Abstract
The importance of interactions between the brain and the gastrointestinal tract has been increasingly recognized in recent years. It has been proposed that dysregulation and abnormalities in the brain-gut axis contribute to the etiology of a variety of central nervous system disorders. Particularly, dysbiosis, or impaired microbiota, has been implicated in multiple neurological and psychological disorders. The present paper reviews current evidence and theories concerning the possible mechanisms by which microbiota dysfunction contributes to the pathogenesis of schizophrenia and major depressive disorder. Clinical trials that investigated the possibility of treating both illnesses by correcting and rebalancing microbiota with probiotics are also reviewed. Overall, despite the accumulated knowledge in this field, more studies are warranted and required to further our understanding of the brain-gut axis and the possibility of targeting microbiota as a treatment option for schizophrenia and major depressive disorder.
Keywords: microbiota, brain-gut axis, psychiatric disorders, schizophrenia, depression
INTRODUCTION
The brain-gut axis conducts signaling between the brain and the gastrointestinal tract, which plays a fundamental role in normal physiological processes [1]. The human gastrointestinal system hosts approximately 1,800 different phyla and 40,000 bacterial species [2], collectively known as microbiota. Despite this substantial diversity, genetic analyses of fecal samples from a variety of individuals have indicated that intestinal bacteria form three distinct clusters, or enterotypes, suggesting that only a limited number of well-balanced and defined microbial community compositions exist across different individuals [3]. As gut microbiota plays an important role in host metabolism and intestinal inflammation responses, dysregulation of microbiota, or dysbiosis, can play a key role in the pathogenesis of inflammatory diseases in the host [4–6] and can serve as an alternative target for the treatment of inflammatory bowel diseases [7].
In recent years, interactions between the brain and gut microbiota have been explored, as microbiota has been found to be an important player in the pathogenesis of various neurological diseases [8] including multiple sclerosis [9, 10], Alzheimer’s disease [11], and Parkinson’s disease [12]. Indeed, microbiota can impact the structure of the brain. Myelination in the prefrontal cortex was found to be upregulated in a line of germ free mice [13]. More recently, Labus et al described a correlation between gut microbial composition and regional brain volume in patients with irritable bowel syndrome [14]. Furthermore, bacteria-derived metabolites could affect the CNS expression of brain-derived neurotrophic factor (BDNF) and other proteins that are important in cognition, which in turn affects host behavior [15]. The brain modulates gut functions including motility, acid secretion, the production of bicarbonates as well as mucus, and the immune response via the autonomic nervous system (ANS), thereby inducing responses to stress in the gastrointestinal system [16]. Particularly, the ANS-mediated modulation of mucus secretion could alter the environments that the microbiota inhabits and profoundly affect its composition and structure [17]. Therefore, the brain and the gut may form a feedback loop mediated by modulation of the microbiota. Mounting evidence suggests that gut microbiota plays a key role in many neuropsychiatric disorders [18, 19]. This review discusses the possible role of gut microbiota in the pathogenesis of schizophrenia and major depressive disorder (MDD), and further summarizes clinical data where the microbiota was targeted as a potential treatment method.
Pathogenesis of schizophrenia and the involvement of gut microbiota
Schizophrenia is a serious psychiatric disorder, characterized by psychosis, in which the involvement of the gut-brain axis has long been recognized [20, 21]. Analyses of the microbiota in oral samples from schizophrenia patients found that the microbiota of these patients were comprised of significantly more Lactobacillus than normal controls [22, 23]. Further analyses indicated that increases in Lactobacillus group bacteria were significantly correlated with the severity of different symptom domains in schizophrenia patients [24]. Based on these studies, a relationship very likely exists between the microbial composition and schizophrenia.
The pathogenesis of schizophrenia is not fully understood; however, a decrease in BDNF expression and the resultant hypoactivity in N-methyl-D-aspartate (NMDA) receptor [25] have been implicated in the pathology of Schizophrenia. In a recent study Li et al first described a decrease in NMDA receptor expression in an animal model, and then by treating the animals with LY39, an agonist of mGluR, corrected the disrupted NMDA receptor expression. Furthermore, they observed that learning deficits and cognitive flexibility improved in treated animals, and thus found a correlation between the expression level of BDNF and NMDA and the symptoms of schizophrenia [26]. In a germ free mice line, decreased expression levels of BDNF and NMDA receptors were found in the cortex and hippocampus [27]. In schizophrenia patients, a decreased level of plasma BDNF has been noted, although findings from different studies have been inconsistent [28, 29]. Overall, the possibility that the microbiota affects BDNF expression and contributes to the development of schizophrenia warrants further investigation.
