Summary
Background
Identifying combinations of the hip and knee joint angles which can selectively recruit specific hamstring muscles may be beneficial for injury prevention or rehabilitation. The purpose of this study was to examine the joint torque and electromyographic (EMG) activity of the semitendinosus (ST) and biceps femoris long head (BFlh).
Methods
Twenty subjects performed maximum isokinetic concentric and eccentric knee flexor efforts at 60°·s−1, 120°·s−1 and 150°·s−1 from three different hip joint angles while surface EMG of ST and BFlh was recorded.
Results
Analysis of variance showed that there are no inter-muscular differences in EMG amplitude across testing conditions (p > .05). Peak EMG occurred near full knee extension for the BFLh and at a higher flexion angle for the ST while exercise from a prone position shifted the peak EMG towards higher knee flexion angle (p < 0.05).
Conclusion
Maximal dynamic knee flexion exercises do not induce a higher EMG amplitude of BFlh or ST. Exercising from a higher hip flexion angle near full knee extension may selectively activate the BFlh.
Level of evidence
IIb.
Keywords: muscle length, EMG-length relationship, hamstring strength, hip, hamstring exercises, knee
Introduction
The hamstring muscle group includes the semimembranosus, the semitendinosus (ST) and the biceps femoris which consists of the long head (BFlh) and the short head. Injury rates are not equal between the individual hamstring muscles and they may vary along the length of each muscle-tendon unit1–3. Most sprint-type hamstring injuries occur during the late swing phase of the sprinting phase, which is characterized by a greater BFlh muscle-tendon stretch than the remaining hamstrings4. This indicates that despite their common bi-articular action around the knee and the hip, there are differences in the function between individual components of the hamstring muscle group.
A few research studies examined the inter-muscular differences in muscle activation patterns during maximum contractions of the knee flexors with conflicting results5, 6. Particularly, some studies reported no differences in maximum EMG amplitude between the ST and BFlh7, 8, whereas others reported the opposite6, 9. Therefore, it is not currently clear whether knee flexion exercises can selectively activate one muscle of the hamstrings more than the others.
Research studies have examined whether intermuscular differences in EMG amplitude are length-dependent6–8. Particularly, BFlh EMG amplitude was reported to increase6 or decrease8 near full extension or to be unaffected by knee flexion angle7. Studies have reported that the ST EMG amplitude is less near full extension6, 7 or that it is unaffected by knee flexion angle8. The implications for these contrasting findings may lead to different recommendations regarding exercise selection for the hamstrings. For example, it has been suggested that the BFlh is a strong knee flexor near knee extension whilst the other muscles are stronger flexors at higher knee flexion angles6. However based on other results, exercise near full knee extension may actually decrease BFlh activation8 or has no particular benefit7.
The effects of hip flexion angle on muscle activation patterns of the hamstrings have received less attention7, 10–12. Particularly, it has been shown that increasing hip flexion angle is associated with a higher peak knee flexion torque7, 10, 11. Lunnen et al.10 reported a decreased hamstring activity as hip flexion angle increased. Worrell et al.13 found that the EMG amplitude of the hamstring muscle during isometric hip extension activity was constant at all hip angles. Similarly, two more recent studies have found inconsistent differences in EMG activation either between different hip angles or between individual hamstring muscles7, 11. In a more recent study, however, rapid knee flexion efforts against a fitball from a prone position have been reported to selectively recruit the BFlh whilst exercises involving combinations of hip and knee motion (such as lunges and single leg Roman dead-lifts) have been reported to recruit the ST14. Nevertheless, the effect of changing hip angle on inter- muscular differences in EMG activity was not clear.
Identifying combinations of the hip and knee joint angles which can selectively recruit specific hamstring muscles (mainly the BFlh) that are at higher injury risk, may be beneficial for injury prevention or rehabilitation. The purpose of this study was to examine the differences in muscle activation of two hamstring muscles during maximum isokinetic tests at different hip joint angles. The main hypothesis was that there will be inter-muscular differences in EMG amplitude at various hip flexion angles.
