symptoms, radiology, pathology and microbiology were collected. Analyses evaluated for PFI-related risks and outcomes.
Results
In 59 PLT, pre-transplant fungal colonization occurred in 6 donors and 15 recipients. Cystic fibrosis (CF) was associated with pre-transplant colonization (p<0.01). Twenty-five (42%) PLT had 26 post-transplant colonizations (median=67d, range=0–750d) with Candida(13), Aspergillus(4), mold(6) or yeast(3). Post-PLT colonization was not associated with CF, age or pre-PLT colonization. Thirteen PFIs occurred in 10(17%) patients, 3 proven (Candida species) and 10 probable (Candida[3], Aspergillus[3], Penicillium[3], and mold[1]). PFI was preceded by post-PLT colonization with the same organism in 4 of 13 PFI, but post-PLT colonization did not predict subsequent PFI(p=0.87). Older age at transplant was a risk for PFI(p<0.01). No mortality was attributed to PFI. Prophylaxis use was not associated with decreased post-PLT colonization (p=0.60) or PFI (p=0.48).
Conclusion
In PLT, PFI and fungal colonization are common but without associated mortality. Post-PLT colonization did not predict PFI. Optimal prevention strategies require additional study.
Keywords: Lung Transplantation, Pediatrics, Fungal Colonization, Pulmonary Fungal Infection (PFI), Prophylaxis
Background
Fungal infection remains a serious complication for patients after lung transplantation. Invasive aspergillosis was reported in 3% to 16% of adult lung transplant recipients, with a mortality rate of 41% to 50%.1–5 In a retrospective multi-centered study of 555 pediatric lung transplant recipients, pulmonary fungal infection (PFI) was reported at similar rates with 10.5% infected by one-year post-transplant. Episodes of acute rejection (AR) and age ≥ 15 years were predictive of subsequent PFI. However, this retrospective study did not evaluate the impact of colonization or preventative therapy.6 A smaller retrospective, single-center study of pediatric patients showed post-transplant colonization rates of 60%, but did not find significant association between colonization and subsequent PFI.7 Adult populations have shown increased incidence of invasive pulmonary aspergillosis following pre-transplantation or post-transplantation colonization by Aspergillus, but risk factors for progression were not well defined.8 The data from these studies relied on retrospective data and were limited by availability of patient records.9 Further, the cohorts of recipients were transplanted before the contemporary era of antifungal prophylaxis. We conducted a prospective, observational cohort study of pediatric lung transplant recipients initially designed to evaluate the impact of respiratory viruses on outcome. As a substudy, we evaluated fungal colonization, pulmonary fungal infections (PFI) and outcomes within two years of transplantation.
Methods
This NIH-funded Clinical Trials in Organ Transplant in Children (CTOTC-03) study was performed at six institutions in the United States from 2009 to 2013 following Institutional Review Board (IRB) approval. This prospective, longitudinal study of pediatric lung transplant recipients collected microbiology, radiology, histology, and clinical data. All cultures were obtained from bronchoalveolar lavage (BAL) fluid or from pleural fluid (on one occasion). Data collection began prior to transplant and continued up to two years post-transplant or until either death or retransplantation, whichever occurred first. Clinical data was collected at scheduled visits including: pre-transplant, at transplant along with relevant donor demographics, and at post-transplant weeks 2, 4, 6, 8 and months 3, 6, 9, 12, 18 and 24. Additionally, data collection occurred with clinical symptoms at unscheduled study visits including events prior to 2 weeks post-transplant.
Therapy Regimens
Patients underwent routine pre-transplant evaluation. Induction and standard immunosuppression were determined by the transplant center and followed International Pediatric Lung Transplant Collaborative (IPLTC) guidelines.10 For induction, three centers used an IL-2 antagonist (43 patients), two used rabbit anti-thymocyte globulin (13 patients), and one administered no induction therapy (3 patients). Triple drug maintenance immunosuppressive therapy included tacrolimus, mycophenolate and prednisone, with standardized levels for tacrolimus. Fungal prophylaxis was determined by the local center.
