Abstract
Introduction
Type 2 diabetes mellitus (T2DM) is a risk factor for Alzheimer's disease. Cerebrospinal fluid (CSF) amyloid β (Aβ) 1-42 is an important Alzheimer's disease biomarker. However, it is inconclusive on how T2DM is related to CSF Aβ1-42.
Methods
Participants with T2DM were selected from the Alzheimer's Disease Neuroimaging Initiative by searching keywords from the medical history database. A two-way analysis of covariance model was used to analyze how T2DM associates with CSF Aβ1-42 or cerebral cortical Aβ.
Results
CSF Aβ1-42 was higher in the T2DM group than the nondiabetic group. The inverse relation between CSF Aβ1-42 and cerebral cortical Aβ was independent of T2DM status. Participants with T2DM had a lower cerebral cortical Aβ in anterior cingulate, precuneus, and temporal lobe than controls.
Discussion
T2DM is positively associated with CSF Aβ1-42 but negatively with cerebral cortical Aβ. The decreased cerebral cortical Aβ associated with T2DM is preferentially located in certain brain regions.
Keywords: Amyloid β, Aβ1-42, Alzheimer's disease, Cerebrospinal fluid (CSF), Type 2 diabetes mellitus
1. Introduction
In 1999, the Rotterdam Study found that type 2 diabetes mellitus (T2DM) could double the risk of Alzheimer's disease (AD) [1]. In 2011, another study reported that AD risk increased 60% in patients with T2DM over the nondiabetics [2]. Further, a high prevalence of T2DM in patients with AD is congruent with T2DM as an AD risk factor [3]. Cerebrospinal fluid (CSF) amyloid β (Aβ) 1-42 has been shown as a sensitive biomarker for diagnosing AD [4] and a strong predictor for people with subjective cognitive complaints to progress to AD [5]. However, a recent study did not find the association between T2DM and CSF Aβ1-42 [6]. Using data from participants enrolled in Alzheimer's Disease Neuroimaging Initiative (ADNI), we investigated the relationship between CSF Aβ1-42 with T2DM and baseline cognition diagnosis. Aβ load in cerebral cortex as well as its subregions was also compared between participants with and without T2DM. Our findings provide important insight into CSF Aβ1-42 as an AD biomarker and its relation to the cerebral cortical Aβ, especially for patients with T2DM.
2. Methods
2.1. ADNI
Demographic and imaging data were downloaded from the ADNI database (adni.loni.usc.edu). As an ongoing project, ADNI was launched in 2003 and has been sponsored by the following agencies: National Institute on Aging, National Institute of Biomedical Imaging and Bioengineering, Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations. The primary goal of the ADNI has been to test whether serial magnetic resonance imaging, positron emission tomography (PET), biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD. In three phases (1, GO, and 2) and from over 50 sites across the United States and Canada, the ADNI has recruited more than 1800 adult participants. The participants are older adults (aged 55–90 years) with normal cognition, MCI, or mild AD. Further information can be found at http://www.adni-info.org/ and in previous reports [7], [8], [9], [10], [11], [12].
2.2. Selection of T2DM participants
The following search terms were used in the medical history database to screen the ADNI participants: diabetes, diabetic, and insulin. Based on the medical history information (age at onset of diabetes, clinical diagnosis, and/or use of diabetic medications), 159 participants from the ADNI were found to have T2DM at the screening visit (Table 1). In these diabetic participants, 76.73% (122/159) were being treated with antidiabetic medications. These diabetic participants had an average fasting glucose level between 110 and 120 mg/dL at the baseline and at 12-, 24-, and 36-month follow-up visits.
Table 1.
