Abstract
Pain interventions with no active ingredient, placebo, are sometimes effective in treating chronic pain conditions. Prior studies on the neurobiological underpinnings of placebo analgesia indicate endogenous opioid release and changes in brain responses and functional connectivity during pain anticipation and pain experience in healthy subjects. Here, we investigated placebo analgesia in healthy subjects and in interictal migraine patients (n = 9) and matched healthy controls (n = 9) using 11C-diprenoprhine Positron Emission Tomography (PET) and simultaneous functional Magnetic Resonance Imaging (fMRI). Intravenous saline injections (the placebo) led to lower pain ratings, but we did not find evidence for an altered placebo response in interictal migraine subjects as compared to healthy subjects.
Highlights
-
•
Simultaneous fMRI and 11C-Diprenoprhine PET of migraineurs and controls before and after placebo analgesia
-
•
Placebo led to an overall reduction in pain ratings, with no significant differences between patients and controls
-
•
Structural changes in migraine, but no effects of placebo or diagnosis on pain fMRI or on opioid receptor binding.
1. Introduction
Placebo analgesia constitutes a great opportunity for clinical treatment, and also a challenge for clinical drug trials. Meta-analyses of the placebo response in acute migraine clinical trials indicates that approximately one in three patients experience pain relief 2 h after being administered a placebo, and six to 9% of patients experience complete pain relief by placebo (Macedo et al., 2006, Loder et al., 2005). For migraine prophylactic clinical trials, approximately one in five patients have a reduction of migraine attacks > 50% after placebo treatment (Macedo et al., 2008, van der Kuy and Lohman, 2002).
Current neurobiological models of placebo analgesia largely rely on data obtained from healthy subjects. In healthy subjects, endogenous opioids mediate part of the placebo analgesic response (Grevert et al., 1983), but the magnitude of the placebo response and related endogenous opioid release varies substantially between subjects. Part of such variability is predicted by opioid receptor expression and endogenous neurotransmitter tone (Wager et al., 2007, Zubieta et al., 2002, Zubieta et al., 2003, Zubieta et al., 2005) and by resting state functional connectivity patterns (Ploner et al., 2010). Also in chronic pain patients, endogenous opioids may contribute to placebo analgesia (Lipman et al., 1990), and among placebo responders, the magnitude of placebo response has been related to the duration of symptoms (Kosek et al., 2017).
Episodic migraine patients offer an ideal clinical population to investigate placebo responses given that there are well described alterations in brain structure (Dai et al., 2015) and in functional responses to pain (Schwedt et al., 2014, Russo et al., 2012) and emotion (Wilcox et al., 2016). There is also some evidence of altered opioidergic function in migraine, in that μ-opioid receptor binding potential is reduced in the medial prefrontal cortex during a migraine attack (DaSilva et al., 2014), and the capacity for pain-induced endogenous opioid release in the periaqueductal gray, a key pain modulating region, is diminished during migraine attacks (Nascimento et al., 2014). Further, the interictal (pain free) state allows for evaluating brain function without the potentially confounding effects of ongoing pain.
We hypothesized that since there is evidence of functional, structural and opioidergic alterations in migraine, alterations in placebo responses would be present. Specifically, we sought to determine: (a) if the magnitude of an experimentally induced conditioned placebo analgesia was similar between healthy subjects, and migraine patients; (b) if functional responses to pain anticipation and pain stimuli is differentially influenced by placebo in healthy subjects and migraine patients; and (c) if endogenous opioid levels and endogenous opioid release induced by placebo administration differentiates migraine patients and healthy subjects. We investigated group effects (migraine versus healthy), placebo analgesia effects, and placebo by group interaction effects on behavioral responses, brain structure, resting state functional connectivity, experimentally evoked responses, and opioid receptor binding potential using simultaneous PET-MRI and the opioid ligand 11C-diprenorphine.
2. Methods
2.1. Subjects
Migraine patients and healthy subjects were recruited through physician contacts and local advertisements. Patient inclusion criteria were acute episodic migraine (with or without aura) meeting the IHS Classification ICHD-II criteria, 3–14 migraines per month for at least 3 yrs. Exclusion criteria were other significant disease, pregnancy, claustrophobia, weight > 107 kg (MRI table limit), moderate to severe depression, significant alcohol history (> 7 units/week), MR incompatible implants, previous significant exposure to ionizing radiation, a history of adverse reactions to opioids, and recent use of recreational drugs as per urine drug screen. Patients with migraine < 72 h prior to the experiment were also excluded to ensure inter-ictal status.
2.2. Patient consent and ethical considerations
Informed consent requires researchers to provide accurate, complete and understandable information about the research procedures. In clinical trials, subjects are typically informed that they may or may not receive an active drug, and/or the probability of receiving an active drug. In this the present study, there was no active drug and all subjects underwent two experiment, one with no drug, and one with placebo (IV saline). Despite some recent studies suggesting that “open-label” placebo, i.e. full disclosure, may still be effective (Kaptchuk et al., 2010), prior studies indicate that placebo effects are stronger if subjects are deceived to believe they get an active drug (Kirsch and Weixel, 1988), or if the probability of receiving and active drug is higher (R. Freeman et al., 2015). The use of deception is not consistent with fully informed consent (Miller et al., 2005), however, participants can be informed prior to deciding whether to volunteer for a study that the experimental procedures will not be described in the full extent. This approach, dubbed “authorized deception,” permits research participants to decide whether they wish to participate in research involving deception and, if so, to knowingly authorize its use. We thus informed subjects, during the consent in process, “that they will obtain either a “powerful pain killer” or placebo”. We further informed subjects about “authorized deception” in the following way: “In some research studies, the investigators cannot tell you exactly what the study is about before you participate in the study. We will describe the tasks in the study in a general way, but we cannot explain the real purpose of the study until after you complete these tasks. When you are done, we will explain why we are doing this study, what we are looking at, and any other information you should know about this study. You will also be able to ask any questions you might have about the study's purpose and the tasks you did. Though we may not be able to explain the real purpose of the study until after you complete the tasks, there are no additional risks to those that have been described in this consent form.” The Partners Human Research Committee IRB (protocol 2012.P-00555) and the Massachusetts General Hospital Radioactive Drug Research Committee (RDRC) approved the study.
2.3. Experimental details
2.3.1. Pre-scan pain calibration
Before scanning, we determined the temperature necessary to elicit an 8 on a 0–10 Visual analogue scale (20 s @ 41–48 °C) delivered to the right forearm of subjects using a Thermal Sensory Analyzer (TSA) (MEDOC, Haifa, Israel). Once the threshold was determined, subjects were informed that this would be the maximum temperature used throughout the entire experiment.
2.3.2. Pre-scan placebo conditioning
After determining the individual pain levels, subject were conditioned to expect analgesia from a placebo drug. We used intravenous normal saline as the placebo, as it has been demonstrated to be the more effective route of administration (Craen et al., 2000). An intravenous line for tracer infusion was inserted in the antecubital fossa of the left arm. This line was also used to infuse normal saline at room temperature, when telling subject they received a “short acting powerful painkiller or placebo”. Prior to infusion, subjects were told they “may feel a slight coolness to the arm and a tingling sensation”. Five minutes after injection, subjects were told that the individually determined pain stimulation was to be repeated to test analgesic success. In fact, at this time we delivered a reduced temperature (2 °C below established threshold) to condition placebo analgesia, in accordance with prior placebo studies (Price et al., 1999). In the counterbalanced “no drug” control condition, no placebo was injected, and subjects receive identical temperature stimulations as determined during the calibration procedure. Subjects were told the pain stimulation was “to ensure the stability of your pain ratings with no drug on board”. The placebo and no-drug procedures were designed to be as similar as possible to one another, and the IV was in place for both trials.
2.3.3. Scanning protocol
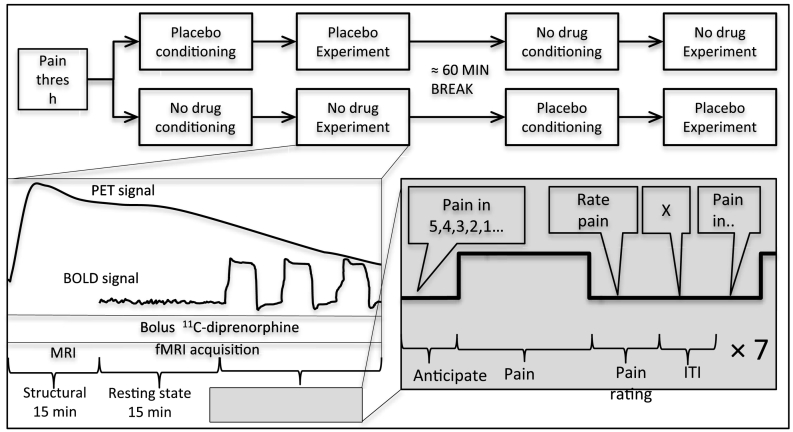
The overall experimental procedure and scanning protocol, adapted from (Wager et al., 2007), is illustrated in Fig. 1. Two scanning sessions, placebo and no drug, were obtained in counterbalanced order, separated by approximately 2.5 h to allow for radiotracer decay, and to minimize residual effects from the previous challenge. A bolus dose of 11C-diprenorphine was delivered, and PET data acquisition commenced shortly before bolus administration in list-mode throughout the scanning period. The first 15 min of scan time were used to acquire structural images, next, two six minute resting state functional MRI scans were collected, and finally a pain anticipation, provocation and rating paradigm, adapted from (Wager et al., 2007), was conducted. The paradigm consisted of a visual countdown cue (jittered 7–14 s duration) indicating that pain delivery was eminent (i.e. “Pain in 5”, “Pain in 4”, …). At the end of the anticipation period, approximately 24.5 s of thermal stimulation (3 s ramp up, 17 s at target, 4.5 s ramp down) was delivered to the dorsum of the left hand using a MR-compatible Medoc Pathway (TSA) (MEDOC, Haifa, Israel). Next, participants received a cue to rate the stimulus intensity on a 0–10 VAS scale using a MR compatible dial in their right hand. The interval between the thermal pain offset and the next pain-delivery cue was 30.5 (± 5 s jitter) seconds to allow physiological recovery and prevent habituation. This anticipation-pain-rating block was repeated seven times in each fMRI run, with four pain stimuli at pain threshold level, and three pain stimuli at two degrees Celsius under pain threshold level. The seven stimuli trial lasted approximately 8 min, and was repeated four times in each of the two sessions (placebo and no drug).