Methods
Subjects
A total of 20 subjects (10 males and 10 females; age 22.93 ± 3.54 years; mass 72.28 ± 5.37 kg; height 1.74 ± 0.08 m) volunteered to participate in this study after signing written informed consent. The participants were healthy and they had no injury of the lower limbs including history of hamstring strain or any other muscle or ligamentous injury of the knee within the past two years. The study was approved by the University’s Institutional Review Board in accordance with the Ethics standards of the journal15.
Procedures
Maximum strength tests were performed on a Cybex Norm isokinetic dynamometer (Humac Norm, Cybex CSMI, Stoughton, MA, U.S.A.). A Biopac MP100 Acquisition Unit (Biopac Systems Inc., Goleta, California) was used to collect and synchronize the torque, EMG and angular position signals at 1000 Hz. The data were analyzed using AcqKnowledge (Version 3.9.1., Biopac System Inc., Goleta, CA, USA). An ultrasonic apparatus (SSD-3500, ALOKA, Japan) with an electronic linear array probe of 7.5 MHz wave frequency and a length of 6 cm was used to identify specific electrodes placement location. The validity of the protocol is reported elsewhere16.
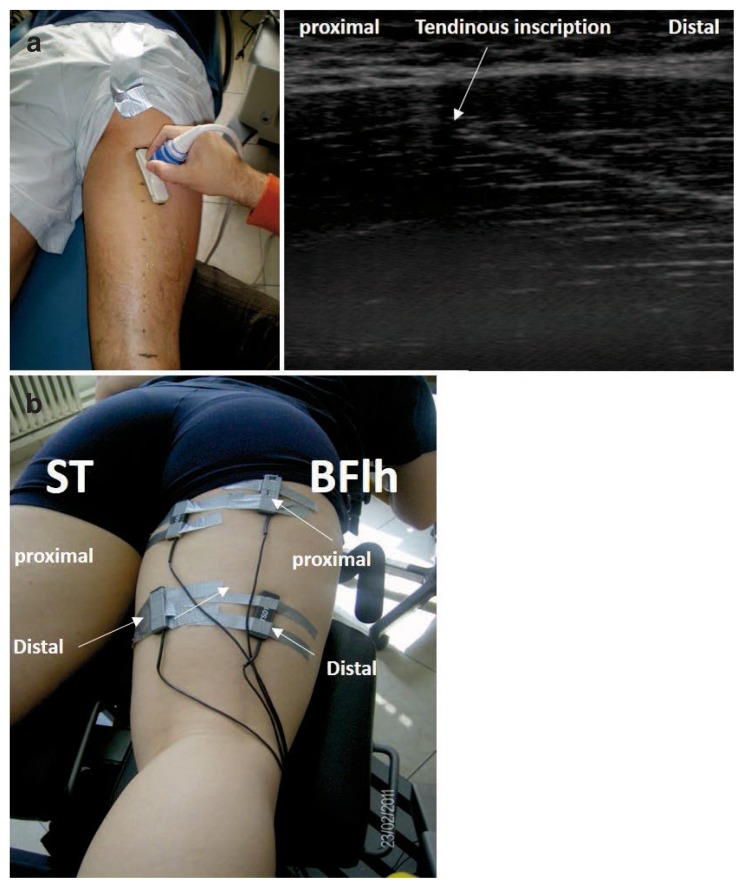
EMG signals were collected using bipolar bar surface electrodes (inter-electrode distance 1 cm, TSD 150B, Biopac System Inc., Goleta, CA, USA). The skin was shaved and cleaned with alcohol wipes. Four pairs of electrodes were placed on the most proximal and distal part of the ST and BFlh, respectively. Particularly, the origins of the ST and BF were identified using ultrasound. Muscle-tendon length was measured as the curved path from the ischial tuberosity (proximal origin) to the fibular head (distal origin of the BFlh) or the medial condyle and fascia cruris (ST) using a flexitape16. The ST is divided in two compartments via a tendinous inscription. Therefore, the apex of the tendinous inscription was identified and subsequently electrodes were positioned proximally and distally to the apex of the tendinous inscription (Fig. 1). These locations corresponded approximately to 65% and 35% of the muscle tendon length. Subsequently, we also placed two pairs of electrodes at 65% and 35% of the whole BLlh muscle length, respectively. The ground electrode was placed over a bony land mark on the lateral epicondyle.