Definitions
Fungal events were defined based on fungal classification guidelines (ISHLT 2010 and EORTC/MSG 2008)11,12 adapted to the available study data. Events were classified by two independent members of the research team with discrepancies adjudicated by a third member.
Pre-transplant colonization
In donors, pre-transplant colonization was defined as a positive culture with a fungal organism from the implanted organ. In recipients, pre-transplant colonization was defined as either: (1) fungal organisms in the explanted organ or (2) previous history of pulmonary fungal colonization or infection as reported in the clinical pre-transplant history.
Post-transplant colonization
Positive culture of a fungal organism without meeting the definition below of PFI based on symptoms, radiology, and histopathology.
Proven PFI
Presence of all of the following: (1) positive culture, (2) symptoms suggestive of PFI, (3) radiological signs or locally visualized purulent sputum without competing etiology, and (4) positive histopathology or cultured organism from sterile tissue.
Probable PFI
Presence of all of the following: (1) Positive culture or galactomannan blood assay, (2) symptoms suggestive of PFI, (3) radiological signs or locally visualized purulent sputum without competing etiology, and (4) negative or absent histopathology.
Acute cellular rejection
Defined according to the ISHLT criteria.13
Prophylaxis
Anti-fungal regimens first prescribed within the first two weeks (≤ 14 days) post-transplant and initiated without prior post-transplant colonization or PFI. Changes to the regimen that occurred within 21 days of the previous medication were considered a continuation of prophylaxis. Prophylaxis regimens lasting more than 30 days from transplant were defined as extended prophylaxis.
Pre-emptive therapy
Initiation of a new anti-fungal medication prescribed within one week (≤ 7 days) after a new post-transplant colonization was reported.
Statistical Analysis
Collected data were managed and stored by RHO Inc. (Chapel Hill, NC), with analyses performed at Cincinnati Children’s Hospital Medical Center with JMP (Version 12.1.0, Cary, NC) and SAS (Version 9.4, Cary, NC). Association was assessed by status of pre-transplant colonization, post-transplant colonization, and PFI. Association with continuous and nominal or ordinal variables were done using 2-tailed t-tests and Fisher’s exact test or chi squared tests, respectively. Survival tests were conducted with Kaplan-Meier and Cox Proportional Hazards (PH) models to determine association between events with death, retransplantation or 730 days post-transplant as censored time points in this time to PFI model, whichever occurred first. Significance was evaluated at a p-value of ≤ 0.05.
Results
In this prospective cohort, 61 patients were enrolled and transplanted between 2009 and 2013 and followed until death, re-transplantation, or 24 months post-transplant, whichever occurred first. Two patients who died within 21 days of transplant were excluded from analysis; neither patient had a post-transplant fungal event prior to death. Two reported fungal infections were excluded: tinea corporis and a positive urine culture for Candida species. Thirty-nine (59%) patients were female, with a mean age of 12.13 years (range = 0.74 to 19.10 years). Cystic fibrosis (CF) was the primary diagnosis pre-transplant in 29 patients (49%). Common pre-transplant diagnoses include cystic fibrosis (CF) in 29 patients (49%), bronchiolitis obliterans in 7 (12%), pulmonary hypertension in 6 (10%), interstitial lung disease in 4 (7%) and surfactant deficiency in 3 (5%). Demographics are reported in Table 1.
Table 1.