Fig. 1.
Overview of experimental setup. All subjects underwent two consecutive PET-MR scan, one with placebo and one with no drug. For both scans, 11C-diprenorphine binding, brain structure, resting state fMRI and fMRI responses to pain anticipation and high or low pain stimulation and pain ratings were measured.
2.3.4. MR scan parameters
All data was collected on a Siemens 3 Tesla MR scanner using a PET-compatible eight-channel head coil. Structural T1 weighted MPRAGE images were collected with the following parameters: voxel size 1 × 1 × 1 mm; TR = 2530 ms; TE = 1.63 ms, 3.49 ms, 5.35 ms and 7.21 ms, flip angle = 7°; rs-fMRI and pain paradigm fMRI images were with the following parameters: multi-slice T2*-weighted echo-planar images, voxel size 3.1 × 3.1 × 3.1 mm, TR = 2000 ms, TE = 30 ms, flip angle = 90°, number of slices = 37. Two sets of 184 volumes (6 min, 14 s) of resting state data were collected for each of the two PET-MR scans. Four sets of 250 volumes (8 min, 26 s) of pain experiment data, as described in Fig. 1, were collected for each of the two PET-MR scans.
2.4. MR data analysis
2.4.1. Brain structure
The two anatomical scans collected in each subject were quality controlled visually and then structurally normalized using the SPM12 default Dartel VBM pipeline (new segment, Dartel template, normalize to MNI space preserving amounts, 8 mm smoothing). Group (HC vs. migraine) differences were evaluated using a flexible factorial design incorporating the two structural scans for each subject, and with age and gender as nuisance variables. Statistical thresholds were set at a voxel-forming threshold of p < 0.001 and a cluster threshold of p < 0.05, family wise error corrected for multiple comparisons.
2.4.2. Resting state analysis
We selected the resting state scans with the lowest relative motion from each subject. There was no significant difference in subject motion between the groups or between the conditions. The resting state scans were analyzed using CONN-fMRI Functional Connectivity Toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012) http://www.nitrc.org/projects/conn, version 17c. Briefly, images were preprocessed using slice timing correction, realignment, normalization, and smoothing (8 mm FWHM Gaussian filter), using SPM12 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm). To address potential spurious correlations in resting-state networks caused by head motion, we used the Artifact Detection Tools (ART, http://www.nitrc.org/projects/artifact_detect). Specifically, an image was defined as an outlier image if the head displacement in x, y, or z direction was > 0.9 mm from the previous frame, or if the global mean intensity in the image was > 5 standard deviations from the mean image intensity for the entire resting scan. Anatomical volumes were segmented into gray matter, white matter, and CSF areas. Denoising of the BOLD time series was done using a combination of CompCor to regress out white matter and CSF signal (5 principal components each), scrubbing derived from the ART tool above, and motion regression (12 regressors: 6 motion parameters + 6 first-order temporal derivatives). The resulting residual BOLD time series were band-pass filtered (0.008 Hz < f < 0.09 Hz).
Based on prior resting state studies of placebo analgesia, we performed seed-based analysis in ten regions: 5 mm radius spherical seeds were placed in the bilateral ventral striatum (MNIXYZ = ± 13, 18, 21) from (Yu et al., 2014), the right midfrontal gyrus (MNIXYZ = 28, 52, 9), the bilateral anterior cingulate cortex (MNIXYZ = ± 3, 40,2) and the posterior cingulate cortex (MNIXYZ = ± 1, − 45, 15) from (Tetreault et al., 2016). We also included seeds in the precentral gyrus (MNIXYZ = 38, 0, 32), the anterior mid-cingulate (MNIXYZ = − 6, 22, 30), and the brainstem/periaqueductal gray area (MNIXYZ = − 2, − 26, − 10), as these areas were consistently implicated in pre-stimuli pain anticipation placebo analgesia in a meta-analysis of functional MR placebo studies (Amanzio et al., 2013).
Main effects of group (healthy subjects versus controls), placebo (no drug versus placebo), the interaction between group and placebo, and correlations to placebo magnitude were evaluated. Significance was set at a voxel-forming threshold of p < 0.001 and a cluster threshold of p < 0.05, family wise error corrected for multiple comparisons.
2.4.3. Pain anticipation and provocation data analysis
Functional scans were preprocessed using slice timing correction, realignment, normalization, and smoothing (8 mm FWHM Gaussian filter), using SPM12. In each condition (placebo & no drug) subjects underwent four sets of pain anticipation and pain stimulation at high and low temperatures (somatosensory control condition), see Fig. 1. The stimuli were modeled as boxcar time series, with additional regressors for temperature ramp-up, ramp-down, pain rating sequence, and six motion regressors.
Contrasts analyzed included pain anticipation, and pain stimulation. Main effects of stimuli (pain anticipation vs. baseline and pain stimulation vs. baseline), group (migraineurs vs. healthy subjects), placebo (no drug vs. placebo), the interaction between group and placebo, and correlations to placebo magnitude were evaluated. Significance was set at a voxel-forming threshold of p < 0.001 and a cluster threshold of p < 0.05, family wise error corrected for multiple comparisons.
2.5. 11C-diprenorphine PET data acquisition and analysis
Up to 12 mCi (8.83 ± 3.8 mCi, N = 36) of 11C-diprenorphine was injected intravenously as a manual bolus for each scan. PET data were acquired for 90 min and stored in list mode format. MR-based attenuation correction maps were created based on the MPRAGE data (Izquierdo-Garcia et al., 2014). PET data were binned into 30 frames of progressively longer duration (10 s × 9, 20 s × 3, 30 s × 3, 1 min × 1, 2 min × 1, 3 min × 1, 5 min × 8, 10 min × 4) that were motion-corrected before reconstruction using motion estimates derived from the MR data (Catana et al., 2011). The corresponding images were reconstructed using the 3D OP-OSEM algorithm with detector efficiency, decay, dead time, attenuation, and scatter corrections applied.
The reconstructed PET volume consisted of 153 slices with 256 × 256 pixels (1.25 × 1.25 × 1.25 mm3).
Kinetic modeling was carried out in PMOD (PMOD Technologies LLC, Switzerland) using the subject-specific bilateral occipital cortices as the reference tissues. Nondisplaceable binding potential maps (BPND), representing the relative amount of specifically bound radioligand to that of non-displaceable radioligand, were calculated from the 90 min of dynamic PET data using the simplified reference tissue model 2 (Wu and Carson, 2002) with the occipital cortex used as the reference tissue.
The BPND maps were moved to the MNI152 space for group analysis based on transformation matrices derived from the simultaneously acquired anatomical MRI images. The resulting 36 BNND maps (9 healthy subjects, 9 migraine subjects, placebo and no-drug conditions), were analyzes in SPM12 using paired t-tests (main effect of placebo), t-test of no-drug condition (main effect of group), a repeated measures flexible factorial model (for interaction effects), and a multiple linear regression model determining relations between placebo magnitude and BPND as well as placebo induced changes in BPND. All models included administered dose as a nuisance variable, and models were evaluated with and without adjusting for global signal, as prior studies have used both methods (Maarrawi et al., 2007, Wey et al., 2014).
Main effects of group (migraineurs versus healthy subjects), placebo (no drug versus placebo) and the interaction between group and placebo were evaluated. Significance was set at a voxel-forming threshold of p < 0.001 and a cluster threshold of p < 0.05, family wise error corrected for multiple comparisons.
3. Results
3.1. Subjects
22 subjects were investigated, whereof complete behavioral MR and PET data was obtained in 18 subjects, whereof 9 had chronic episodic migraine, see Table 1 for details.
Table 1.
Subject characteristics.
| Migraine subjects | Healthy controls | Difference | |
|---|---|---|---|
| n (men/women) | 4/5 | 3/6 | n.s. |
| Age yrs. (± SD) | 25.9 (± 4) | 25.6 (± 4) | n.s. |
| Ethnicity | 56% white (22% Hisp.) | 66% white (11% Hisp.) | |
| 33% black | 35% black | ||
| 11% more than one race | |||
| Weight kg (± SD) | 78.9 (± 18) | 70.1 (± 14) | n.s. |
| BMI | 26.7 (± 6) | 24.4 (± 4) | n.s. |
| BDI | 6.2 (± 2) | 3.1 (± 4) | n.s. |
| HAI | 0.9 (± 1) | 0.6 (± 1) | n.s. |
| BPI | 10.4 (± 12) | 1.0 (± 2) | p = 0.04 |
| Migraines/month | 5.2 (± 3) | n/a | |
| Disease duration | 13.1 (± 9) yrs. | n/a | |
| Aura | 3/9 | n/a |
Hisp. Hispanic, BMI body mass index, BDI Beck Depression Inventory, HAI Hamilton Anxiety Inventory, BPI Brief Pain Inventory.