Converging lines of evidence indicate that the immune system could play an important role in the etiology of schizophrenia. Features of an autoimmune process, as well as diffuse non-specific immune system overactivation and activation of different types of T-helper cells, have been found in subgroups of schizophrenia patients [30]. A link between schizophrenia and an inflammatory-immune response has been further corroborated by the findings that antipsychotics produce anti-inflammatory effects in schizophrenia patients [31] and that non-steroid anti-inflammatory drugs could reduce the severity of schizophrenia type symptoms, especially in patients with a relatively more strongly altered immune response [32–34]. Troll-like receptors (TLR) mediate the innate immune response. TLR4 responds to lipopolysaccharides (LPSs), an important component of the outer membrane of Gram-negative bacteria, and triggers an inflammatory response [35]. Because TLRs are involved in the maintenance of intestinal epithelial homeostasis and protection from injuries as a result of the immune response to commensal bacteria, TLRs recognize the gut microbiota in healthy subjects [36, 37]. However, abnormal interactions between TLRs and gut microbiota could lead to chronic inflammation [37]. Thus, changes in the composition of microbiota as a feature of schizophrenia might induce an immune response mediated by TLRs and contribute to symptoms of the disorder.
Dysregulation in the metabolic pathway of tryptophan has been implicated in the pathogenesis of schizophrenia. Kynurenic acid is a tryptophan metabolite and an NMDA receptor antagonist [38]. An increased level of kynurenic acid in the central nervous system has been found in schizophrenia patients [39, 40]; however, a deficit of peripheral kynurenic acid has been proposed to be associated with a relapse [41]. Furthermore, an increase in the level of anthranilic acid, a downstream metabolite of kynurenic acid, has been found in schizophrenia patients, particularly a subgroup of patients with autoimmune presentation [42]. Interestingly, one study reported that the immune response to gluten, as assessed by measurement of serum antigliatin IgG level, was positively correlated with the kynurenine level and the kynurenine/tryptophan ratio [43]. Gut microbiota can substantially influence the level of plasma tryptophan [44–46], and thereby affect tryptophan metabolism. Although the exact role of the kynurenic pathway of tryptophan metabolism in the pathogenesis of schizophrenia has not been elucidated, some studies report that the upregulation of the pathway is associated with schizophrenia [47, 48]. Taken together, there is evidence pointing to the possibility that increased kynurenine level in the CNS is associated with Schizophrenia, and that this increase could lead to downregulation of NMDA activity or promotion of an immune response.
The cause of dysbiosis has also been investigated. As it is widely believed that an infant’s first exposure to bacteria is to the mother’s microbiota during vaginal delivery, it has been speculated that dysbiosis could result from a lack of exposure that occurs in a cesarean section birth. However, further analyses did not find that birth by cesarean section was correlated with schizophrenia [49, 50]. Nevertheless, the development of gut microbiota is shaped by early-life events, including mode of delivery, type of feeding, the application of antibiotics to the mother during delivery, and the gender of the infant [51–53]; therefore, the link between schizophrenia and dysbiosis requires further examination.
Pathogenesis of MDD and gut microbiota
Mounting evidence supports the existence of a correlation between gut microbiota and the development of MDD [54, 55]. A study of fecal samples from MDD patients documented the occurrence of over- and under- presentation of the orders of Bacteroidales and Lachnospiraceae, respectively. The study further described a significant correlation that existed between depression and one clade in each of the genera Oscillibacter and Alistipes [56]. Subsequent analyses found that the bacteria that are associated with MDD were also associated with an increased level of isovaleric acid [56, 57]. Previous studies have shown that this colon-derived, short-chain fatty acid acetate can cross the blood-brain barrier and interact directly with the hypothalamus and the central homeostasis mechanism [58]. Moreover, valeric acid can act as an inverse agonist of the adenosine A1 receptor and influence the release of neurotransmitters in the brain [59]. Taken together, these studies indicate that changes in the gut microbiota may lead to an altered level of valeric acid, which in turn can affect the brain and contribute to the development of MDD.
Another recent study confirmed that microbial composition in MDD patients differs from that of non-depressed individuals. Analyses of fecal samples from MDD patients found a weak negative correlation between the relative abundance of Faecalibacterium and the severity of the depressive symptoms, and that the MDD patients had increased levels of Enterobacteriaceae and Alistipes but reduced levels of Faecalibacterium [60]. While the results of these studies are not completely consistent, for example, the variations in the reported gut bacterial species that are associated with MDD, they all underscore the connection between microbiota and MDD.
The level of tryptophan, a precursor of serotonin, is influenced by gut microbiota [44–46]. Specifically, in an animal study, treatment with Bifidobacteria infantis resulted in a significant change in serotonin metabolism in the brain [44]. The role of serotonin and its receptors in MDD has long been established [61, 62], and gut microbiota probably contribute to the development of MDD by affecting the tryptophan level.