Recipient Demographics by Post-Transplant Event
| Demographics | All Subjects (n=59) |
No event (n=31) |
Colonization (n=18) |
OR (95% CI) | p- value |
PFI (n=10) |
OR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|---|
| Sex | 1.29 (0.40–4.22) | 0.77 | 1.92 (0.42–8.84) | 0.48 | ||||
| female | 35 | 17 | 11 | 7 | ||||
|
| ||||||||
| Age (mean in yrs) | 12.13 | 10.96 | 11.85 | 1.03(0.92–1.15)a | 0.58 | 16.26 | 1.34 (1.03–1.74) | < 0.01 |
|
| ||||||||
| Race | ||||||||
| White | 48 | 26 | 13 | - | - | 9 | - | - |
| Black | 6 | 4 | 1 | 0.50 (0.05–4.94) | 1.00 | 1 | 0.72 (0.07–7.34) | 1.00 |
| Asian | 2 | 0 | 2 | 0.13 | 0 | - | - | |
| Unknown | 3 | 1 | 2 | 4.0 (0.33–48.3) | 0.29 | 0 | 0 | 1.00 |
|
| ||||||||
| Primary Diagnosis | ||||||||
| CF | 29 | 15 | 7 | 0.68 (0.21–2.21) | 0.56 | 7 | 2.49 (0.54–11.43) | 0.29 |
|
| ||||||||
| PGD ≥ Grade 2 | ||||||||
| Yes | 33 | 16 | 12 | 0.53 (0.16–1.78) | 0.38 | 5 | 1.07 (0.26–4.44) | 1.00 |
|
| ||||||||
| Transplant type | 1.17 (0.10–13.92) | 1.00 | 0.62 (0.05–7.67) | 1.00 | ||||
| Double lung | 55 | 29 | 17 | 9 | ||||
| Heart-lung | 4 | 2 | 1 | 1 | ||||
|
| ||||||||
| Pre-tx recipient colonization | 15 | 5 | 5 | 2.00 (0.49–8.17) | 0.46 | 5 | 5.20 (1.08–24.9) | 0.044 |
|
| ||||||||
| Hospitalization post-tx | ||||||||
| >14 days | 47 | 23 | 15 | 0.58 (0.13–2.52) | 0.72 | 9 | 0.32 (0.04–2.93) | 0.41 |
CF = Cystic Fibrosis; PGD = Primary Graft Dysfunction (at any time within 72 hours of transplant)
Colonization p-value comparing colonization to neither
PFI p-value comparing PFI to neither
Unit odds ratio reported
Pre-transplant Colonization
Of the 59 patients in the cohort, six donor organs had positive pre-transplant cultures. Donors were colonized by Aspergillus (1), Candida (3) and unspecified yeast (2).
Twenty positive cultures were identified in 15 recipients prior to transplantation. Recipients were colonized by Aspergillus (10), Candida (7), unspecified mold (2) and unspecified yeast (1) (Table 2). Three patients were colonized with both Aspergillus and Candida, and one patient was colonized by three organisms pre-transplant: Candida albicans, Candida parapsilosis, and Aspergillus fumigatus. Additionally, two of the above patients had positive cultures from both the donor and recipient. C. albicans was recovered from donor and recipient in one subject, while another subject had Aspergillus in the donor and yeast in the recipient. A primary diagnosis of CF was significantly associated with pre-transplant colonization (p < 0.01; OR 11.38, 95% CI 2.27 to 56.93); however, CF was not specifically associated with pre-transplant colonization with Aspergillus species.
Table 2.
Culture results Pre- and Post-transplant
| Species | Pre-Transplant | Post-transplant colonization (n=26) |
Post-transplant PFI (n=13) |
|
|---|---|---|---|---|
|
| ||||
| Donor (n=6) |
Recipient (n=20) |
|||
| Mold (n=27) | ||||
| Aspergillus (n=18) | ||||
| A. flavus | 0 | 1 | 0 | 1 |
| A. fumigatus | 0 | 7 | 3 | 2 |
| A. Unspecified | 1 | 2 | 1 | 0 |
| Penicillium (n=8) | 0 | 0 | 5 | 3 |
| Scedosporium/p. boydii (n=1) | 0 | 0 | 1 | 0 |
| Unspecified Mold (n=3) | 0 | 2 | 0 | 1 |
| Yeast (n=34) | ||||
| Candida (n=28) | ||||
| C. albicans | 2 | 5 | 4 | 1 |
| C. glabrata | 0 | 0 | 2 | 3 |
| C. krusei | 0 | 0 | 2 | 0 |
| C. lusitaniae | 0 | 1 | 1 | 0 |
| C. parapsilosis | 0 | 1 | 1 | 1 |
| C. tropicalis | 1 | 0 | 0 | 0 |
| Candida Unspecified | 0 | 0 | 3 | 1 |
| Yeast Unspecified (n=6) | 2 | 1 | 3 | 0 |
Post-transplant Colonization
Twenty-five patients (42%) had 26 post-transplant colonizations with Candida (13), other yeasts (3), Aspergillus (4), Penicillium (5), and unspecified mold (1) (Table 2). One patient had two separate colonization events with C. albicans and Penicillium, respectively. Events occurred at a median of 67 days post-transplant (range = 0 to 750 days) and lasted a median of 37 days (range = 9 to 315 days). Duration was determined through center report of resolution of the event. Four colonizations had no reported end date and may have persisted beyond the study period.