Fourteen of the participants were not on any medications; one healthy control was on Atorvastatin and one on oral contraceptives. Three migraineurs reported taking non-steroidal anti-inflammatory drugs (NSAIDS: but not within 12 h prior to study visit), one was on oral contraceptives and one was on SSNRI (Duloxetin).
3.2. Pain thresholds, ratings and placebo effects
All patients were investigated in the inter-ictal phase. Healthy subjects selected on average 45.9 (± 1.6)°C as the highest tolerable temperature for pain stimulation, and migraine subjects selected 45.6 (± 1.2)°C, with no significant differences between migraine subjects and control subjects (p = 0.6). This temperature was rated as a 0–10 VAS of 6.2 (± 2.1) during the trials without placebo. During the trials with placebo, the average pain rating was 5.6 (± 2.1), a placebo effect of 12.5% (± 26%, p = 0.02). There was no significant difference in the magnitude of the placebo effect between patients and controls (13.8% reduction in migraineurs, 11.2% reduction in controls). Placebo effects ranged from − 1.4 to 3.9 on VAS ratings (or − 39% to 56%). In other words, some subjects reported an increase in pain ratings (nocebo effect), while the majority reported a decrease in pain ratings. Based on a median split (6.7% decrease), three of the nine healthy subjects and six of the nine migraineurs were classified as placebo responders. Notably, two healthy subjects and two migraineurs had placebo induced increases in pain ratings (more than a 6.7% increase), and were classified as nocebo responders.
3.3. Data QC
Structural data were of sufficient quality in all subjects. At least one resting state fMRI run was collected in each subject in the no-drug and in the placebo condition. For the pain anticipation and provocation paradigm, there were a total of 144 runs (18 subjects, 8 runs per subject), each consisting of 7 anticipation and provocation trials. Due to technical errors, fMRI data were lost in 8 of the 144 runs (5.5%). PET data of sufficient quality were obtained in 18 subjects in both the no-drug and the placebo condition.
3.4. Structural results
3.4.1. Group effects
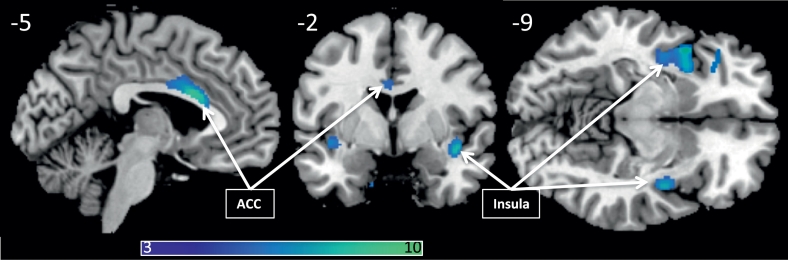
There was significantly reduced gray matter density in the migraine group in the middle and anterior cingulate gyrus, bilateral insular cortices, the left inferior temporal gyrus, the right cerebellum and left precentral gyrus. No regions displayed increased gray matter density in the migraine group as compared to the healthy group, see Fig. 2 and Table 2 for details. Correlations to placebo magnitude: There was no significant correlation between the magnitude of the placebo response and gray matter structure, nor any interactions between placebo response magnitude and group gray matter alterations.
Fig. 2.
Regions with lower gray matter density in migraine subjects (PFWE < 0.05), overlaid on a template structural image, displayed at MNIxyz (− 5, − 2, − 9). The color bar depicts T-values.
Table 2.
Regions with lower gray matter density in migraine subjects as compared to healthy controls. Coordinates in parenthesis are sub-peaks within the above larger cluster.
| MNIxyz | PFWE | Cluster voxels | T | Peak region | ||
|---|---|---|---|---|---|---|
| − 15 | 4 | − 33 | 0.002 | 107 | 9,40 | Left Uncus |
| − 6 | 16 | 22 | < 0.001 | 575 | 9.02 | Anterior cingulate |
| − 44 | 10 | − 9 | < 0.001 | 875 | 8.96 | Superior temporal gyrus |
| (− 36 | 12 | 14) | 7.87 | Left middle insula/inferior frontal gyrus | ||
| (− 39 | − 4 | − 8) | 6.87 | Left middle insula | ||
| 42 | − 2 | − 12 | < 0.001 | 202 | 7.31 | Right middle insula/temporal pole |
| − 48 | − 16 | − 30 | 0.004 | 72 | 6.65 | Left inferior temporal gyrus |
| − 36 | 38 | − 4 | 0.002 | 103 | 6.49 | Left middle frontal gyrus |
| (− 33 | 30 | − 6) | 6.49 | Left inferior frontal gyrus/anterior insula | ||
3.5. Resting state functional connectivity (rsFC) results
For clarity, seed regions, as defined in the methods above, are indicated in bold.
3.5.1. Group effects
There was significantly lower rsFC in migraine subjects than in healthy subjects between the left ventral striatum and the left superior lateral occipital cortex, (MNI(XYZ) = (− 20, − 70, 58), 499 voxels, PFWE = 0.000016).
We further observed significantly higher rsFC in migraine subjects than in healthy subjects in three seeds: the right middle frontal gyrus to the left occipital pole (MNI(XYZ) = (2, − 102, 8), 296 voxels, PFWE = 0.002), the left anterior cingulate to the left middle temporal gyrus (MNI(XYZ) = (− 46, − 16, − 18), 282 voxels, PFWE = 0.002), and the right anterior cingulate to the right frontal pole (MNI(XYZ) = (30, 64, 14), 164 voxels, PFWE = 0.039).
3.5.2. Placebo effects
Placebo as compared to no-drug, led to a significant increase in rsFC of the brainstem PAG to a cluster encompassing the bilateral occipital pole, the bilateral cuneal cortex, the right intracalcarine cortex and the right lingual gyrus (MNI(XYZ) = (4, − 88, 22), 2838 voxels, PFWE < 0.001), and to a cluster encompassing the right superior lateral occipital cortex and right angular gyrus (MNI(XYZ) = (38, − 62, 36), 223 voxels, PFWE = 0.0018). When on placebo, there was also a significant decrease in rsFC of the left ventral striatum to the bilateral superior frontal gyrus (MNI(XYZ) = (6, 22, 54), 216 voxels, PFWE = 0.0027).
3.5.3. Placebo × group interactions
RsFC of the right ventral striatum to the right superior lateral occipital cortex (MNI(XYZ) = (26, − 70, 38), 138 voxels, PFWE = 0.031) displayed an interaction effect, where healthy subjects had an increase in connectivity when on placebo, and migraine subjects displayed no change. RsFC of the left ventral striatum to the right posterior cingulate gyrus (MNI(XYZ) = (− 2, − 42, 48), 124 voxels, PFWE = 0.046), also displayed an interaction effect, where healthy subjects had an increase in connectivity when on placebo, and migraine subjects displayed a slight decrease. A third interaction effect was observed in rsFC of the right middle frontal gyrus to the right and left lateral occipital and cuneal cortex (MNI(XYZ) = (20, − 74, 44), 246 voxels, PFWE = 0.0031, and MNI(XYZ) = (− 10, − 80, 40), 155 voxels, PFWE = 0.032) where healthy subjects displayed an increase in connectivity when on placebo, and migraine subjects displayed a decrease. A forth interaction effect was evident in rsFC of the right precentral gyrus to the right parietal operculum and right anterior supramarginal gyrus (MNI(XYZ) = (54, − 26, 18), 184 voxels, PFWE = 0.0012), where healthy subjects displayed an decrease in connectivity when on placebo, and migraine subjects displayed a slight increase.
3.5.4. Predictors of placebo magnitude
In the no-drug condition, there was a significant positive correlation between subsequent individual placebo analgesia and rsFC of the left anterior cingulate to the precuneus and posterior cingulate (MNI(XYZ) = (− 4, − 50, 44), 166 voxels, PFWE = 0.032).
In the no-drug condition, there was a significant negative correlation between subsequent individual placebo analgesia and rsFC of the right ventral striatum to the middle frontal gyrus (MNI(XYZ) = (28, 30, 10), 137 voxels, PFWE = 0.043).
3.5.5. Placebo induces changes in rsFC in relation to placebo magnitude
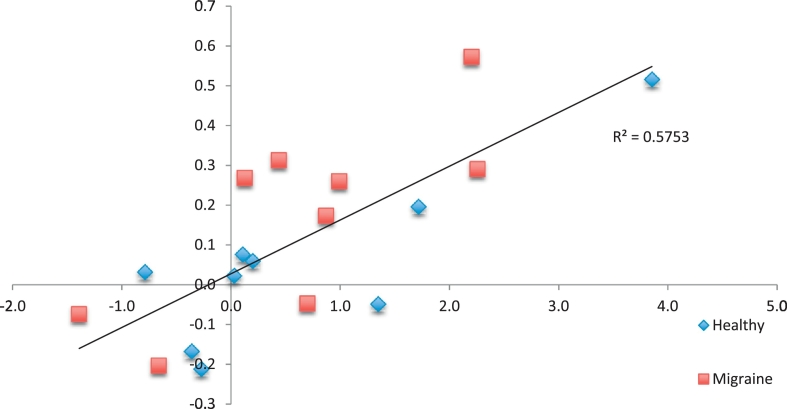
There was a significant correlation between the magnitude of placebo induced change in pain ratings and functional connectivity of the left anterior mid cingulate and the left putamen and left anterior insula (MNI(XYZ) = (− 26, 8, 8), 289 voxels, PFWE = 0.0009), see Fig. 3.
Fig. 3.
Relationship between placebo induced changes in pain ratings (x-axis, ΔVAS) and placebo induced changes in rsFC between the left anterior mid-cingulate and the left putamen/anterior insula (y-axis, β).