Additionally, intestinal dysbiosis and a resultant “leaky gut” condition can lead to an increased immune response, and contribute to the etiology of MDD [63]. Given the role gut microbiota plays in the gut inflammatory response, it is highly likely that gut microbiota contribute to the pathogenesis of MDD by influencing the immune system and the inflammation response.
Further investigation of the correlation between MDD and the composition of microbiota is required to gain a better understanding of the mechanisms by which microbiota contribute to mood disorders.
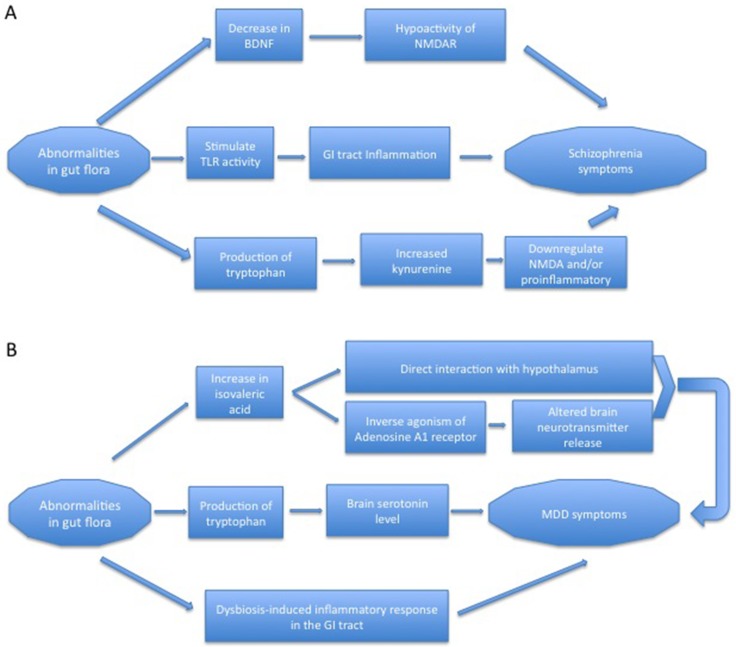
Overall, gut flora may contribute to the pathogenesis of schizophrenia and MDD through the production of key molecules and/or promotion of intestinal immune response (Figure 1).
Figure 1. Possible mechanisms of gut microbiota in the pathogenesis of schizophrenia and MDD.
(A) Gut flora possibly contributes to the pathogenesis of schizophrenia through modulation of BDNF, the immune response in the gut, and the kynurenine pathway of tryptophan metabolism. (B) Gut flora possibly contributes to the pathogenesis of MDD through altering the levels of valeric acid and tryptophan, and the gut immune response.
Targeting microbiota in major psychiatric disorders
As we gain a clearer understanding of the relationship between dysbiosis and the development of schizophrenia and MDD, initial steps are being taken to explore the possibility of targeting microbiota as treatment options for both [64]. This review focuses on the use of probiotics in clinical trials that assessed the clinical benefits of probiotics in the treatment of schizophrenia and MDD. Relevant clinical trials are summarized in Table 1.
Table 1. Clinical trials with probiotics in the treatment of schizophrenia and MDD.
| Study | Population | Design | Intervention | Outcome |
|---|---|---|---|---|
| Schizophrenia | ||||
| Dickerson FB, et al., 2014 [63] * | Schizophrenia patients | Randomized, placebo-controlled | Adjunctive probiotics vs placebo | No improvement in Schizophrenia symptoms, but improved GI functions |
| Tomaskik J, et al., 2015 [64] * | Schizophrenia patients | Biomarker analysis | Adjunctive probiotics vs placebo | GI function improvement might be mediated by modulation of the immune response |
| Severance EG, et al., 2017 [65] * | Schizophrenia patients | Longitudinal study | Adjunctive probiotic vs placebo | GI function improvement associated with a decrease in C. albicans IgG; a trend of improved positive symptoms |
| MDD | ||||
| Benton D, et al., 2007 [66] | Healthy volunteer | Double-blind, placebo-controlled | Probiotics vs placebo | Mood improvement in participants in the probiotic group who had poor mood initially |
| Messaoudi M, et al., 2011 [67] | Healthy volunteer | double-blind, placebo-controlled, randomized parallel group study | Probiotics | Improved psychological distress |
| Chung Y-C, et al., 2014 [68] | Elderly volunteer | double-blind, randomized | Fermented milk | Did not improve depression |
| Akkasheh G, et al., 2016 [69] | MDD patients | Double-blind, placebo-controlled | Supplement probiotics vs placebo | Significantly improved MDD symptoms |
| Bambling M, et al., 2017 [70] | SSRI-resistant MDD patients | Cohort study | Combined supplement of probiotics and magnesium orotate, co-administered with an SSRI | Significantly improved MDD symptoms, and relapse when patients ceased to take probiotics while on SSRI |
| Romjin AR, et al., 2017 [71] | MDD patients | Double-blind, randomized placebo-controlled | Probiotics as a primary treatment | No effect was noted in any of the psychological and functional assessments |
MDD: major depressive disorder; SSRI: selective serotonin reuptake inhibitor
*The trials are connected and analyzed the same intention-to-treat population
An initial randomized, placebo-controlled clinical study investigated the effects of supplemental probiotics on the symptoms of schizophrenia and gastrointestinal function [65]. Recruited schizophrenia patients were given colony-forming adjunctive probiotics (Lactobacillus rhamnosus strain CG and Bifidobacterium animalis subsp. lactis Bb12) or placebos for 14 weeks. The study found that while offering no improvement in schizophrenia symptoms, administration of adjunctive probiotics was associated with improved gastrointestinal function. Biomarker analysis of the study participants found that the beneficial effects of probiotics are possibly mediated by modulation of the intestinal immune response [66]. A subsequent longitudinal study of the same population showed that the level of C. albicans IgG in male patients was significantly lowered, which was associated with improvements in the gastrointestinal complaints of male patients [67]. That study also reported improvement trends in the positive symptoms of schizophrenia [67]. Although the results from the clinical trials were promising, given the paucity of clinical data, the true efficacy and clinical benefits of probiotics in the treatment of schizophrenia patients remain to be validated by future clinical studies.