There were six individual patients presenting with a post-transplant event by the same class as a pre-transplant event (i.e. a pre-transplant mold and subsequent post-transplant mold). Three were colonized within one week of transplant, one with the same species (Candida lusitaniae). More than one month elapsed between transplant and colonization for the other three patients, with two colonized pre- and post-transplant by the same organism (A. fumigatus and C. albicans, respectively). However, pre-transplant colonization, either in the donor or recipient, was not statistically significantly associated with subsequent post-transplant colonization by the same class of organism. CF was not significantly associated with post-transplant colonization.
Post-transplant Pulmonary Fungal Infections (PFI)
Ten of the 59 patients in the cohort (17%) had a total of 13 PFIs post-transplant (10 probable, 3 proven) Proven PFIs, confirmed through histopathology, all occurred within 6 months of transplant and were caused by C. parapsilosis (1), C. glabrata (1), and C. albicans (1) (Tables 2 & 3). Probable PFIs were caused by Candida glabrata (2), unspecified Candida (1), Aspergillus fumigatus (2), A. flavus (1), Penicillium (3) and unspecified mold (1). Probable PFI occurred within six and twelve months post-transplant in six and seven patients, respectively. The remaining three probable PFIs occurred more than 12 months post-transplant. No mortality was attributed to proven or probable PFI in this cohort.
Table 3.
Proven and Probably PFI after Pediatric Lung Transplantation
| ID | Demographics | Pre-Tx cultures (R=recipient; D=Donor) |
Early extended Prophylaxis |
Event Type | Time to event (event length) |
Organisms | Therapy |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 16.3 y.o.; M; CF | R: Mold* | (no opportunity for prophylaxis) | Probable | 0 (20) | Mold* | Ampho (i); Vori |
|
|
|||||||
| 1 | Proven | 5 (15) | C. parapsilosis | Ampho (i); Vori | |||
|
| |||||||
| 2 | 18.2 y.o.; F; CF | R: A. flavus | Vori | Proven | 172 (11) | C. glabrata | Vori |
|
| |||||||
| 3 | 17.5 y.o.; F; CF | D: C. tropicalis | Ampho (i); Vori; Micafungin | Probable | 172 (188) | Candida Species | Ampho (i); Micafungin |
|
|
|||||||
| 3 | Probable | 727 (uk) | C. glabrata | Micafungin | |||
|
| |||||||
| 4 | 14.7 y.o.; F; CF | R: A. fumigatus | Ampho (i); Vori | Probable | 489 (55) | Penicillium | |
|
| |||||||
| 5 | 18.0 y.o.; F; Complex Congenital Heart Disease | Anidulafungin | Probable | 181(uk) | A. fumigatus | Vori | |
|
|
|||||||
| 5 | Probable | 380 (123) | Penicillium | Anidulafungin | |||
|
| |||||||
| 6 | 17.7 y.o.; M; CF | Vori; Anidulafungin | Probable | 64 (uk) | Penicillium | Vori | |
|
| |||||||
| 7 | 8.0 y.o.; F; IPF | Probable | 126 (95) | A. fumigatus | Ampho (i); Vori | ||
|
| |||||||
| 8 | 16.1 y.o.; F; CF | R: Yeast | Probable | 10 (69) | A. flavus | Fluconazole; Vori; Ampho (i & IV) | |
|
| |||||||
| 9 | 19.1 y.o.; F; PPHTN | Proven | 10 (59) | C. albicans | |||