3.6. Evoked responses, pain anticipation
3.6.1. Main effect of anticipation
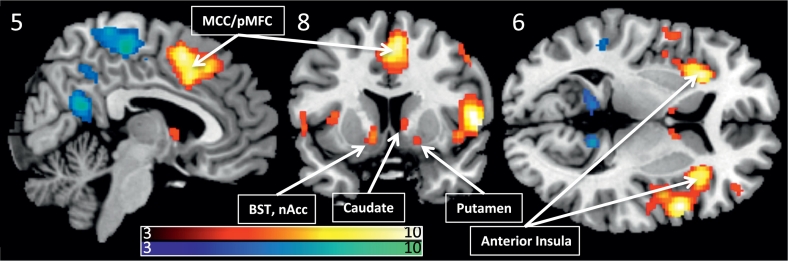
Anticipating a painful stimuli led to significant (pFWE < 0.05) activation of the right supramarginal gyrus, the bilateral anterior insula, the middle cingulate gyrus, left post-central gyrus, the bilateral middle frontal gyrus, the left pre-central gyrus, cerebellum, the bilateral caudate, right amygdala, see Fig. 4. Significant de-activations were observed in the precuneus, posterior cingulate, fusiform gyrus, bilateral angular gyrus, left superior frontal gyrus, left precentral gyrus, bilateral parahippocampal gyrus.
Fig. 4.
Significant anticipatory activations and deactivations combined over migraine subjects and healthy controls (PFWE < 0.05), overlaid on a template structural image, displayed at MNIxyz (5, 8, 6). The color bars depicts T-values. BST Bed nucleus of the Stria Terminalis, nAcc nucleus Accumbens.
3.6.2. Group effects
During anticipation, migraine patients (vs. controls) displayed significantly higher activation of the left occipital cortex lingual gyrus ((MNI(XYZ) = (− 18, − 88, 16), 1975 voxels, PFWE < 0.001, T = 7.62), and the right occipital cortex cuneus ((MNI(XYZ) = (22, − 86, 22), 188 voxels, PFWE < 0.018, T = 5.26).
3.6.3. Main effect of placebo
During anticipation, there was a significant effect of placebo on anticipatory activation of the right precentral gyrus ((MNI(XYZ) = (34, − 6, 46), 211 voxels, PFWE < 0.011, T = 5.35) indicating significantly reduced activation during trials in which all subjects had received placebo.
3.6.4. Placebo and group interactions
There was no significant group by condition interaction.
3.6.5. Correlations to placebo magnitude
There were no significant correlations between the magnitude of the placebo response and placebo induced changes in anticipatory responses.
3.7. Evoked responses, pain
3.7.1. Main effect of pain stimulus
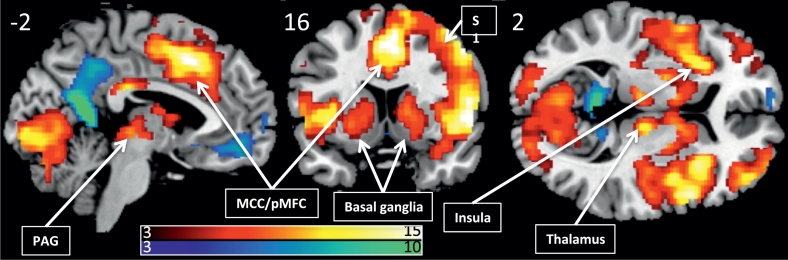
The painful stimuli evoked significant functional responses in regions typically associated with pain processing, including the middle cingulate, the thalamus, the periaqueductal gray, the insula, primary and secondary somatosensory cortices, see Fig. 5.
Fig. 5.
Pain induced activations and deactivations across all subjects (PFWE < 0.05), overlaid on a template structural image, displayed at MNIxyz (− 2, 16, 2). The color bars depicts T-values.
3.7.2. Group effects
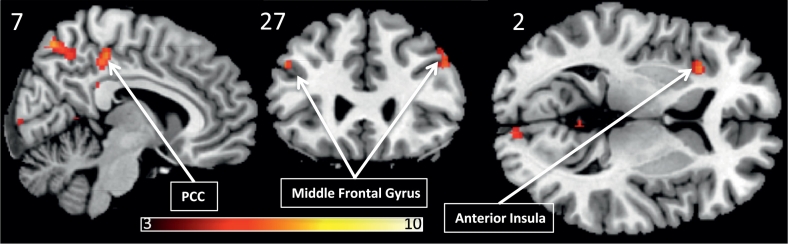
Across both the baseline and the placebo condition, healthy subjects displayed greater pain induced activation of several regions, including the right superior parietal lobule, the bilateral supramarginal gyrus, the bilateral occipital poles, the left anterior insula, the precuneus, the medial precentral gyrus, the bilateral middle frontal gyrus and the left hippocampus, see Fig. 6 and Table 3 for details. There were no regions where migraine patients displayed hyperactivation to pain as compared to healthy subjects.
Fig. 6.
Regions where healthy controls displayed higher activation than migraine subjects (PFWE < 0.05), overlaid on a template structural image, displayed at MNIxyz (− 2, 16, 2). The color bar depicts T-values.
Table 3.
Regions with higher BOLD responses to pain in healthy controls versus migraine subjects. Subpeaks within the same cluster are indicated in parenthesis.
| MNIxyz | PFWE | Cluster voxels | T | Peak region | ||
|---|---|---|---|---|---|---|
| 42 | − 50 | 60 | < 0.001 | 557 | 7.98 | R. Inf. Parietal Lobule |
| (38 | − 50 | 36) | 7.75 | R. Inf. Parietal Lobule | ||
| (52 | − 46 | 50) | 7.58 | R. Inf. Parietal Lobule | ||
| 58 | − 30 | 50 | < 0.001 | 85 | 7.32 | R. Postcentral Gyrus |
| 18 | − 94 | − 8 | < 0.001 | 103 | 7.17 | R. Lingual Gyrus |
| (8 | − 92 | 2) | 5.77 | R. Lingual Gyrus | ||
| − 20 | − 98 | − 4 | < 0.001 | 54 | 6.91 | L. Cuneus |
| − 32 | 22 | 6 | < 0.001 | 46 | 6.79 | L. Anterior insula |
| 6 | − 68 | 56 | < 0.001 | 82 | 6.76 | R. Sup. Parietal Lobule |
| (4 | − 56 | 48) | 5.72 | R. Precuneus | ||
| 6 | − 32 | 50 | < 0.001 | 94 | 6.74 | R. Frontal Paracentral Lobule |
| − 58 | − 26 | 34 | < 0.001 | 123 | 6.67 | L. Inf. Parietal Lobule |
| (− 58 | − 32 | 46) | 5.99 | L. Inf. Parietal Lobule | ||
| (− 46 | − 38 | 40) | 5.47 | L. Inf. Parietal Lobule | ||
| − 52 | 18 | 32 | < 0.001 | 81 | 6.51 | L. Middle Frontal Gyrus |
| (− 48 | 26 | 32) | 6.37 | L. Middle Frontal Gyrus | ||
| (− 38 | 34 | 34) | 5.85 | L. Superior Frontal Gyrus | ||
| 46 | 30 | 38 | < 0.001 | 49 | 6.51 | R. Middle Frontal Gyrus |
| − 20 | − 34 | − 6 | 0.001 | 23 | 6.35 | L. Parahippocampal Gyrus |
| 4 | − 52 | 6 | 0.001 | 22 | 6.34 | R. Posterior Cingulate Gyrus |
| − 20 | − 58 | 58 | 0.001 | 32 | 6.23 | L. Precuneus |
| 46 | 50 | 10 | 0.003 | 16 | 6.03 | R. Middle Frontal Gyrus |
| (46 | 42 | 8) | 5.03 | R. Middle Frontal Gyrus | ||
| 36 | 56 | − 10 | < 0.001 | 34 | 5.92 | R. Middle Frontal Gyrus |
| 54 | − 30 | 24 | 0.005 | 12 | 5.92 | R. Inf. Parietal Lobule |
| − 36 | − 48 | 36 | 0.001 | 28 | 5.91 | L. Supramarginal Gyrus |
| 30 | 52 | 30 | 0.004 | 14 | 5.82 | R. Superior Frontal Gyrus |
| − 38 | − 60 | 56 | 0.002 | 21 | 5.72 | L. Superior Parietal Lobule |
| 14 | − 62 | 58 | 0.003 | 15 | 5.41 | R. Precuenus |
| − 2 | − 36 | 30 | 0.004 | 13 | 5.32 | L. Posterior Cingulate Gyrus |
| − 46 | − 48 | 52 | 0.002 | 18 | 5.30 | L. Inf. Parietal Lobule |
3.7.3. Placebo effects
There were no significant effects of placebo on pain induced responses, no group-by-condition interactions and no correlations between placebo magnitude and pain induced activations.