Probiotics have been shown to improve mood in the healthy population. A double-blind, placebo-controlled trial investigated the effects of probiotics on mood [68]. Healthy volunteers were given daily milk drinks containing Lactobacillus casei or a placebo for three weeks. At the end of the study period, the subgroup of participants receiving probiotics, whose mood was initially poor, showed improvements. In another study, the administration of probiotics (L. helveticus R0052 and B. longum R0175) reduced anxiety-like behavior in a murine model and improved psychological distress in healthy volunteers [69]. However, Chuang et al. reported that in healthy elderly Korean volunteers, the consumption of Lactobacillus helveticus-fermented milk was not associated with improved depression outcomes, as assessed with the geriatric depression scale -short form [70].
Studies that investigated the clinical benefits of probiotics in MDD have also yielded conflicting results. Akkasheh et al. reported the results of a pioneer clinical trial of probiotics used as a supplement in MDD patients [71]. In this double-blind, placebo-controlled study, MDD patients received placebos or supplement probiotic capsules containing Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum, for eight weeks. At the end of the study period, patients receiving the probiotic capsules showed significant improvements in depression symptoms, as assessed by using the Beck Depression Inventory score [71]. More recently, a cohort study examined the effects of combined supplements of probiotics and magnesium orotate in a small group of selective serotonin reuptake inhibitor (SSRI)-resistant MDD patients [72]. At the end of the eight-week study period, the majority of the cohort demonstrated significantly improved depression symptoms and quality of life. However, relapse was observed while the patients were on a selective serotonin reuptake blocker but ceased to take the supplement probiotics and magnesium orotate [72]. However, a double-blind, randomized, placebo-controlled clinical trial that assessed the effects of probiotics as a primary treatment for MDD did not find that a combination of probiotics improved depression symptoms [73]. Participants of this trial were given placebo or probiotic formulas that contained L. helveticus R0052 and B. longum R0175 for eight weeks, and the severity of depression symptoms was assessed by the Montgomery-Åsberg Depression Rating Scale, the Improved Clinical Global Impressions Scale, and QIDS-SR16. Additionally, global function and abdominal symptoms were assessed, as well as the status of various biomarkers for inflammation. At the end of the eight-week study period, no significant difference was noted in any of the measurements in the psychological and functional outcomes or biomarkers between the placebo and probiotic groups. Overall, although treatment of MDD with probiotics showed some promise, because the number of clinical trials was very limited and a relatively small population was included in the available trials, it is difficult to conclude whether or not probiotics offer clinical benefits for MDD patients. More studies using larger populations will be required to fully examine the possibility of using probiotics as a treatment for MDD.
CONCLUSIONS
Recent studies have recognized and underscored the importance of interactions between the brain and the gut, mediated by gut microbiota, in the pathogenesis of psychiatric disorders such as schizophrenia and MDD. Gut microbiota can affect the brain by releasing essential amino acids and participating in the immune and inflammatory responses. The brain, in turn, can modify the conditions of the microbial habitat and may influence the composition of the specific bacterial species present in the microbiota. Therefore, the microbiota has become a valid target in the search for effective treatments for schizophrenia and MDD. To date, only very few well-controlled clinical studies have been conducted, and the results from different clinical trials were not always consistent. Further studies are required to fully understand the intricate interactions between the members of the brain-gut axis and to validate/invalidate the clinical benefits of probiotics in the treatment of schizophrenia and MDD. Studies comparing the gut microbial composition of patients both during a psychiatric disorder episode (MDD or schizophrenia) and when in remission should shed light on the interactions between the gut flora and the brain during an active disease state. This type of study will also help to identify the species of bacteria that offer benefits to patients with psychiatric disorders. In order to understand the clinical benefits of probiotics, it will be helpful to establish to what extent the host gut flora could be modified by probiotic consumption, with or without dietary modification, and whether the effects are long-term. Furthermore, the molecular interactions between the brain and the gut flora could be explored if an established schizophrenia animal model with defined gastrointestinal microbial composition is available. Overall, more mechanistic studies are necessary for the development of probiotics as an effective therapy.