Participant demographics and clinical information
| Participant features | T2DM | Nondiabetics |
|---|---|---|
| N | 77 | 735 |
| Age (<75:75–80:>80) (Mean ± SD) |
53 (68.83%):18 (23.38%):6 (7.80%) (70.48 ± 6.71) |
454 (61.77%):179 (24.35%):102 (13.88%) (72.17 ± 7.37) |
| Gender (M: F) | 46:31 | 384:351 |
| APOE ɛ4 carrier status (+/−) | 39:37 | 327:404 |
| APOE genotype (ɛ2/ɛ2: ɛ2/ɛ3: ɛ3/ɛ3: ɛ2/ɛ4: ɛ3/ɛ4: ɛ4/ɛ4) | 0 (0%):4 (5.19%):33 (42.86%):1 (1.30%): 32 (41.56%):6 (7.80%) | 1 (0.14%):59(8.03%):344 (46.80%):9 (1.22%): 245 (33.33%):73 (9.93%) |
| Education | 15.74 ± 2.44 | 16.31 ± 2.62 |
| HC: MCI: AD | 10:54:2 | 151:493:7 |
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein epsilon; HC, healthy control; MCI, mild cognitive impairment; T2DM, type 2 diabetes mellitus; SD, standard deviation.
2.3. CSF Aβ1-42 measures
CSF samples were collected by following standard procedures stated in the ADNI protocols. AD biomarkers including Aβ1-42 were measured at the ADNI Biomarkers Core located at the University of Pennsylvania. In brief, all CSF samples were collected from the participants after at least a 6-hour fasting period. The CSF samples were analyzed by following storing, shipping, and testing procedures and with parallel strict quality control steps. To date, eight batches of data on CSF biomarkers have been released from the Biomarkers Core. Only baseline CSF Aβ1-42 and corresponding cerebral cortical Aβ PET measures were being analyzed in the present study.
2.4. 18F florbetapir AV45 PET imaging and analysis
Preprocessed florbetapir imaging data were downloaded from the LONI ADNI site (http://adni.loni.usc.edu). Data preprocessing information is available online (adni.loni.ucla.edu/about-data-samples/image-data/). Briefly, image data were acquired in four 5-min frames 50–70 minutes after injection of approximately 10 mCi of 18F florbetapir, the four frames were co-registered to one another, averaged, interpolated to a uniform image and voxel size (160 × 106 × 96, 1.5 mm3), and smoothed to a uniform resolution (8 mm FWHM) to account for differences between scanners [13].
For quantifying cerebral cortical Aβ, preprocessed florbetapir image data and co-registered structural magnetic resonance images were analyzed using Freesurfer software, version 4.5.0 (surfer.nmr.mgh.harvard.edu/) as described before [14] and online (adni.loni.ucla.edu/research/pet-post-processing/). The mean Aβ retention, measured by the florbetapir AV45 standardized uptake value ratio, was normalized to the whole cerebellum as a summary measure of florbetapir retention for each participant.
2.5. Statistical analysis, tables, and figures
SPSS software (version 24.0) was used for all statistical analyses. First, a two-way analysis of covariance model was utilized to evaluate effects of T2DM and baseline diagnostic group (healthy control [HC], MCI, and AD) on CSF Aβ1-42. Age, gender, and apolipoprotein epsilon (APOE) ε4 carrier status (+/−) were controlled as possible confounding factors (Table 1). Data were shown in the form of mean ± standard deviation, and P < .05 was considered as significant in all statistical analyses. Figures were created using Microsoft Excel or SigmaPlot (version 10.0).
2.6. Standard protocol approvals, registrations, and patient consents
Written informed consent was obtained from all participants (or guardians of participants) participating in the study according to the Declaration of Helsinki (consent for research).
3. Results
CSF Aβ1-42 was affected by both T2DM (P = .001) and baseline diagnosis (P = .001). CSF Aβ1-42 in the T2DM group was 204.35 ± 9.40 pg/mL (95% CI: 185.89–222.80 pg/mL, n = 76), which is higher than its level in the nondiabetic group of 168.59 ± 4.49 pg/mL (95% CI: 159.76–177.40 pg/mL, n = 731, P = .001) (Fig. 1). It is worthy to note that T2DM did not interact with baseline diagnosis significantly for its effects on CSF Aβ1-42 (P = .053).
Fig. 1.
Participants with T2DM have a higher CSF Aβ1-42 than those without T2DM. Abbreviations: Aβ42, Aβ 1-42; CSF, cerebrospinal fluid; T2DM, type 2 diabetes mellitus.