3.8. PET diprenorphine BPND
3.8.1. Group, placebo, and interaction effects
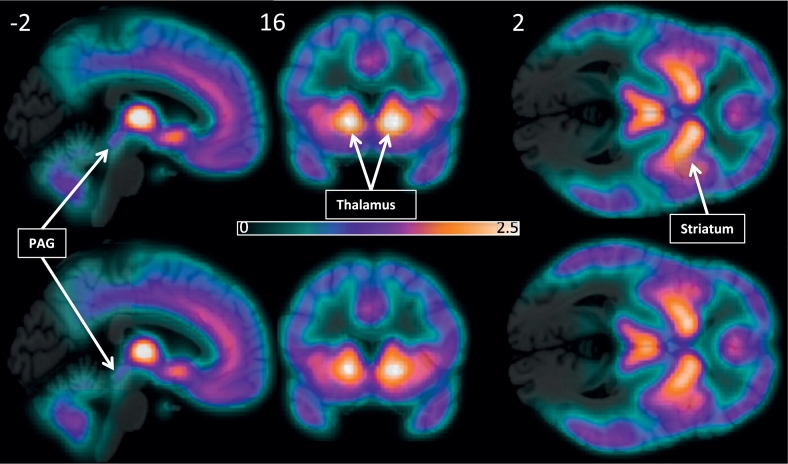
There were no significant differences in baseline BPND between the migraineurs and healthy controls (Fig. 7), no significant changes in BPND between the no-drug and the placebo conditions, no significant placebo-by-group interactions, no significant correlations between baseline opioid BPND and the magnitude of placebo analgesia, and no significant correlations between placebo-induced changes in opioid BPND and the magnitude of placebo analgesia.
Fig. 7.
Average opioid receptor BPND in healthy subjects (top) and migraine subjects (bottom) overlaid on a template structural image. The color bar indicates 11C-diprenorphine BPND.
4. Discussion
With 18 subjects investigated with repeated PET-MR measures, this was one of the largest studies to date to employ simultaneous molecular and functional imaging. The study did not find not find evidence for an altered placebo response in migraine subjects as compared to healthy subject, either in terms of placebo induced reductions in pain ratings, in functional brain responses to pain anticipation or functional responses to thermal pain, or in placebo induced alterations in diprenorphine BPND. We did, however, observe some group-by-condition interactions in resting state functional connectivity, discussed below. Group comparisons with nine subjects in each cell yield low statistical power (Poldrack et al., 2017), so our results, and lack of results, are not conclusive. Below we discuss these findings in the context of four domains: i) Pain ratings; ii) opioid receptor binding; iii) resting and functional responses; and iv) gray matter alterations.
4.1. Pain ratings
We did not observe any group differences in pain thresholds or pain ratings between migraine subjects and healthy controls, in line with prior studies in the inter-ictal phase (Uglem et al., 2016). The current study used acute pain and acute placebo, enhanced by conditioning, as has been the case in most prior investigation on placebo mechanisms. The magnitude of the placebo analgesia in the present study was 13% on a VAS rating scale of pain intensity, equivalent of a standard mean difference (SMD) of 0.29. This degree of analgesia is lower than that of many prior placebo mechanism studies, where a recent meta-analysis found that placebo analgesia had a SMD of 1.73 in experimental pain (Forsberg et al., 2017). However, prior studies of experimental placebo analgesia have reported placebo analgesia in a broad range (for example 2% (Wager et al., 2004) 5,5% (Johansen et al., 2003), 5.6% (Wrobel et al., 2016), 9.8% (Aslaksen et al., 2011), 22% (Wager et al., 2004) and 5–35% (Colloca and Benedetti, 2006) VAS pain reductions). Notably, comparable placebo responses have been observed in children and adolescents (Wrobel et al., 2015), and in the elderly (Wrobel et al., 2016). Moreover, in the current study, while subjects on average displayed a decrease in pain ratings when on placebo, the degree of placebo response ranged from 56% reductions (clear placebo effect) to a 39% increase, indicative of a nocebo response. Modeling placebo effects as a continuum from nocebo to placebo did not yield any significant relationships. It is possible that placebo and nocebo engage different neuronal mechanism (S. Freeman et al., 2015), which thereby cannot be captured in linear regression models.
Neuroimaging studies typically investigate immediate placebo analgesic effects on experimental pain in healthy controls; patient trials typically focus on reductions of clinical pain in longer duration trials. In such trials, when placebo is compared to no treatment, there is a much more modest placebo analgesic response of 6.5% (Hrobjartsson and Gotzsche, 2001). Mechanism studies often enhance placebo responses using conditioning and/or suggestions, and thus, placebo analgesic effects are typically higher in mechanism studies than in placebo control studies (Vase et al., 2009). This is not explained by experimental pain being more susceptible to placebo analgesia than clinical pain, on the contrary, in a direct comparison, placebo was more effective at reducing lower back pain than at reducing cold pressor pain (Charron et al., 2006). In the current study, migraine subjects were pain free at the time of investigation, so clinical pain was not involved.
In the present study, placebo conditioning, no-drug conditioning, and testing was done on the same skin region, raising the possibility that the conditioning stimuli may have sensitized or de-sensitized the skin region. For placebo conditioning, subjects received a stimulus at 2 °C below threshold, and for the no-drug conditioning, subjects received a stimulus at the pre-determined pain threshold. Skin sensitization could thus be more prominent in the no-drug condition, and bias results towards higher pain ratings during the no-drug session. We did not observe evidence for skin sensitization or de-sensitization. While pain ratings were typically highest for the very first stimuli in a series (salience effect), there was no significant decrease in pain ratings over time, and no time-by-group-by-treatment interactions.
4.2. Opioid receptor binding
We did not observe any differences between migraine subjects and healthy controls diprenorphine baseline BPND. Prior studies on patient populations have observed reduced binding, as evidenced in neuropathic pain (Jones et al., 2004), post-stroke pain (Willoch et al., 2004), peripheral neuropathy (Maarrawi et al., 2007) and fibromyalgia (Harris et al., 2007). Further, successful treatment can normalize opioid BPND (Harris et al., 2009, Jones et al., 1999, Jones et al., 1994). DaSilva et al. (2014) recently demonstrated μOR activation in the medial prefrontal cortex during the ictal phase in seven migraine patients, and Nascimento et al. (2014) found μOR activation of the midbrain PAG correlated to the magnitude of ictal trigeminal allodynia in the same cohort. In the current study, patients were investigated in the inter-ictal state. There was no ongoing clinical pain, potentially thereby minimizing differences between healthy subjects and migraine subjects.
It is well established the administration of the opioid antagonist naloxone can decreases placebo analgesia (Amanzio and Benedetti, 1999, Levine et al., 1978, Eippert et al., 2009), and prior studies indicate that endogenous opioid tone influences the magnitude of placebo analgesia (Zubieta et al., 2005, Lipman et al., 1990). We did not find evidence of this in the present study. In previous opioid receptor imaging studies on placebo (Wager et al., 2007, Zubieta et al., 2005, Scott et al., 2008, Zubieta and Stohler, 2009), the μ-opioid specific agonist 11C-carfentanil (Frost et al., 1985) has been used, whereas in the present study, we used the μ-, κ- and δ-receptor antagonist 11C-diprenorphine (Jones et al., 1985). While changes in diprenorphine BPND have been observed in response to acupuncture (Dougherty et al., 2008) and sustained cuff pain (Wey et al., 2014), diprenorphine appears less sensitive than carfentanil to endogenous opioid release (Quelch et al., 2014), potentially explaining the current null-effects. It is possible that more potent and longer duration stimuli, such as ongoing clinical pain, are needed to alter opioid receptor BPND as measured by 11C-diprenorphine.
It is not possible to state evidence for the null hypothesis (placebo does not change 11C-diprenorphine BPND) using conventional significance testing. Bayes factor is an attractive alternative to the conventional t-test, that can indicate preference for either the null or the alternative hypothesis (Rouder et al., 2009). As a follow-up analysis, we extracted BPND levels for the anterior cingulate, the right and left thalamus, and for the PAG, and calculated Bayes factor using the Jeffrey-Zellner-Siow (JZS) Prior, as implemented in (Rouder et al., 2009) (http://pcl.missouri.edu/bf-one-sample). Bayes factor was 4.11 for the ACC, 3.98 for the left and 3.95 for the right thalamus, and 3.56 for the PAG, all in favor of the null hypothesis.
4.3. Functional MRI - resting state
Prior resting state functional connectivity studies in migraine have demonstrated altered connectivity patterns, primarily in regions involved in pain processing, see (Schwedt et al., 2015) for a recent review. In the present study, we observed group differences in the no-drug condition between migraine subjects and healthy controls in rsFC of the left ventral striatum to occipital regions. These results were not predicted, as a prior study of rsFC of striatal regions in migraine found increased connectivity to the ACC and decreased connectivity to the anterior insula (Chen et al., 2016). We further observed an increased connectivity between right middle frontal gyrus and the left occipital pole, somewhat in line with a results from a prior study on 21 migraine subjects (without aura) that observed increased rsFC between occipital regions and the medial prefrontal cortex (Jin et al., 2013). As such, future adequately powered studies on rsFC of occipital and prefrontal regions are warranted. We did not, however, replicate prior studies indicative of altered rsFC of the brainstem periaqueductal gray region in migraine (Li et al., 2016).
Despite the lack of behavioral differences between patients and controls in placebo magnitude, we observed a placebo by group interactions on resting-state connectivity patterns in four of the nine seed regions investigated. Notably, on placebo, the control subjects displayed an increase in connectivity between seeds in the right ventral stratum and the right middle frontal gyrus to occipital region, where migraine subjects did not show such an increase. Prior studies on healthy subjects have found that placebo aimed at reducing anxiety (Petrovic et al., 2005) or disgust (Schienle et al., 2014) can alter visual cortex responses to emotional stimuli in healthy subjects. In our sample, three out of nine patients' experienced visual aura associated with migraine attacks, and it has been suggested that cortical spreading depression of the visual cortex also occurs in migraine without aura. As such, we cannot assert an interpretation of the observed lack of placebo-induced increases in occipital cortex connectivity in migraine, but this may be an interesting area of future studies.