Acknowledgments
This work was supported by a grant from the Tianjin Health Bureau Foundation (2014KR02 to C.Z.) , Key Projects of Natural Science Foundation of Tianjin, China(17JCZDJC35700 to C.Z.) , Projects of Natural Science Foundation of Tianjin, ( 16JCYBJC24200 to J.L.) and Tianjin Health Key Program (13KG118 to J.L).
Author contributions
CZ and YY: conceptual design and writing of the draft manuscript; FL, SC and LW: conceptual design and writing of the final manuscript; RJ, HT and JL collected and examined the enrolled articles in this review.
CONFLICTS OF INTEREST
The authors declare no conflicts of interests
REFERENCES
- 1.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–14. doi: 10.1038/nrgastro.2009.35. https://doi.org/10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24:4–10. doi: 10.1097/MOG.0b013e3282f2b0e8. https://doi.org/10.1097/MOG.0b013e3282f2b0e8. [DOI] [PubMed] [Google Scholar]
- 3.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. https://doi.org/10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasubramanian I, Gao N. From sensing to shaping microbiota: insights into the role of NOD2 in intestinal homeostasis and progression of Crohn's Disease. Am J Physiol Gastrointest Liver Physiol. 2017 doi: 10.1152/ajpgi.00330.2016. ajpgi.00330.2016. https://doi.org/10.1152/ajpgi.00330.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seregin SS, Golovchenko N, Schaf B, Chen J, Pudlo NA, Mitchell J, Baxter NT, Zhao L, Schloss PD, Martens EC, Eaton KA, Chen GY. NLRP6 Protects Il10-/- Mice from Colitis by Limiting Colonization of Akkermansia muciniphila. Cell Rep. 2017;19:733–45. doi: 10.1016/j.celrep.2017.03.080. https://doi.org/10.1016/j.celrep.2017.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundin J, Ohman L, Simren M. Understanding the Gut Microbiota in Inflammatory and Functional Gastrointestinal Diseases. Psychosom Med. 2017 doi: 10.1097/PSY.0000000000000470. https://doi.org/10.1097/psy.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 7.Meighani A, Hart BR, Bourgi K, Miller N, John A, Ramesh M. Outcomes of Fecal Microbiota Transplantation for Clostridium difficile Infection in Patients with Inflammatory Bowel Disease. Dig Dis Sci. 2017 doi: 10.1007/s10620-017-4580-4. https://doi.org/10.1007/s10620-017-4580-4. [DOI] [PubMed] [Google Scholar]
- 8.Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E. The gut microbiome in human neurological disease: A review. Ann Neurol. 2017;81:369–82. doi: 10.1002/ana.24901. https://doi.org/10.1002/ana.24901. [DOI] [PubMed] [Google Scholar]
- 9.Adamczyk-Sowa M, Medrek A, Madej P, Michlicka W, Dobrakowski P. Does the Gut Microbiota Influence Immunity and Inflammation in Multiple Sclerosis Pathophysiology? J Immunol Res. 2017;2017:7904821. doi: 10.1155/2017/7904821. https://doi.org/10.1155/2017/7904821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrillo-Salinas FJ, Mestre L, Mecha M, Feliu A, Del Campo R, Villarrubia N, Espejo C, Montalban X, Alvarez-Cermeno JC, Villar LM, Guaza C. Gut dysbiosis and neuroimmune responses to brain infection with Theiler's murine encephalomyelitis virus. Sci Rep. 2017;7:44377. doi: 10.1038/srep44377. https://doi.org/10.1038/srep44377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–8. doi: 10.1016/j.neurobiolaging.2016.08.019. https://doi.org/10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Petrov VA, Saltykova IV, Zhukova IA, Alifirova VM, Zhukova NG, Dorofeeva YB, Tyakht AV, Kovarsky BA, Alekseev DG, Kostryukova ES, Mironova YS, Izhboldina OP, Nikitina MA, et al. Analysis of Gut Microbiota in Patients with Parkinson's Disease. Bull Exp Biol Med. 2017;162:734–7. doi: 10.1007/s10517-017-3700-7. https://doi.org/10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 13.Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ, Clarke G, Cryan JF. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6:e774. doi: 10.1038/tp.2016.42. https://doi.org/10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, Acosta J, Luna RA, Aagaard K, Versalovic J, Savidge T, Hsiao E, Tillisch K, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. 2017;5:49. doi: 10.1186/s40168-017-0260-z. https://doi.org/10.1186/s40168-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohlich EE, Farzi A, Mayerhofer R, Reichmann F, Jacan A, Wagner B, Zinser E, Bordag N, Magnes C, Frohlich E, Kashofer K, Gorkiewicz G, Holzer P. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun. 2016;56:140–55. doi: 10.1016/j.bbi.2016.02.020. https://doi.org/10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–9. doi: 10.1136/gut.47.6.861. https://doi.org/10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macfarlane S, Dillon JF. Microbial biofilms in the human gastrointestinal tract. J Appl Microbiol. 2007;102:1187–96. doi: 10.1111/j.1365-2672.2007.03287.x. https://doi.org/10.1111/j.1365-2672.2007.03287.x. [DOI] [PubMed] [Google Scholar]