Figure 1 a, b.
a) Example image from the experimental set up for the ST. Ultrasound was used to identify the tendinous inscription of the semitendinosus (ST) and electrode locations were marked on the skin proximally (~65% of the muscle length) and distally (approximately 35% of the muscle-tendon length) to the tendinous inscription (please note that participant is not secured on the dynamometer chair for illustration purposes). b) An example of electrode positions on the ST and biceps femoris long head (BFlh).
The subject lied in a prone position on a customized chair attachment which allowed performance of knee joint motion from different hip joint angles. Maximum strength testing of the knee flexors of the right (preferred) limb was performed when the hip joint was positioned at 0° (neutral position), 45° and 90° of hip flexion. The hip joint angle was checked with a standard analogue goniometer to an accuracy of ± 1°. The thigh, pelvis and trunk were stabilized with straps. The axis of knee rotation was aligned with the lateral femoral condyle. The knee range of motion was set from 0° (full extension) to 90° of knee flexion. Prior to the test, the limb was weighed at 30° using the static gravitational correction procedure recommended by the manufacturer. The main testing protocol included performance of 5 concentric and eccentric knee flexion efforts at angular speeds of 60°·s−1 and 120°·s−1 and 150°·s−1. The sequence of tests was randomized across types of muscle actions, angular speeds and hip flexion angles. The subjects were instructed to exert maximal effort through the whole range of motion during the test. Between the sets, there was a time interval of 1 min to minimize any fatigue effect. Prior to the main testing the participants were given a familiarization session by performing 5 submaximal efforts at 120°·s−1 in each of the above hip testing positions.
The protocol also included three maximal isometric contraction (MVC) efforts of the knee flexors from a hip flexion angle of 0° and knee angle at 45° in order to normalize the raw EMG signals. This angle was selected because it is characterized by a greater torque during isokinetic tests and a similar EMG amplitude of the ST and BFlh6–8. The position of the electrodes did not change during the test and all tests were performed by the same investigator.
The EMG signal was amplified (gain × 1000) with an input impedance of 10 MΩ and a common rejection ratio of 130dB. The signal was filtered using a band pass filter (low 15 Hz and high 450 Hz) to avoid noise from other electrical devices and movement artifacts. Following, full-wave rectification, the root mean square (RMS) was calculated with a sliding window of 10 samples. The resulting RMS signal was further smoothed using a 10-point moving average such that the peak RMS signal value is easier to identify. The reliability of peak torque and surface EMG measurements during isokinetic and isometric contractions using a similar protocol is high as reported elsewhere17. The repetition with the gravity-corrected maximum joint torque was analyzed from the five repetitions. The range of motion was restricted from 10° to 80° to avoid inertial effects. For EMG normalization we used the average RMS recorded during the MVC for a period of 2 seconds where the recorded torque remained relatively consistent. Data from proximal and distal electrode pairs were averaged to yield a single representative RMS value of each muscle, for each testing condition. For each testing condition, the maximum torque, the peak normalized RMS signal (RMSpeak) and the knee flexion angle of peak RMS (RMSangle) throughout the range of motion were used for further analysis.
Statistical analyses
A three-way analysis of variance (ANOVA) was used to examine the differences in peak torque between hip flexion angles (0°, 45°, 90°), angular velocities (60°, 120°, 150°·s−1) and types of muscle action (concentric, eccentric).
For EMG comparisons, a four-way ANOVA was used to examine differences in whole ST and BFlh RMSpeak between hip joint angles, angular velocities and types of muscle action. A similar ANOVA was used to compare RMSangle across testing conditions. Significant F values were followed by Tukey’s post hoc tests to determine the significance between group means. The level of significance was set at p < .05.