Functional connectivity metrics may be useful to predict subsequent placebo responses (Yu et al., 2014, Tetreault et al., 2016, Schmidt-Wilcke et al., 2014) using various methods (regional homogeneity of the ventral striatum in during conditioned analgesia in experimental pain (Yu et al., 2014), degree count of the right medial frontal gyrus predicting pill placebo responses in knee OA pain (Tetreault et al., 2016), and pregenual ACC to dorsolateral prefrontal resting state connectivity predicting fibromyalgia pill placebo (Schmidt-Wilcke et al., 2014)). As our paradigm was different than the above studies, we did not attempt a direct replication. However, we found that the magnitude of placebo analgesia was correlated to baseline rsFC between the left anterior cingulate and the posterior cingulate, and that placebo analgesia was negatively correlated to right ventral striatum to middle frontal gyrus baseline rsFC, thus implicating similar structures as the prior studies suggestive of a role for both attention and reward process in placebo susceptibility.
We are not aware of any studies investigating alterations in rsFC induced by acute experimental placebo analgesia. Here, we observed that the magnitude of placebo analgesia was correlated to placebo-induced changes in rsFC between the left anterior mid-cingulate and the left putamen. It has been suggested that part of the cortico-basal ganglia-thalamo-cortical loops is to relate cognitive information from neocortex to the putamen, which in turn influences activity of large areas of the cerebral cortex processing nociceptive information (Starr et al., 2011). Thus, it is conceivable that information flow between the mid-cingulate and the putamen sets the stage for how a nociceptive stimulus will be experienced, and modulating this information flow can either decreases (placebo) or increases (nocebo) how subsequent pain is experienced.
4.4. Pain anticipation functional brain responses
Anticipating pain led to significant activations of the bilateral anterior insula, the middle cingulate gyrus and left amygdala, highly consistent with prior studies on pain anticipation (see Palermo et al., 2015 for a recent meta-analysis). We further found that during pain anticipation, migraine patients displayed a higher activation of the regions of the primary visual cortex. Such hyperactivations have previously been reported in migraine patients with aura, but not in patients without aura (Cucchiara et al., 2015, Datta et al., 2013). Further, visual cortex activation during pain anticipation has been related to visual cortex hyperactivation in healthy subjects that are pain fearful (Yang et al., 2016). In the current study, only three of the nine patients had aura, and patients were not more anxious than healthy subjects as per self-report, so the interpretation of the observed group difference is not clear.
Further, there was a main effect of placebo on pain anticipatory activation of the right precentral gyrus, with reduced activations when on placebo. This has not been reported in prior imaging studies investigating placebo analgesia effects on anticipatory activation (Wager et al., 2004, Geuter et al., 2013, Watson et al., 2009). There was however no placebo effects on pain anticipation in the dorsolateral prefrontal cortex, brainstem or cingulate, as previously reported in studies on healthy subjects (Wager et al., 2004, Geuter et al., 2013, Watson et al., 2009). Moreover, there were no significant correlations between the magnitude of the placebo response and placebo induced changes in anticipatory responses.
4.5. Pain functional brain responses
Migraine patients and healthy controls displayed similar activation patterns to pain stimulation in the somatosensory cortices, mid-cingulate and brain stem, but healthy subjects displayed greater activation than migraineurs to pain stimuli, including the posterior cingulate, anterior insula and parahippocampal gyrus. This is in contrast to some prior studies indicating hyperactivation of these areas in migraine subjects (Schwedt et al., 2014, Russo et al., 2012). In contrast to prior studies, placebo did not affect functional pain responses, nor did we observe a correlation between the magnitude of the placebo response and changes in functional pain responses. It should be noted that prior studies have typically had larger samples, and/or excluded placebo non-responders from the analysis, and employed more lenient statistical thresholds.
4.6. Gray matter volume
Our results on gray matter density confirm prior studies and meta-analyses indicating gray matter reductions in posterior insular and anterior cingulate regions (Dai et al., 2015). However we did not replicate prior findings by Schweinhardt et al. (2009) indicating a relation between the magnitude of placebo response and gray matter density in the insula and striatum, either at the pre-defined corrected statistical threshold of pFWE < 0.05, or at an exploratory uncorrected threshold of p < 0.05. As the study by Schweinhardt et al. was larger (22 subjects) and more homogenous (all healthy males aged 22 ± 4 yrs.), and employed a more lenient statistical threshold, the present study may have had insufficient statistical power to provide a replication.
4.7. Conclusions
This study replicated prior studies on group differences in brain structure in migraineurs, but we found no evidence for alterations in opioid receptor expression at baseline. We further observed the expected main effects of pain anticipation and pain stimulation, and our experimental paradigm induced a small but significant placebo analgesic response in terms of pain ratings. We did not find substantial evidence for an altered placebo response in interictal migraine subjects as compared to healthy subjects, either in behavior, resting state metrics, functional responses or opioidergic function. Prior neuroimaging studies on placebo analgesia have typically employed larger and more homogenous populations, but also used more lenient statistical thresholds. The intra- and inter-individual variability of acute experimental placebo responses is high, and studies on patient populations add further variability due to differences in clinical severity. Thus, future studies need to be adequately powered (Button et al., 2013) and preferentially evaluate migraine subjects during more than one phase of migraine.
Acknowledgments
Acknowledgements
The authors would like to thank the staff at the Martinos center, and the participants of the study.
The work was supported by grant NCCIH 5R21AT007530-02 (DB), NINDS K24 NS064050 (DB).
Contributors
CL, LB, JH and DB designed the study. CL and MP collected the data. CL, CC and DBC analyzed the data. CL, CC, DBC, LB, JH and DB interpreted the results and wrote the manuscript.
Conflict of interest
DB consults for Biogen. The other authors have no potential conflicts of interest.
References
- Amanzio M., Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J. Neurosci. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. (Epub 1998/12/31. PubMed PMID: 9870976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzio M., Benedetti F., Porro C.A., Palermo S., Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum. Brain Mapp. 2013;34(3):738–752. doi: 10.1002/hbm.21471. (PubMed PMID: 22125184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslaksen P.M., Bystad M., Vambheim S.M., Flaten M.A. Gender differences in placebo analgesia: event-related potentials and emotional modulation. Psychosom. Med. 2011;73(2):193–199. doi: 10.1097/PSY.0b013e3182080d73. (Epub 2011/01/11. PubMed PMID: 21217098) [DOI] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P., Mokrysz C., Nosek B.A., Flint J., Robinson E.S., Munafo M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. (PubMed PMID: 23571845) [DOI] [PubMed] [Google Scholar]
- Catana C., Benner T., van der Kouwe A., Byars L., Hamm M., Chonde D.B., Michel C.J., El Fakhri G., Schmand M., Sorensen A.G. MRI-assisted PET motion correction for neurologic studies in an integrated MR-PET scanner. Eur. J. Nucl. Med. 2011;52(1):154–161. doi: 10.2967/jnumed.110.079343. (Epub 2010/12/30. PubMed PMID: 21189415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron J., Rainville P., Marchand S. Direct comparison of placebo effects on clinical and experimental pain. Clin. J. Pain. 2006;22(2):204–211. doi: 10.1097/01.ajp.0000161526.25374.e5. (PubMed PMID: 16428957) [DOI] [PubMed] [Google Scholar]
- Chen Z., Chen X., Liu M., Liu S., Shu S., Ma L., Altered Yu S. Functional connectivity of the marginal division in migraine: a resting-state fMRI study. J. Headache Pain. 2016;17(1):89. doi: 10.1186/s10194-016-0682-1. (PubMed PMID: 27670428; PMCID: PMC5037100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L., Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124(1–2):126–133. doi: 10.1016/j.pain.2006.04.005. (PubMed PMID: 16701952) [DOI] [PubMed] [Google Scholar]
- de Craen A.J., Tijssen J.G., de Gans J., Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J. Neurol. 2000;247(3):183–188. doi: 10.1007/s004150050560. (Epub 2000/04/29. PubMed PMID: 10787112) [DOI] [PubMed] [Google Scholar]
- Cucchiara B., Datta R., Aguirre G.K., Idoko K.E., Detre J. Measurement of visual sensitivity in migraine: validation of two scales and correlation with visual cortex activation. Cephalalgia. 2015;35(7):585–592. doi: 10.1177/0333102414547782. (PubMed PMID: 25187033) [DOI] [PubMed] [Google Scholar]