|
| |||||||
| 10 | 17.0 y.o.; M; CF | R: C. albicans; R: Aspergillus species | Ampho (i); Vori | Probable | 40 (uk) | C. glabrata | Ampho (i) |
CF=Cystic Fibrosis; M=Male; F=Female; uk=Unknown end date of infection; Ampho=Amphotericin; Vori=voriconazole; (i)=Route-Inhaled; IV=Route-Intravenous;
= positive galactomannan blood immunoassay
Older age at transplant was significantly associated with PFI (p < 0.01; OR 1.34, CI 1.09 to 1.87). As age appeared to be confounded by underlying diagnosis, CF and non-CF patients were assessed separately. Age at transplant was found to be significant for CF patients (p = 0.01; OR 1.61, CI 0.97 to 2.69), but not significant for non-CF patients (p = 0.09; OR 1.21, CI 0.92 to 1.59). Pre-transplant colonization with any fungal organism was significantly associated with post-transplant PFI, however no correlation was evident between pre-transplant colonization and PFI when accounting for fungal organism; association between pre-transplant colonization and subsequent PFI with the same organism was not statistically significant (p = 0.70; OR 1.32, 0.29 to 5.93). After transplant, post-transplant colonization preceded PFI with the same species in four cases suggesting the potential for progression from colonization to PFI, with colonization preceding onset of PFI by a median of 75 days (range = 16 to 172 days). However, post-transplant colonization was not statistically associated with a subsequent PFI with the same organism (p = 1.00; OR 1.13, CI 0.28 to 4.50). Twelve patients had events of acute cellular rejection, but rejection preceded PFI in only three cases. Acute cellular rejection was not associated with subsequent PFI (p = 0.80; hazard ratio 1.19).
Prophylaxis
For prophylaxis analyses, one additional patient with an early PFI (< 7 days) was excluded. Of the remaining 58 patients, 39 patients (67%) received initial prophylaxis. Patients often received multiple medications throughout the course of their prophylaxis. Eight patients received prophylaxis for less than 30 days, while the remaining 31 patients (53% of the 58 patients) received prophylaxis in continuity for 30 or more days. Excluding 11 patients without specific medication end dates, prophylaxis courses in the remaining 28 patients had a median duration of 82.5 days (range = 5 to 741 days).
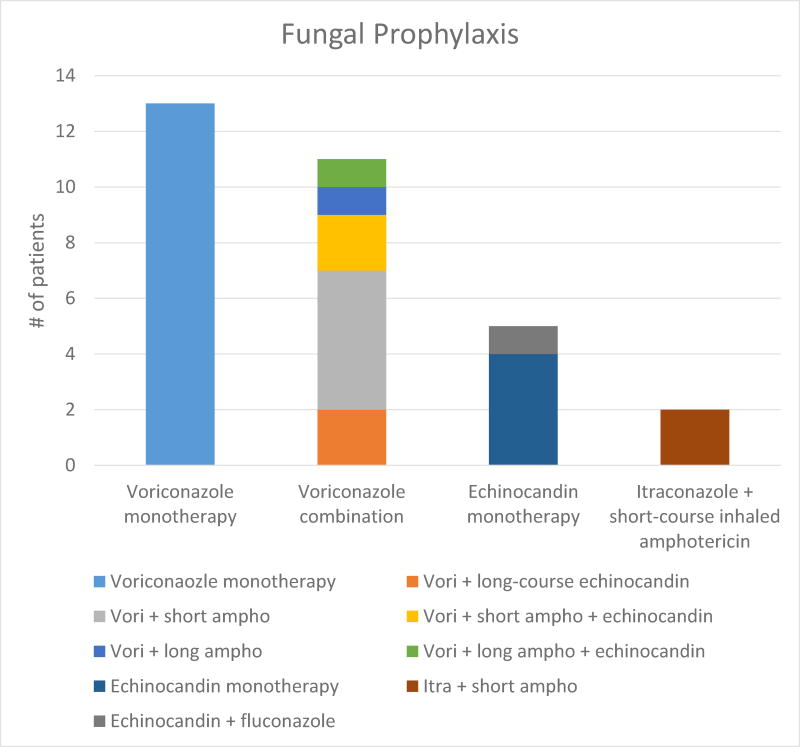
For the 31 patients who received extended prophylaxis (≥ 30 days), the most common regimen was voriconazole monotherapy, given to 13 patients. Eleven additional patients received voriconazole in combination with another prophylactic agent including inhaled amphotericin and echinocandins (Figure 1). Additionally, five patients received echinocandins as their primary prophylactic agent, one in combination with fluconazole. Two received itraconazole with amphotericin. Eighteen patients (58%) who received extended prophylaxis did not have any post-transplant fungal event (colonization or PFI).