Results
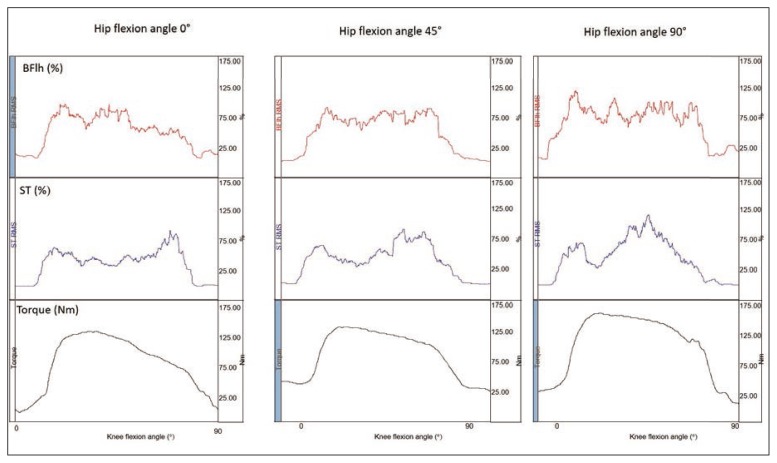
An example of normalized RMS and torque measurements is displayed in Figure 2. The recorded peak torque at different hip angular positions, angular velocities and types of muscle action are presented in Table I. The ANOVA results showed a non-significant three-way interaction effect (F4, 76 = 0.33, p > .05). There was a statistically significant main effect for hip angle (F2, 38 = 20.03, p < .05), action (eccentric greater than concentric, F1, 19 = 52.97, p < .05) and angular velocity (F2, 38 = 26.17, p < .05). Post-hoc Tukey tests indicated that the torque at 0° hip angle was significantly lower than that recorded at 45° and 90° and the torque at 60°·s−1 was significantly lower compared to those recorded at 120°·s−1 and 150°·s−1 (p < .05).
Figure 2.
An example of torque and normalized RMS of the semitendinosus (ST) and biceps femoris long head (Bflh) during a concentric knee flexion trial at 60°.s−1.
Table I.
Peak knee flexion torque (Nm) at different hip joint positions, types of muscle action and angular velocities (*value collapsed for types of muscle action and angular velocity significantly lower compared with values at other hip flexion angles; & value collapsed for hip joint angle and angular velocity significantly different compared to the corresponding eccentric value; ^values collapsed across angles and types of muscle action were significantly lower than value at 60°·s−1).
| Hip flexion angle (°) | ||||
|---|---|---|---|---|
|
| ||||
| 0 | 45 | 90 | Mean (action) | |
| Concentric 60°·s−1 | 109.31 ± 26.27 | 128.09 ± 31.81 | 128.51 ± 37.68 | 112.22 ± 30.72^ |
| Concentric 120°·s−1 | 97.82 ± 24.50 | 114.81 ± 29.63 | 115.70 ± 32.17^ | |
| Concentric 150°·s−1 | 92.38 ± 24.04 | 113.19 ± 36.96 | 110.13 ± 33.34^ | |
| Eccentric 60°·s−1 | 124.59 ± 37.26 | 151.65 ± 40.66 | 149.92 ± 41.11 | 135.97 ± 38.56 |
| Eccentric 120°·s−1 | 119.32 ± 33.03 | 143.99 ± 41.27 | 140.78 ± 37.97^ | |
| Eccentric 150°·s−1 | 116.72 ± 29.10 | 142.82 ± 40.08 | 133.94 ± 33.72^ | |
| Mean value (angle) | 110,02 ± 27.33* | 132.43 ± 36.81 | 129.83 ± 35.11 | - |
Inter-muscular EMG comparison
The recorded normalized RMSpeak values ranged from 74.17 ± 29.96% MVC to 124.28 ± 33.25% MVC for the BFlh and from 74.72 ± 28.70% MVC to 125.49 ± 44.85% MVC for the ST (Tab. II). The ANOVA results showed a non-significant four-way interaction effect (F4, 76 = 2.41, p > .05). No main effect for muscle (F1, 19 = 0.03, p > .05) and hip flexion angle (F2, 38 = 1.17, p > .05) were also found. There were statistically significant effects of muscle action, where the eccentric RMS was lower than the corresponding concentric (F1, 19 = 52.97, p < .05) and angular velocity (F2, 38 = 26.17, p < .05) (normalized RMSpeak increased with increasing angular velocity).
Table II.