- 18.Fetissov SO, Dechelotte P. The new link between gut-brain axis and neuropsychiatric disorders. Curr Opin Clin Nutr Metab Care. 2011;14:477–82. doi: 10.1097/MCO.0b013e32834936e7. https://doi.org/10.1097/MCO.0b013e32834936e7. [DOI] [PubMed] [Google Scholar]
- 19.Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders With Suspected Immune Dysregulation. Clin Ther. 2015;37:984–95. doi: 10.1016/j.clinthera.2015.04.002. https://doi.org/10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemani K, Hosseini Ghomi R, McCormick B, Fan X. Schizophrenia and the gut-brain axis. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:155–60. doi: 10.1016/j.pnpbp.2014.08.018. https://doi.org/10.1016/j.pnpbp.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. 2015;17:27. doi: 10.1007/s11920-015-0574-0. https://doi.org/10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, Schroeder JR, Yolken RH, Crandall KA. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ. 2015;3:e1140. doi: 10.7717/peerj.1140. https://doi.org/10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yolken RH, Severance EG, Sabunciyan S, Gressitt KL, Chen O, Stallings C, Origoni A, Katsafanas E, Schweinfurth LA, Savage CL, Banis M, Khushalani S, Dickerson FB. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophr Bull. 2015;41:1153–61. doi: 10.1093/schbul/sbu197. https://doi.org/10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz E, Maukonen J, Hyytiainen T, Kieseppa T, Oresic M, Sabunciyan S, Mantere O, Saarela M, Yolken R, Suvisaari J. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2017 doi: 10.1016/j.schres.2017.04.017. https://doi.org/10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal R, Kalmady SV, Venkatasubramanian G. In SilicoModel-driven Assessment of the Effects of Brain-derived Neurotrophic Factor Deficiency on Glutamate and Gamma-Aminobutyric Acid: Implications for Understanding Schizophrenia Pathophysiology. Clin Psychopharmacol Neurosci. 2017;15:115–25. doi: 10.9758/cpn.2017.15.2.115. https://doi.org/10.9758/cpn.2017.15.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li ML, Gulchina Y, Monaco SA, Xing B, Ferguson BR, Li YC, Li F, Hu XQ, Gao WJ. Juvenile treatment with a novel mGluR2 agonist/mGluR3 antagonist compound, LY395756, reverses learning deficits and cognitive flexibility impairments in adults in a neurodevelopmental model of schizophrenia. Neurobiol Learn Mem. 2017;140:52–61. doi: 10.1016/j.nlm.2017.02.004. https://doi.org/10.1016/j.nlm.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–75. doi: 10.1113/jphysiol.2004.063388. https://doi.org/10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui H, Jin Y, Wang J, Weng X, Li C. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia: A systematic review. Shanghai Arch Psychiatry. 2012;24:250–61. doi: 10.3969/j.issn.1002-0829.2012.05.002. https://doi.org/10.3969/j.issn.1002-0829.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16:960–72. doi: 10.1038/mp.2010.88. https://doi.org/10.1038/mp.2010.88. [DOI] [PubMed] [Google Scholar]
- 30.Strous RD, Shoenfeld Y. Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. J Autoimmun. 2006;27:71–80. doi: 10.1016/j.jaut.2006.07.006. https://doi.org/10.1016/j.jaut.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Tourjman V, Kouassi E, Koue ME, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, Potvin S. Antipsychotics' effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013;151:43–7. doi: 10.1016/j.schres.2013.10.011. https://doi.org/10.1016/j.schres.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Sommer IE, de Witte L, Begemann M, Kahn RS. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73:414–9. doi: 10.4088/JCP.10r06823. https://doi.org/10.4088/JCP.10r06823. [DOI] [PubMed] [Google Scholar]