Figure 1.
Fungal prophylaxis of Pediatric Lung Transplantation
Of the 15 patients with pre-transplant colonization, 10 received extended prophylaxis post-transplant. Of the 25 patients who had post-transplant colonization, 12 received extended prophylaxis. In addition, 6 of the 9 patients with PFI occurring more than one week post-transplant had received extended prophylaxis. Extended antifungal prophylaxis was not associated with a decrease in post-transplant colonization (p = 0.60; OR 0.68, CI 0.24 to 1.92) or PFI (p = 0.48; OR 0.52, CI 0.24 to 2.33) when compared to those who did not receive extended antifungal prophylaxis.
Pre-emptive Therapy
Pre-emptive therapy was commonly administered to patients following colonization. Of the 25 patients colonized, 15 received pre-emptive treatment (60%), with new medication initiated within one week of colonization event onset. Six patients who were already taking an anti-fungal medication at the time of colonization had a new antifungal initiated. Initial pre-emptive therapy was most often echinocandin monotherapy (47%) or an enchinocandin in combination with inhaled amphotericin (7%). Patients also received fluconazole (20%), voriconazole monotherapy (13%) or voriconazole in combination with amphotericin (7% inhaled, 7% inhaled and intravenous). Two of the four patients with colonization that progressed to PFI received pre-emptive therapy. However, the association between pre-emptive therapy and subsequent PFI was not statistically significant (p = 1.00; OR 0.62, CI 0.07 to 5.28).
Discussion
Pulmonary fungal infections pose a significant risk to lung transplant recipients.2,6,9,14 PFI has an incidence of up to 16% with mortality in up to 55% of adult populations.3,5,14 Colonization occurs in up to 60% of pediatric patients with an incidence of PFI around 20%.6,7 In this prospective cohort, we found a slightly lower rate of colonization (42%) with a similar rate of PFI (17%). Interestingly, no mortality was attributed to PFI in this cohort. Several reasons for the absence of attributable mortality can be hypothesized. Introduction of newer anti-fungal agents, increased clinical attention to PFI in the current era and enrollment in a prospective observational clinical trial could have impacted the treatment strategies and timing of interventions, affecting death from PFI. Furthermore, only 25% of PFIs were caused by Aspergillus, suggesting Candida and Penicillium PFIs may have lower mortality rates.
As shown in prior pediatric studies, older age was associated with pre-transplant colonization and PFI. In this cohort, acute rejection was not associated with PFI, which contrasts data from prior retrospective studies.6,7 Differences between the prior retrospective studies and the current prospective cohort include era of transplant, immunosuppression utilized and incidence of acute rejection, which was small in the current cohort. Colonization pre-transplant was associated with a primary diagnosis of CF, however neither CF nor pre-transplant colonization predicted post-transplant colonization with specific organisms. Further investigation of the interaction between CF and fungal events is necessary and could potentially include evaluation of prolonged antibiotic exposure and environmental exposure.