Mean group normalized (as percentage of maximum isometric contraction) RMS values of the biceps femoris long head (BFlh) and semitendinosus (ST) at different hip joint positions, types of muscle action and angular velocities.
| Hip flexion angle 0° | Hip flexion angle 45° | Hip flexion angle 90° | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ST | BFlh | ST | BFlh | ST | BFlh | |
| Concentric 60°·s−1 | 122.40 ± 40.74 | 122.99 ± 39.05 | 111.98 ± 40.23 | 106.37 ± 23.25 | 113.02 ± 33.19 | 110.78 ± 29.42 |
| Concentric 120°·s−1 | 119.00 ± 45.37 | 112.78 ± 37.81 | 113.86 ± 41.08 | 115.31 ± 36.84 | 120.75 ± 40.22 | 122.03 ± 31.93 |
| Concentric 150°·s−1 | 125.49 ± 44.85 | 109.04 ± 35.83 | 122.49 ± 32.82 | 121.67 ± 35.53 | 121.88 ± 36.56 | 124.28 ± 33.25 |
| Eccentric 60°·s−1 | 100.51 ± 33.82 | 92.40 ± 29.15 | 91.48 ± 25.59 | 89.66 ± 29.76 | 88.38 ± 27.63 | 89.75 ± 27.67 |
| Eccentric 120°·s−1 | 90.76 ± 28.21 | 88.20 ± 29.43 | 76.41 ± 24.51 | 82.96 ± 35.56 | 79.98 ± 20.79 | 90.46 ± 22.28 |
| Eccentric 150°·s−1 | 84.73 ± 24.63 | 87.73 ± 38.03 | 74.72 ± 28.70 | 74.17 ± 29.96 | 76.94 ± 29.69 | 79.38 ± 22.33 |
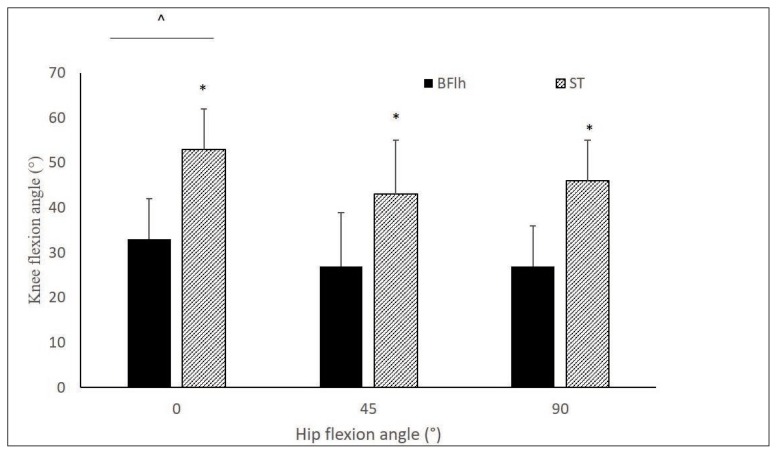
The ANOVA results showed a non-significant fourway interaction effect (F4, 76 = 0.74, p > .05) on RMSangle values. No main effect for speed and type of muscle action were found (p > 0.05). However, there was a statistically significant main effect for hip angle (F2, 38 = 24.12, p > .05) and muscle (F1, 19 = 57.29, p < .05). Therefore, data collapsed for muscle action and angular speed, are presented in Figure 3. Posthoc Tukey tests showed that the RMSangle at hip flexion angle of 0° was significantly higher than that obtained from hip angles of 45° and 90° (p < .05). Furthermore, the RMSangle was lower for the BFLh than the ST.
Figure 3.
Mean group knee flexion angle (°) of peak RMS of the biceps femoris long head (BFlh) and semitendinosus (ST) at different hip joint positions. Data are averaged across types of muscle action and angular speeds (*significantly different compared with BFlh; ^ significantly different compared to hip flexion angle of 45° and 90°).
Discussion
The main findings of this study were that there were no inter-muscular differences in peak surface EMG amplitude between the ST and BFlh muscles during maximum isokinetic knee flexion tests from various hip flexion angles. However, the peak RMS occurred at a lower knee flexion angle for the BFlh than the ST whereas the peak RMS of both muscles occurred at a more flexed knee joint angle when the test was performed from the prone position compared with the other hip testing positions.