- 33.Meyer U, Schwarz MJ, Muller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96–110. doi: 10.1016/j.pharmthera.2011.06.003. https://doi.org/10.1016/j.pharmthera.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–7. doi: 10.4088/JCP.09m05117yel. https://doi.org/10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 35.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 36.Caso JR, Balanza-Martinez V, Palomo T, Garcia-Bueno B. The Microbiota and Gut-Brain Axis: Contributions to the Immunopathogenesis of Schizophrenia. Curr Pharm Des. 2016;22:6122–33. doi: 10.2174/1381612822666160906160911. [DOI] [PubMed] [Google Scholar]
- 37.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. https://doi.org/10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–79. [PubMed] [Google Scholar]
- 39.Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav. 2007;92:203–9. doi: 10.1016/j.physbeh.2007.05.025. https://doi.org/10.1016/j.physbeh.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- 41.Szymona K, Zdzisinska B, Karakula-Juchnowicz H, Kocki T, Kandefer-Szerszen M, Flis M, Rosa W, Urbanska EM. Correlations of Kynurenic Acid, 3-Hydroxykynurenine, sIL-2R, IFN-alpha, and IL-4 with Clinical Symptoms During Acute Relapse of Schizophrenia. Neurotox Res. 2017 doi: 10.1007/s12640-017-9714-0. https://doi.org/10.1007/s12640-017-9714-0. [DOI] [PubMed] [Google Scholar]
- 42.Oxenkrug G, van der Hart M, Roeser J, Summergrad P. Anthranilic Acid: A Potential Biomarker and Treatment Target for Schizophrenia. Ann Psychiatry Ment Health. 2016;4 [PMC free article] [PubMed] [Google Scholar]
- 43.Okusaga O, Fuchs D, Reeves G, Giegling I, Hartmann AM, Konte B, Friedl M, Groer M, Cook TB, Stearns-Yoder KA, Pandey JP, Kelly DL, Hoisington AJ, et al. Kynurenine and Tryptophan Levels in Patients With Schizophrenia and Elevated Antigliadin Immunoglobulin G Antibodies. Psychosom Med. 2016;78:931–9. doi: 10.1097/PSY.0000000000000352. https://doi.org/10.1097/psy.0000000000000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–74. doi: 10.1016/j.jpsychires.2008.03.009. https://doi.org/10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–73. doi: 10.1038/mp.2012.77. https://doi.org/10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 46.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–703. doi: 10.1073/pnas.0812874106. https://doi.org/10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–94. doi: 10.1177/0269881108089583. https://doi.org/10.1177/0269881108089583. [DOI] [PubMed] [Google Scholar]
- 48.Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074:25–37. doi: 10.1016/j.brainres.2005.12.056. https://doi.org/10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 49.Fond G, Bulzacka E, Boyer L, Llorca PM, Godin O, Brunel L, Andrianarisoa MG, Aouizerate B, Berna F, Capdevielle D, Chereau I, Denizot H, Dorey JM, et al. Birth by cesarean section and schizophrenia: results from the multicenter FACE-SZ data-set. Eur Arch Psychiatry Clin Neurosci. 2016 doi: 10.1007/s00406-016-0708-3. https://doi.org/10.1007/s00406-016-0708-3. [DOI] [PubMed]
- 50.O'Neill SM, Curran EA, Dalman C, Kenny LC, Kearney PM, Clarke G, Cryan JF, Dinan TG, Khashan AS. Birth by Caesarean Section and the Risk of Adult Psychosis: A Population-Based Cohort Study. Schizophr Bull. 2016;42:633–41. doi: 10.1093/schbul/sbv152. https://doi.org/10.1093/schbul/sbv152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bridgman SL, Azad MB, Field CJ, Haqq AM, Becker AB, Mandhane PJ, Subbarao P, Turvey SE, Sears MR, Scott JA, Wishart DS, Kozyrskyj AL. Fecal Short-Chain Fatty Acid Variations by Breastfeeding Status in Infants at 4 Months: Differences in Relative versus Absolute Concentrations. Front Nutr. 2017;4:11. doi: 10.3389/fnut.2017.00011. https://doi.org/10.3389/fnut.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, Sears MR, Mandhane PJ, Turvey SE, Subbarao P, Becker AB, Scott JA, Kozyrskyj AL. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. Bjog. 2016;123:983–93. doi: 10.1111/1471-0528.13601. https://doi.org/10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 53.Martin R, Makino H, Cetinyurek Yavuz A, Ben-Amor K, Roelofs M, Ishikawa E, Kubota H, Swinkels S, Sakai T, Oishi K, Kushiro A, Knol J. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS One. 2016;11:e0158498. doi: 10.1371/journal.pone.0158498. https://doi.org/10.1371/journal.pone.0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22:361–8. doi: 10.3748/wjg.v22.i1.361. https://doi.org/10.3748/wjg.v22.i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–48. doi: 10.1038/mp.2016.50. https://doi.org/10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–62. doi: 10.1111/nmo.12378. https://doi.org/10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 57.Szczesniak O, Hestad KA, Hanssen JF, Rudi K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. 2016;19:279–83. doi: 10.1179/1476830515Y.0000000007. https://doi.org/10.1179/1476830515y.0000000007. [DOI] [PubMed] [Google Scholar]