Post-transplant colonization was not associated with subsequent PFI. In adult lung transplant populations, colonization is a significant risk factor for PFI by Aspergillus species; however risk factors for progression were not well defined through retrospective studies.2,8,15 Previous studies have often limited analysis to PFI caused by molds, highlighting a difference from the current cohort that reports proven PFI with Candida species. This suggests differences between pediatric and adult lung transplant recipients may exist. Although pre-transplant colonization was significantly associated with PFI, these events were not the same organism or even class of organism (yeast verses mold) limiting prediction of future events and intervention strategies. Proximity of pre-transplant colonization diagnosis to transplantation was not considered in this pediatric cohort. Additionally, unrecognized colonization of implanted lungs with inconsistent procurement of cultures from the explanted lungs could impact interpretation of the data. In both adult literature and this study, PFI most commonly occurred within the first six months post-transplant. While adult populations show a primary diagnosis of CF and early post-transplant colonization as significant risk factors for subsequent PFI, this was not the case in this cohort.9
Multicenter, descriptive studies report wide variability in prevention strategies, but voriconazole monotherapy was the most common regimen similar to prophylaxis in this pediatric cohort.16,17 Extended prophylaxis (≥ 30 days) was not associated with prevention of colonization or PFI. However, with the variable prophylactic strategies including at least 9 different regimens, conclusions should not be drawn at this time. Pre-emptive therapy initiated within one week of a new positive fungal culture occurred frequently (60% of colonization events). Nonetheless, pre-emptive therapy was not associated with prevention of subsequent PFI. Again, in a size-limited cohort in which six different pre-emptive strategies were employed, uncertainty exists. Findings in a single-center retrospective study in adults contrast this finding, with use of a single culture-directed pre-emptive therapy regimen significantly improving outcome one year post-transplant. This study reported similar frequency of pre-emptive therapy use, which was primarily voriconazole monotherapy, although deviation from the regimen was common.8 Prospective studies with consistent prophylactic and pre-emptive treatment strategies would be needed to address this issue.
Pediatric lung transplantation is a rare event. Although this prospective observational study captured a substantial number (~40%) of the first-time pediatric lung transplants performed in the United States during the study period, the sample size remains limited. With only 13 cases of PFI, multivariate analysis would have not been robust. Missing data may have led to an underestimation of PFI incidence. However, careful monitoring and data audits should have limited underestimation of colonization and PFI. Additionally, unscheduled study visits were included in the data, meaning events were documented that may have otherwise been unreported in a retrospective cohort. Pre-transplant colonization of the recipient may have been overestimated, because any positive fungal culture prior to transplant was included. This was assumed because a fungal colonization could continue to exist indefinitely, especially with a primary diagnosis of CF and issues of airway clearance. However, pre-transplant colonization could have been underestimated as not every patient had pre-transplant cultures performed. Obtaining fungal cultures from the explanted lung in every case may have better estimated pre-transplant colonization.
In lung transplant patients, PFI occurs commonly post-transplant, but in this cohort was not associated with mortality. Older age (≥ 15 years) at transplant especially in patients with a diagnosis of CF was found to be significantly associated with developing PFI. While post-transplant colonization is common, it was not associated with subsequent PFI. Prophylaxis and pre-emptive therapy are common, but the choice of both prophylaxis and pre-emptive therapy varied considerably in agent and duration. Prophylaxis was not associated with a decrease in PFI or colonization in this population. In future research, more uniform anti-fungal prophylaxis practices could allow for comparisons of prevention strategies, and a larger cohort could allow for multivariate analysis of PFI risk factors, as well as confirm the low rate of mortality observed in this study.
Acknowledgments
This research was performed as a project of the Clinical Trials in Organ Transplantation in Children a collaborative clinical research project headquartered at the National Institute of Allergy and Infectious Diseases (U01 Grant AI077810 awarded to S. Sweet).
The CTOT-03 consortium members thank the following personnel for the support of the work: Boston Children’s Hospital, Boston MA: Dawei Jiang; Children’s Hospital of Philadelphia: Rosa Kim, Sara Nguyen; Lucile Packard Children’s Hospital at Stanford, Palo Alto, CA: Elisabeth Merkel; Nationwide Children’s Hospital, Columbus, OH: Todd Astor, Stephen Kirkby, Ashley Nance, Kerri Nicholson, Susan Meyer; St. Louis Children’s Hospital, St. Louis, MO: Colleen Eisenbarger; Texas Children’s Hospital, Houston, TX: George Mallory, Mea Ebenbichler.
Abbreviations
- PFI
pulmonary fungal infection
- PLT
pediatric lung transplant
- ISHLT
International Society of Heart and Lung Transplantation
- CF
cystic fibrosis
- AR
acute rejection
Footnotes
Disclosures
None of the authors have any disclosures.
Previously Presented at the SURF Capstone Poster Symposium, University of Cincinnati Medical Center, Cincinnati, Ohio and Annual Meeting of the International Society of Heart and Lung Transplantation, San Diego, CA 2017.