In contrast to our research hypothesis, our findings showed no inter-muscular difference in EMG amplitude during isokinetic maximum knee flexion efforts performed from various hip flexion angles (Tab. II). This is in agreement with some studies7, 8, 11 but in contrast to others6, 9. Comparisons of our data with those reported by previous studies should take into consideration some methodological differences between studies. For example, in this present study as well as other studies7, 11 we measured the maximum EMG amplitude during knee flexion during tests performed from different hip joint positions. This differs significantly to other designs which examined the changes in EMG throughout knee joint range of motion (irrespective of hip flexion angle)6, 9, those who examined the EMG during hip joint motion (with a fixed knee joint angle)10, 12, 13 and those who assessed the overall EMG during a combined hip and knee joint exercise14. Second, different studies used a different method to normalize the EMG. Particularly, the EMG signal was not normalized11, it was normalized as a percentage of the EMG recorded during a particular range of the motion (75–90° of knee flexion) 6 or a series of dynamic isokinetic tests8, a MVC (present study and9) or during a manual knee flexion test7. Further, the present as well as other studies assessed the EMG using a 90° range of motion7 while others used a higher range of motion6, 8, 10. This might have an effect on EMG amplitude of the hamstrings, especially that of the ST6–8. Finally, some studies reported EMG patterns8, 12, 14 during exercises performed against body weight or external resistance of submaximal loads which differ significantly compared to the maximum isokinetic tests applied in the present study.
Since the increase in hip joint angle is accompanied by an increase in exerted knee flexion torque (Tab. I), one may suggest that this increase is not due to a higher EMG amplitude of a particular hamstring muscle. The increase in hip flexion angle results in a more lengthened muscle-tendon unit and, hence, a higher torque11. Previous researchers commented that the higher force generation capacity of the hamstrings as hip flexion angle increases is mainly due to a higher contribution of the passive (elastic) elements of the muscle and a higher moment-arm around the hip18, 19 rather than an increase in the neuromuscular activation7, 11. Nevertheless, hip flexion angle had an effect on the RMSangle, as the peak RMS occurred at a lower knee flexion angle when the test was performed from the 45° and 90° position compared with the prone position (Fig. 3). In a recent study, Guex et al.11 suggested that exercising from a higher hip flexion angle using a hamstrings strengthening device functioning with a higher hip flexion angles might have additional potential benefits as it is accompanied by a higher torque demands, especially under eccentric conditions. In addition, hamstring injury is observed during the late swing phase, where the hip is flexed by 70° and the knee is flexed by 20–40°4. Consequently, our findings provide further evidence that performing knee flexion exercises near full extension from a higher hip flexion angle may provide a more optimal strengthening of the hamstrings for injury prevention purposes than exercising from a prone position.
The results showed that the RMS occurred earlier in the knee joint range of motion for the BFlh compared to ST (Fig. 3). The increase in BFlh EMG amplitude near knee extension is in agreement with some studies6 but it is contrast to others7, 8. Similarly, the decline of peak ST EMG amplitude near full extension agrees with some studies5, 6 but not with others7. Our results appear to confirm previous suggestions that exercising near full knee extension appears to promote BFlh activation5 while ST peak activation is achieved by performing knee flexion exercises at mid-range to higher knee flexion angles. The reasons for this selective activation at different parts of the range of motion are not clear. Prior to any physiological explanation, it should be mentioned that a common limitation of studies using surface EMG is that the recorded signal may be affected by changes in electrode location relative to the muscle fibers19. Nevertheless, the lower activation of ST near full extension has been attributed to the lower moment-arm of this muscle6,20. However, there is evidence that when the moment-arm decreases, the EMG activity increases21 and therefore, this suggestion remains unverified. In addition, there is some evidence that the BFlh muscle fibers display a higher passive elongation as the knee approaches full extension than the ST muscle fibers22. In theory, this would signify a higher change in contractile muscle fascicle length for the BFlh than the ST when the hamstrings contract from a higher muscle-tendon length. In this case, one may suggest that the increase in BFlh EMG activity is an attempt of the neuromuscular system to contract the stretched muscle fascicles. Evidence of EMG changes with muscle length varies amongst studies and is largely muscle-dependent5, 23, 24 while motor unit activation at different muscle lengths of the hamstring muscles has not been systematically investigated. Consequently, further research is necessary to identify factors that contribute to differences in neuromuscular activation patterns between the hamstring muscle group components.