- 58.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. https://doi.org/10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lacher SK, Mayer R, Sichardt K, Nieber K, Muller CE. Interaction of valerian extracts of different polarity with adenosine receptors: identification of isovaltrate as an inverse agonist at A1 receptors. Biochem Pharmacol. 2007;73:248–58. doi: 10.1016/j.bcp.2006.09.029. https://doi.org/10.1016/j.bcp.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 60.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94. doi: 10.1016/j.bbi.2015.03.016. https://doi.org/10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 61.van Praag HM. Serotonin precursors in the treatment of depression. Adv Biochem Psychopharmacol. 1982;34:259–86. [PubMed] [Google Scholar]
- 62.de Montigny C, Blier P. Effects of antidepressant treatments on 5-HT neurotransmission: electrophysiological and clinical studies. Adv Biochem Psychopharmacol. 1984;39:223–39. [PubMed] [Google Scholar]
- 63.Slyepchenko A, Maes M, Jacka FN, Kohler CA, Barichello T, McIntyre RS, Berk M, Grande I, Foster JA, Vieta E, Carvalho AF. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother Psychosom. 2017;86:31–46. doi: 10.1159/000448957. https://doi.org/10.1159/000448957. [DOI] [PubMed] [Google Scholar]
- 64.Fond G, Boukouaci W, Chevalier G, Regnault A, Eberl G, Hamdani N, Dickerson F, Macgregor A, Boyer L, Dargel A, Oliveira J, Tamouza R, Leboyer M. The "psychomicrobiotic": Targeting microbiota in major psychiatric disorders: A systematic review. Pathol Biol (Paris) 2015;63:35–42. doi: 10.1016/j.patbio.2014.10.003. https://doi.org/10.1016/j.patbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 65.Dickerson FB, Stallings C, Origoni A, Katsafanas E, Savage CL, Schweinfurth LA, Goga J, Khushalani S, Yolken RH. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;16 doi: 10.4088/PCC.13m01579. https://doi.org/10.4088/PCC.13m01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomasik J, Yolken RH, Bahn S, Dickerson FB. Immunomodulatory Effects of Probiotic Supplementation in Schizophrenia Patients: A Randomized, Placebo-Controlled Trial. Biomark Insights. 2015;10:47–54. doi: 10.4137/BMI.S22007. https://doi.org/10.4137/bmi.s22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Severance EG, Gressitt KL, Stallings CR, Katsafanas E, Schweinfurth LA, Savage CL, Adamos MB, Sweeney KM, Origoni AE, Khushalani S, Dickerson FB, Yolken RH. Probiotic normalization of Candida albicans in schizophrenia: A randomized, placebo-controlled, longitudinal pilot study. Brain Behav Immun. 2017;62:41–5. doi: 10.1016/j.bbi.2016.11.019. https://doi.org/10.1016/j.bbi.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61:355–61. doi: 10.1038/sj.ejcn.1602546. https://doi.org/10.1038/sj.ejcn.1602546. [DOI] [PubMed] [Google Scholar]
- 69.Messaoudi M, Lalonde R, Violle N, Javelot H, Desor D, Nejdi A, Bisson JF, Rougeot C, Pichelin M, Cazaubiel M, Cazaubiel JM. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–64. doi: 10.1017/S0007114510004319. https://doi.org/10.1017/s0007114510004319. [DOI] [PubMed] [Google Scholar]
- 70.Chung YC, Jin HM, Cui Y, Kim DS, Jung JM, Park JI, Jung ES, Choi EK, Chae SW. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. Journal of Functional Foods. 2014;10:465–74. https://doi.org/https://doi.org/10.1016/j.jff.2014.07.007. [Google Scholar]
- 71.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, Memarzadeh MR, Asemi Z, Esmaillzadeh A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315–20. doi: 10.1016/j.nut.2015.09.003. https://doi.org/10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Bambling M, Edwards SC, Hall S, Vitetta L. A combination of probiotics and magnesium orotate attenuate depression in a small SSRI resistant cohort: an intestinal anti-inflammatory response is suggested. Inflammopharmacology. 2017;25:271–4. doi: 10.1007/s10787-017-0311-x. https://doi.org/10.1007/s10787-017-0311-x. [DOI] [PubMed] [Google Scholar]
- 73.Romijn AR, Rucklidge JJ, Kuijer RG, Frampton C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry. 2017:4867416686694. doi: 10.1177/0004867416686694. https://doi.org/10.1177/0004867416686694. [DOI] [PMC free article] [PubMed] [Google Scholar]