References
- 1.Husni RN, Gordon SM, Longworth DL, et al. Cytomegalovirus infection is a risk factor for invasive aspergillosis in lung transplant recipients. Clin Infect Dis. 1998;26(3):753–755. doi: 10.1086/514599. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed4&NEWS=N&AN=1998111717. [DOI] [PubMed] [Google Scholar]
- 2.Cahill BC, Hibbs JR, Savik K, et al. Aspergillus airway colonization and invasive disease after lung transplantation. Chest. 1997;112(5):1160–1164. doi: 10.1378/chest.112.5.1160. [DOI] [PubMed] [Google Scholar]
- 3.Mehrad B, Paciocco G, Martinez FJ, Ojo TC, Iannettoni MD, Lynch JP. Spectrum of Aspergillus infection in lung transplant recipients: Case series and review of the literature. Chest. 2001;119(1):169–175. doi: 10.1378/chest.119.1.169. [DOI] [PubMed] [Google Scholar]
- 4.Minari A, Husni R, Avery RK, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis. 2002;4(4):195–200. doi: 10.1034/j.1399-3062.2002.t01-2-02002.x. [DOI] [PubMed] [Google Scholar]
- 5.Singh N, Husain S. Aspergillus infections after lung transplantation: clinical differences in type of transplant and implications for management. J Hear Lung Transplant. 2003;22(3):258–266. doi: 10.1016/S1053-2498(02)00477-1. [DOI] [PubMed] [Google Scholar]
- 6.Danziger-Isakov LA, Worley S, Arrigain S, et al. Increased Mortality After Pulmonary Fungal Infection Within the First Year After Pediatric Lung Transplantation. J Hear Lung Transplant. 2008;27(6):655–661. doi: 10.1016/j.healun.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M, Worley S, Mallory GB, Jr, et al. Fungal infections in pediatric lung transplant recipients: colonization and invasive disease. J Hear Lung Transpl. 2009;28(11):1226–1230. doi: 10.1016/j.healun.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SM H-M, C C, M-L L, et al. The Effectiveness of Culture-Directed Preemptive Anti-Aspergillus Treatment in Lung Transplant Recipients at One Year After Transplant. Transplantation. 2015;99(11):2387–2393. doi: 10.1097/TP.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 9.Husain S, Sole A, Alexander BD, et al. The 2015 International Society for Heart and Lung Transplantation Guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: Executive summary. J Hear Lung Transplant. 2016;35(3):261–282. doi: 10.1016/j.healun.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb S, Benden C, Sweet S, et al. ISHLT Monograph Series: Pediatric Lung Transplantation. 2013 [Google Scholar]
- 11.Ascioglu S, Rex JH, de Pauw B, et al. Defining Opportunistic Invasive Fungal Infections in Immunocompromised Patients with Cancer and Hematopoietic Stem Cell Transplants: An International Consensus. Clin Infect Dis. 2002;34(1):7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 12.Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Hear Lung Transplant. 2011;30(4):361–374. doi: 10.1016/j.healun.2011.01.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Hear Lung Transplant. 2007;26(12):1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18(1):44–69. doi: 10.1128/CMR.18.1.44-69.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmi M, Love RB, Welter D, Cornwell RD, Meyer KC. Aspergillus infection in lung transplant recipients with cystic fibrosis: risk factors and outcomes comparison to other types of transplant recipients. Chest. 2003;123(3):800–808. doi: 10.1378/chest.123.3.800. [DOI] [PubMed] [Google Scholar]
- 16.Singh N, Huprikar S, Burdette SD, Morris MI, Blair JE, Wheat LJ. Donor-derived fungal infections in organ transplant recipients: Guidelines of the American society of transplantation, infectious diseases community of practice. Am J Transplant. 2012;12(9):2414–2428. doi: 10.1111/j.1600-6143.2012.04100.x. [DOI] [PubMed] [Google Scholar]
- 17.Mead L, Danziger-Isakov LA, Michaels MG, Goldfarb S, Glanville AR, Benden C. Antifungal prophylaxis in pediatric lung transplantation: An international multicenter survey. Pediatr Transplant. 2014;18(4):393–397. doi: 10.1111/petr.12263. [DOI] [PubMed] [Google Scholar]