This study has some limitations. First, surface EMG measurements can be affected by cross-talk from surrounding muscles thus having an effect on intermuscular comparisons. In this study, we used an ultrasonographic imaging device to locate the specific electrode area under the examination. Electrode cables and electrodes were secured to the skin in order to reduce potential error artifacts. Finally, the small inter-electrode distance (1 cm) selected in this study may significantly reduce cross-talk25. In addition, a common issue for investigations utilizing surface electrodes to analyse dynamic joint movements is that the electrode movement relative to the tendinous structures and motor points may result in variations in the recorded surface EMG signal26. It should, however, be mentioned that the ST muscle has two distinct motor points, while both BFlh and ST have long aponeuroses and tendons which may add more complexity in the analysis of the surface EMG signal15.
Conclusions
Increasing hip angle was accompanied by a higher peak knee flexion torque, no change in the amplitude of surface EMG and a shift of peak EMG towards knee extension. Peak BFlh EMG activity occurred at lower knee flexion angles while peak ST EMG activity was observed at a higher knee flexion angle. Within the limitation of the surface EMG technique, maximal dynamic knee flexion exercises performed from a higher hip flexion angle may enhance selective activation of the BFlh near full knee extension.
References
- 1.Comin J, Malliaras P, Baquie P, Barbour T, Connell D. Return to competitive play after hamstring injuries involving disruption of the central tendon. Am J Sports Med. 2013;41(1):111–115. doi: 10.1177/0363546512463679. [DOI] [PubMed] [Google Scholar]
- 2.Dolman B, Verrall G, Reid I. Physical principles demonstrate that the biceps femoris muscle relative to the other hamstring muscles exerts the most force: implications for hamstring muscle strain injuries. Muscles Ligaments Tendons J. 2014;4(3):371–377. [PMC free article] [PubMed] [Google Scholar]
- 3.Malliaropoulos NG. Non contact Hamstring injuries in sports. Muscles Ligaments Tendons J. 2012;2(4):309–311. [PMC free article] [PubMed] [Google Scholar]
- 4.Thelen DG, Chumanov ES, Sherry MA, Heiderscheit BC. Neuromusculoskeletal models provide insights into the mechanisms and rehabilitation of hamstring strains. Exerc Sport Sci Rev. 2006;34(3):135–141. doi: 10.1249/00003677-200607000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Kellis E, Katis A. Hamstring antagonist moment estimation using clinically applicable models: Muscle dependency and synergy effects. J Electromyogr Kinesiol. 2008;18(1):144–153. doi: 10.1016/j.jelekin.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Onishi H, Yagi R, Oyama M, Akasaka K, Ihashi K, Handa Y. EMG-angle relationship of the hamstring muscles during maximum knee flexion. J Electromyogr Kinesiol. 2002;12(5):399–406. doi: 10.1016/s1050-6411(02)00033-0. [DOI] [PubMed] [Google Scholar]
- 7.Mohamed O, Perry J, Hislop H. Relationship between wire EMG activity, muscle length, and torque of the hamstrings. Clin Biomech. 2002;17:569–579. doi: 10.1016/s0268-0033(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 8.Higashihara A, Ono T, Kubota J, Fukubayashi T. Differences in the electromyographic activity of the hamstring muscles during maximal eccentric knee flexion. Eur J Appl Physiol. 2010;108(2):355–362. doi: 10.1007/s00421-009-1242-z. [DOI] [PubMed] [Google Scholar]
- 9.Ono T, Okuwaki T, Fukubayashi T. Differences in activation patterns of knee flexor muscles during concentric and eccentric exercises. Res Sport Med. 2010;18(February 2014):188–198. doi: 10.1080/15438627.2010.490185. [DOI] [PubMed] [Google Scholar]
- 10.Lunnen JD, Yack J, Le Veau BF. Relationship between muscle length, muscle activity, and torque of the hamstring muscles. Phys Ther. 1981;61:190–195. doi: 10.1093/ptj/61.2.190. [DOI] [PubMed] [Google Scholar]