- Dai Z., Zhong J., Xiao P., Zhu Y., Chen F., Pan P., Shi H. Gray matter correlates of migraine and gender effect: a meta-analysis of voxel-based morphometry studies. Neuroscience. 2015;299:88–96. doi: 10.1016/j.neuroscience.2015.04.066. (PubMed PMID: 25943478) [DOI] [PubMed] [Google Scholar]
- DaSilva A.F., Nascimento T.D., DosSantos M.F., Lucas S., van Holsbeec K.H., DeBoer M., Maslowski E., Love T., Martikainen I.K., Koeppe R.A., Smith Y.R., Zubieta J.K. Association of mu-opioid activation in the prefrontal cortex with spontaneous migraine attacks - brief report I. Ann. Clin. Transl. Neurol. 2014;1(6):439–444. doi: 10.1002/acn3.65. (PubMed PMID: 25072055; PMCID: PMC4110741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R., Aguirre G.K., Hu S., Detre J.A., Cucchiara B. Interictal cortical hyperresponsiveness in migraine is directly related to the presence of aura. Cephalalgia. 2013;33(6):365–374. doi: 10.1177/0333102412474503. (PubMed PMID: 23359872; PMCID: PMC3658127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D.D., Kong J., Webb M., Bonab A.A., Fischman A.J., Gollub R.L. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav. Brain Res. 2008;193(1):63–68. doi: 10.1016/j.bbr.2008.04.020. (PubMed PMID: 18562019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F., Bingel U., Schoell E.D., Yacubian J., Klinger R., Lorenz J., Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. (PubMed PMID: 19709634) [DOI] [PubMed] [Google Scholar]
- Forsberg J.T., Martinussen M., Flaten M.A. The placebo analgesic effect in healthy individuals and patients: a meta-analysis. Psychosom. Med. 2017;79(4):388–394. doi: 10.1097/PSY.0000000000000432. (PubMed PMID: 27922566) [DOI] [PubMed] [Google Scholar]
- Freeman R., Emir B., Parsons B. Predictors of placebo response in peripheral neuropathic pain: insights from pregabalin clinical trials. J. Pain Res. 2015;8:257–268. doi: 10.2147/JPR.S78303. (PubMed PMID: 26082659; PMCID: PMC4459620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S., Yu R., Egorova N., Chen X., Kirsch I., Claggett B., Kaptchuk T.J., Gollub R.L., Kong J. Distinct neural representations of placebo and nocebo effects. NeuroImage. 2015;112:197–207. doi: 10.1016/j.neuroimage.2015.03.015. (PubMed PMID: 25776211; PMCID: PMC4408248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost J.J., Wagner H.N., Jr., Dannals R.F., Ravert H.T., Links J.M., Wilson A.A., Burns H.D., Wong D.F., McPherson R.W., Rosenbaum A.E. Imaging opiate receptors in the human brain by positron tomography. J. Comput. Assist. Tomogr. 1985;9(2):231–236. doi: 10.1097/00004728-198503000-00001. (Epub 1985/03/01. PubMed PMID: 2982931) [DOI] [PubMed] [Google Scholar]
- Geuter S., Eippert F., Hindi Attar C., Buchel C. Cortical and subcortical responses to high and low effective placebo treatments. NeuroImage. 2013;67:227–236. doi: 10.1016/j.neuroimage.2012.11.029. (PubMed PMID: 23201367; PMCID: PMC3578963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevert P., Albert L.H., Goldstein A. Partial antagonism of placebo analgesia by naloxone. Pain. 1983;16(2):129–143. doi: 10.1016/0304-3959(83)90203-8. (PubMed PMID: 6308540) [DOI] [PubMed] [Google Scholar]
- Harris R.E., Clauw D.J., Scott D.J., McLean S.A., Gracely R.H., Zubieta J.K. Decreased central mu-opioid receptor availability in fibromyalgia. J. Neurosci. 2007;27(37):10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. (Epub 2007/09/15. PubMed PMID: 17855614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.E., Zubieta J.K., Scott D.J., Napadow V., Gracely R.H., Clauw D.J. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs) NeuroImage. 2009;47(3):1077–1085. doi: 10.1016/j.neuroimage.2009.05.083. (Epub 2009/06/09. PubMed PMID: 19501658; PMCID: 2757074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrobjartsson A., Gotzsche P.C. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N. Engl. J. Med. 2001;344(21):1594–1602. doi: 10.1056/NEJM200105243442106. (PubMed PMID: 11372012) [DOI] [PubMed] [Google Scholar]
- Izquierdo-Garcia D., Hansen A.E., Forster S., Benoit D., Schachoff S., Furst S., Chen K.T., Chonde D.B., Catana C. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. Eur. J. Nucl. Med. 2014;55(11):1825–1830. doi: 10.2967/jnumed.113.136341. (PubMed PMID: 25278515; PMCID: PMC4246705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Yuan K., Zhao L., Zhao L., Yu D., von Deneen K.M., Zhang M., Qin W., Sun W., Tian J. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26(1):58–64. doi: 10.1002/nbm.2819. (PubMed PMID: 22674568) [DOI] [PubMed] [Google Scholar]
- Johansen O., Brox J., Flaten M.A. Placebo and nocebo responses, cortisol, and circulating beta-endorphin. Psychosom. Med. 2003;65(5):786–790. doi: 10.1097/01.psy.0000082626.56217.cf. (PubMed PMID: 14508021) [DOI] [PubMed] [Google Scholar]
- Jones A.K., Luthra S.K., Pike V.W., Herold S., Brady F. New labelled ligand for in-vivo studies of opioid physiology. Lancet. 1985;2(8456):665–666. doi: 10.1016/s0140-6736(85)90028-5. (Epub 1985/09/21. PubMed PMID: 2863651) [DOI] [PubMed] [Google Scholar]
- Jones A.K., Cunningham V.J., Ha-Kawa S., Fujiwara T., Luthra S.K., Silva S., Derbyshire S., Jones T. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br. J. Rheumatol. 1994;33(10):909–916. doi: 10.1093/rheumatology/33.10.909. (Epub 1994/10/01. PubMed PMID: 7921749) [DOI] [PubMed] [Google Scholar]
- Jones A.K., Kitchen N.D., Watabe H., Cunningham V.J., Jones T., Luthra S.K., Thomas D.G. Measurement of changes in opioid receptor binding in vivo during trigeminal neuralgic pain using [11C] diprenorphine and positron emission tomography. J. Cereb. Blood Flow Metab. 1999;19(7):803–808. doi: 10.1097/00004647-199907000-00011. (Epub 1999/07/21. PubMed PMID: 10413036) [DOI] [PubMed] [Google Scholar]
- Jones A.K., Watabe H., Cunningham V.J., Jones T. Cerebral decreases in opioid receptor binding in patients with central neuropathic pain measured by [11C]diprenorphine binding and PET. Eur. J. Pain. 2004;8(5):479–485. doi: 10.1016/j.ejpain.2003.11.017. (Epub 2004/08/25. PubMed PMID: 15324779) [DOI] [PubMed] [Google Scholar]
- Kaptchuk T.J., Friedlander E., Kelley J.M., Sanchez M.N., Kokkotou E., Singer J.P., Kowalczykowski M., Miller F.G., Kirsch I., Lembo A.J. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One. 2010;5(12):e15591. doi: 10.1371/journal.pone.0015591. (Epub 2011/01/05. PubMed PMID: 21203519; PMCID: 3008733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I., Weixel L.J. Double-blind versus deceptive administration of a placebo. Behav. Neurosci. 1988;102(2):319–323. doi: 10.1037//0735-7044.102.2.319. (PubMed PMID: 3365327) [DOI] [PubMed] [Google Scholar]
- Kosek E., Rosen A., Carville S., Choy E., Gracely R.H., Marcus H., Petzke F., Ingvar M., Jensen K.B. Lower placebo responses after long-term exposure to fibromyalgia pain. J. Pain. 2017 doi: 10.1016/j.jpain.2017.02.434. (PubMed PMID: 28279705) [DOI] [PubMed] [Google Scholar]
- Levine J.D., Gordon N.C., Fields H.L. The mechanism of placebo analgesia. Lancet. 1978;2(8091):654–657. doi: 10.1016/s0140-6736(78)92762-9. (Epub 1978/09/23. PubMed PMID: 80579) [DOI] [PubMed] [Google Scholar]
- Li Z., Liu M., Lan L., Zeng F., Makris N., Liang Y., Guo T., Wu F., Gao Y., Dong M., Yang J., Li Y., Gong Q., Liang F., Kong J. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci. Rep. 2016;6 doi: 10.1038/srep20298. (PubMed PMID: 26839078; PMCID: PMC4738255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman J.J., Miller B.E., Mays K.S., Miller M.N., North W.C., Byrne W.L. Peak B endorphin concentration in cerebrospinal fluid: reduced in chronic pain patients and increased during the placebo response. Psychopharmacology. 1990;102(1):112–116. doi: 10.1007/BF02245754. (Epub 1990/01/01. PubMed PMID: 2144051) [DOI] [PubMed] [Google Scholar]
- Loder E., Goldstein R., Biondi D. Placebo effects in oral triptan trials: the scientific and ethical rationale for continued use of placebo controls. Cephalalgia. 2005;25(2):124–131. doi: 10.1111/j.1468-2982.2004.00817.x. (PubMed PMID: 15658949) [DOI] [PubMed] [Google Scholar]
- Maarrawi J., Peyron R., Mertens P., Costes N., Magnin M., Sindou M., Laurent B., Garcia-Larrea L. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain. 2007;127(1–2):183–194. doi: 10.1016/j.pain.2006.10.013. (Epub 2006/12/02. PubMed PMID: 17137714) [DOI] [PubMed] [Google Scholar]
- Macedo A., Farre M., Banos J.E. A meta-analysis of the placebo response in acute migraine and how this response may be influenced by some of the characteristics of clinical trials. Eur. J. Clin. Pharmacol. 2006;62(3):161–172. doi: 10.1007/s00228-005-0088-5. (Epub 2006/01/13, PubMed PMID: 16402240) [DOI] [PubMed] [Google Scholar]
- Macedo A., Banos J.E., Farre M. Placebo response in the prophylaxis of migraine: a meta-analysis. Eur. J. Pain. 2008;12(1):68–75. doi: 10.1016/j.ejpain.2007.03.002. (Epub 2007/04/25, PubMed PMID: 17451980) [DOI] [PubMed] [Google Scholar]