- 11.Guex K, Gojanovic B, Millet GP. Influence of hip-flexion angle on hamstrings isokinetic activity in sprinters. J Athl Train. 2012;47(4):390–395. doi: 10.4085/1062-6050-47.4.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono T, Higashihara A, Fukubayashi T. Hamstring functions during hip-extension exercise assessed with electromyography and magnetic resonance imaging. Res Sport Med. 2011;19(1):42–52. doi: 10.1080/15438627.2011.535769. [DOI] [PubMed] [Google Scholar]
- 13.Worrell TW, Karst G, Adamczyk D, Moore R, Stanley C, Steimel B, et al. Influence of joint position on electromyographic and torque generation during maximal voluntary isometric contractions of the hamstrings and gluteus maximus muscles. J Orthop Sport Phys Ther. 2001;31(12):730–740. doi: 10.2519/jospt.2001.31.12.730. [DOI] [PubMed] [Google Scholar]
- 14.Tsaklis P, Malliaropoulos N, Mendiguchia J, Korakakis V, Tsapralis K, Pyne D, et al. Muscle and intensity based hamstring exercise classification in elite female track and field athletes: implications for exercise selection during rehabilitation. Open Access J Sport Med. 2015;6:209–217. doi: 10.2147/OAJSM.S79189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal - Basic principles and recommendations in clinical and field science research: 2016 update. MLTJ. 2016;6(1):1–5. doi: 10.11138/mltj/2016.6.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellis E, Galanis N, Natsis K, Kapetanos G. Validity of architectural properties of the hamstring muscles: Correlation of ultrasound findings with cadaveric dissection. J Biomech. 2009;42(15):2549–2554. doi: 10.1016/j.jbiomech.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Kellis E, Kouvelioti V, Ioakimidis P. Reliability of a practicable EMG-moment model for antagonist moment prediction. Neurosci Lett. 2005;383(3):266–271. doi: 10.1016/j.neulet.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Arnold AS, Salinas S, Asakawa DJ, Delp SL. Accuracy of muscle moment arms estimated from MRI-based musculoskeletal models of the lower extremity. Comput Aided Surg. 2000;5(2):108–119. doi: 10.1002/1097-0150(2000)5:2<108::AID-IGS5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.O’Neill MC, Lee LF, Larson SG, Demes B, Stern JT, Umberger BR. A three-dimensional musculoskeletal model of the chimpanzee (Pan troglodytes) pelvis and hind limb. J Exp Biol. 2013;216(Pt 19):3709–3723. doi: 10.1242/jeb.079665. [DOI] [PubMed] [Google Scholar]
- 20.Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol. 2005;98(1):120–131. doi: 10.1152/japplphysiol.00894.2004. [DOI] [PubMed] [Google Scholar]
- 21.Herzog W, Read LJ. Lines of action and moment arms of the major force-carrying structures crossing the human knee joint. J Anat. 1993;182(2):213–230. [PMC free article] [PubMed] [Google Scholar]
- 22.Nourbakhsh MR, Kukulka CG. Relationship between muscle length and moment arm on EMG activity of human triceps surae muscle. J Electromyogr Kinesiol. 2004;14(2):263–273. doi: 10.1016/S1050-6411(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 23.Kellis E. Biceps femoris and semitendinosus tendon/aponeurosis strain during passive and active (isometric) conditions. J Electromyogr Kinesiol. 2016;26 doi: 10.1016/j.jelekin.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Pasquet B, Carpentier A, Duchateau J, Pasquet B. Change in muscle fascicle length influences the recruitment and discharge rate of motor units during isometric contractions. J Neurophysiol. 2005;94(5):3126–3133. doi: 10.1152/jn.00537.2005. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy PM, Cresswell AG. The effect of muscle length on motor-unit recruitment during isometric plantar flexion in humans. Exp Brain Res. 2001;137(1):58–64. doi: 10.1007/s002210000623. [DOI] [PubMed] [Google Scholar]
- 26.Winter DA, Fuglevand AJ, Archer SE. Crosstalk in surface electromyography: Theoretical and practical estimates. J Electromyogr Kinesiol. 1994;4(1):15–26. doi: 10.1016/1050-6411(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 27.Zuniga EN, Truong XT, Simons DG. Effects of skin electrode position on averaged electromyographic potentials. Arch Phys Med Rehabil. 1970;51(5):264–272. [PubMed] [Google Scholar]