- Miller F.G., Wendler D., Swartzman L.C. Deception in research on the placebo effect. PLoS Med. 2005;2(9):e262. doi: 10.1371/journal.pmed.0020262. (Epub 2005/09/22. PubMed PMID: 16173830; PMCID: 1198039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento T.D., DosSantos M.F., Lucas S., van Holsbeeck H., DeBoer M., Maslowski E., Love T., Martikainen I.K., Koeppe R.A., Smith Y.R., Zubieta J.K., DaSilva A.F. Mu-opioid activation in the midbrain during migraine allodynia - brief report II. Ann. Clin. Transl. Neurol. 2014;1(6):445–450. doi: 10.1002/acn3.66. 10.1002/acn3.66. (PubMed PMID: 25328905; PMCID: PMC4184673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo S., Benedetti F., Costa T., Amanzio M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Hum. Brain Mapp. 2015;36(5):1648–1661. doi: 10.1002/hbm.22727. (PubMed PMID: 25529840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P., Dietrich T., Fransson P., Andersson J., Carlsson K., Ingvar M. Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. (PubMed PMID: 15953423) [DOI] [PubMed] [Google Scholar]
- Ploner M., Lee M.C., Wiech K., Bingel U., Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc. Natl. Acad. Sci. U. S. A. 2010;107(1):355–360. doi: 10.1073/pnas.0906186106. (PubMed PMID: 19948949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Baker C.I., Durnez J., Gorgolewski K.J., Matthews P.M., Munafo M.R., Nichols T.E., Poline J.B., Vul E., Yarkoni T. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 2017;18(2):115–126. doi: 10.1038/nrn.2016.167. (PubMed PMID: 28053326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D.D., Milling L.S., Kirsch I., Duff A., Montgomery G.H., Nicholls S.S. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83(2):147–156. doi: 10.1016/s0304-3959(99)00081-0. (Epub 1999/10/27. PubMed PMID: 10534585) [DOI] [PubMed] [Google Scholar]
- Quelch D.R., Katsouri L., Nutt D.J., Parker C.A., Tyacke R.J. Imaging endogenous opioid peptide release with [11C]carfentanil and [3H]diprenorphine: influence of agonist-induced internalization. J. Cereb. Blood Flow Metab. 2014;34(10):1604–1612. doi: 10.1038/jcbfm.2014.117. (PubMed PMID: 25005876; PMCID: PMC4269718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder J.N., Speckman P.L., Sun D., Morey R.D., Iverson G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 2009;16(2):225–237. doi: 10.3758/PBR.16.2.225. (PubMed PMID: 19293088) [DOI] [PubMed] [Google Scholar]
- Russo A., Tessitore A., Esposito F., Marcuccio L., Giordano A., Conforti R., Truini A., Paccone A., d'Onofrio F., Tedeschi G. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J. Neurol. 2012;259(9):1903–1912. doi: 10.1007/s00415-012-6438-1. (PubMed PMID: 22349864) [DOI] [PubMed] [Google Scholar]
- Schienle A., Ubel S., Scharmuller W. Placebo treatment can alter primary visual cortex activity and connectivity. Neuroscience. 2014;263:125–129. doi: 10.1016/j.neuroscience.2014.01.016. (PubMed PMID: 24440751) [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Ichesco E., Hampson J.P., Kairys A., Peltier S., Harte S., Clauw D.J., Harris R.E. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. NeuroImage Clin. 2014;6:252–261. doi: 10.1016/j.nicl.2014.09.007. (PubMed PMID: 25379438; PMCID: PMC4215460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt T.J., Chong C.D., Chiang C.C., Baxter L., Schlaggar B.L., Dodick D.W. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014;34(12):947–958. doi: 10.1177/0333102414526069. (PubMed PMID: 24627432; PMCID: PMC4163130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt T.J., Chiang C.C., Chong C.D., Dodick D.W. Functional MRI of migraine. Lancet Neurol. 2015;14(1):81–91. doi: 10.1016/S1474-4422(14)70193-0. (PubMed PMID: 25496899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinhardt P., Seminowicz D.A., Jaeger E., Duncan G.H., Bushnell M.C. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. J. Neurosci. 2009;29(15):4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. (PubMed PMID: 19369556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D.J., Stohler C.S., Egnatuk C.M., Wang H., Koeppe R.A., Zubieta J.K. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry. 2008;65(2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. (PubMed PMID: 18250260) [DOI] [PubMed] [Google Scholar]
- Starr C.J., Sawaki L., Wittenberg G.F., Burdette J.H., Oshiro Y., Quevedo A.S., McHaffie J.G., Coghill R.C. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134(Pt 7):1987–2004. doi: 10.1093/brain/awr117. (PubMed PMID: 21616963; PMCID: PMC3122370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreault P., Mansour A., Vachon-Presseau E., Schnitzer T.J., Apkarian A.V., Baliki M.N. Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biol. 2016;14(10) doi: 10.1371/journal.pbio.1002570. (PubMed PMID: 27788130; PMCID: PMC5082893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uglem M., Omland P.M., Nilsen K.B., Tronvik E., Stovner L.J., Hagen K., Linde M., Sand T. Does pain sensitivity change by migraine phase? A blinded longitudinal study. Cephalalgia. 2016 doi: 10.1177/0333102416679955. (PubMed PMID: 27919023) [DOI] [PubMed] [Google Scholar]
- van der Kuy P.H., Lohman J.J.A. Quantification of the placebo response in migraine prophylaxis. Cephalalgia. 2002;22(4):265–270. doi: 10.1046/j.1468-2982.2002.00363.x. (PubMed PMID: 12100088) [DOI] [PubMed] [Google Scholar]
- Vase L., Petersen G.L., Riley J.L., III, Price D.D. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain. 2009;145(1–2):36–44. doi: 10.1016/j.pain.2009.04.008. (PubMed PMID: 19559529) [DOI] [PubMed] [Google Scholar]
- Wager T.D., Rilling J.K., Smith E.E., Sokolik A., Casey K.L., Davidson R.J., Kosslyn S.M., Rose R.M., Cohen J.D. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. (PubMed PMID: 14976306) [DOI] [PubMed] [Google Scholar]
- Wager T.D., Scott D.J., Zubieta J.K. Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. U. S. A. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. (PubMed PMID: 17578917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A., El-Deredy W., Iannetti G.D., Lloyd D., Tracey I., Vogt B.A., Nadeau V., Jones A.K. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145(1–2):24–30. doi: 10.1016/j.pain.2009.04.003. (PubMed PMID: 19523766; PMCID: PMC2743811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wey H.Y., Catana C., Hooker J.M., Dougherty D.D., Knudsen G.M., Wang D.J., Chonde D.B., Rosen B.R., Gollub R.L., Kong J. Simultaneous fMRI-PET of the opioidergic pain system in human brain. NeuroImage. 2014;102(Pt 2):275–282. doi: 10.1016/j.neuroimage.2014.07.058. (PubMed PMID: 25107855; PMCID: PMC4348014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. (PubMed PMID: 22642651) [DOI] [PubMed] [Google Scholar]
- Wilcox S.L., Veggeberg R., Lemme J., Hodkinson D.J., Scrivani S., Burstein R., Becerra L., Borsook D. Increased functional activation of limbic brain regions during negative emotional processing in migraine. Front. Hum. Neurosci. 2016;10:366. doi: 10.3389/fnhum.2016.00366. (PubMed PMID: 27507939; PMCID: PMC4960233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoch F., Schindler F., Wester H.J., Empl M., Straube A., Schwaiger M., Conrad B., Tolle T.R. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 2004;108(3):213–220. doi: 10.1016/j.pain.2003.08.014. (Epub 2004/03/20. PubMed PMID: 15030940) [DOI] [PubMed] [Google Scholar]
- Wrobel N., Fadai T., Sprenger C., Hebebrand J., Wiech K., Bingel U. Are children the better placebo analgesia responders? An experimental approach. J. Pain. 2015;16(10):1005–1011. doi: 10.1016/j.jpain.2015.06.013. (PubMed PMID: 26220308) [DOI] [PubMed] [Google Scholar]
- Wrobel N., Fadai T., Brassen S., Bingel U. Preserved capacity for placebo analgesia in the elderly. J. Pain. 2016;17(12):1318–1324. doi: 10.1016/j.jpain.2016.08.012. (PubMed PMID: 27616608) [DOI] [PubMed] [Google Scholar]
- Wu Y., Carson R.E. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J. Cereb. Blood Flow Metab. 2002;22(12):1440–1452. doi: 10.1097/01.WCB.0000033967.83623.34. (PubMed PMID: 12468889) [DOI] [PubMed] [Google Scholar]
- Yang Z., Jackson T., Huang C. Neural activation during anticipation of near pain-threshold stimulation among the pain-fearful. Front. Neurosci. 2016;10:342. doi: 10.3389/fnins.2016.00342. (PubMed PMID: 27489536; PMCID: PMC4951481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Gollub R.L., Vangel M., Kaptchuk T., Smoller J.W., Kong J. Placebo analgesia and reward processing: integrating genetics, personality, and intrinsic brain activity. Hum. Brain Mapp. 2014;35(9):4583–4593. doi: 10.1002/hbm.22496. (PubMed PMID: 24578196; PMCID: PMC4107077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J.K., Stohler C.S. Neurobiological mechanisms of placebo responses. Ann. N. Y. Acad. Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. (PubMed PMID: 19338509; PMCID: 3073412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J.K., Smith Y.R., Bueller J.A., Xu Y., Kilbourn M.R., Jewett D.M., Meyer C.R., Koeppe R.A., Stohler C.S. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J. Neurosci. 2002;22(12):5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. (PubMed PMID: 12077205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J.K., Heitzeg M.M., Smith Y.R., Bueller J.A., Xu K., Xu Y., Koeppe R.A., Stohler C.S., Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. (Epub 2003/02/22. PubMed PMID: 12595695) [DOI] [PubMed] [Google Scholar]
- Zubieta J.K., Bueller J.A., Jackson L.R., Scott D.J., Xu Y., Koeppe R.A., Nichols T.E., Stohler C.S. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. (PubMed PMID: 16120776) [DOI] [PMC free article] [PubMed] [Google Scholar]