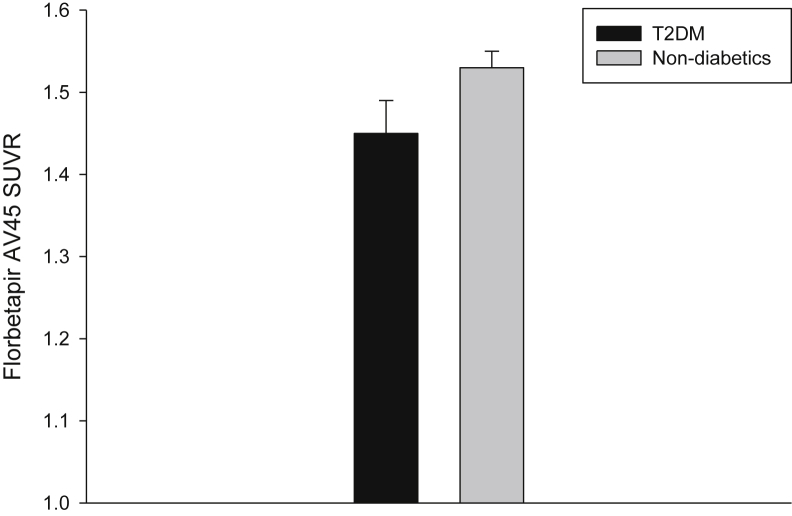
Then cerebral cortical Aβ was analyzed to examine its correlation to the CSF Aβ1-42 for participants with and without T2DM, respectively. For participants with T2DM, the correlation coefficient was −0.669 (n = 77, P < .005). By contrast, the correlation coefficient was −0.705 (n = 735, P < .005) for participants without T2DM. For participants with T2DM, cerebral cortical Aβ (indicated by the florbetapir AV45 standardized uptake value ratio) was 1.45 ± 0.04 (95% CI: 1.38–1.52, n = 74), which is significantly lower than the counterpart measure in participants without T2DM of 1.53 ± 0.02 (95% CI: 1.50–1.57, n = 724, P = .045) (Fig. 2).
Fig. 2.
Cortical Aβ, measured by the florbetapir AV45 SUVR, was compared between participants with and without T2DM. Abbreviations: SUVR, standardized uptake value ratio; T2DM, type 2 diabetes mellitus.
Subsequently, cerebral cortical Aβ load was compared in different brain regions (anterior cingulate, frontal lobe, limbic lobe, occipital lobe, parietal lobe, posterior cingulate, precuneus, and temporal lobe) to examine if the differences between participants with and without T2DM were preferentially distributed in some regions than others (Table 2). A lower load of Aβ was seen in participants with T2DM than in those without T2DM from all examined brain regions (Table 2). However, significant differences were only observed in brain regions of anterior cingulate, precuneus, and temporal lobe between participants with and without T2DM.
Table 2.
The mean Aβ, measured by the florbetapir AV45 standardized uptake value ratio, was compared between participants with and without T2DM in different brain regions
| Brain region | T2DM (n = 74) | Nondiabetics (n = 724) | P value |
|---|---|---|---|
| Anterior cingulate | 1.64 ± 0.05 (95% CI: 1.54–1.74) | 1.75 ± 0.02 (95% CI: 1.70–1.80) | .043 |
| Frontal lobe | 1.47 ± 0.04 (95% CI: 1.39–1.54) | 1.55 ± 0.02 (95% CI: 1.51–1.59) | .054 |
| Limbic lobe | 1.57 ± 0.04 (95% CI: 1.50–1.65) | 1.64 ± 0.02 (95%CI: 1.61–1.68) | .104 |
| Occipital lobe | 1.35 ± 0.03 (95% CI: 1.28–1.41) | 1.42 ± 0.02 (95% CI: 1.39–1.45) | .057 |
| Parietal lobe | 1.43 ± 0.04 (95% CI: 1.34–1.51) | 1.52 ± 0.02 (95% CI: 1.48–1.56) | .062 |
| Posterior cingulate | 1.50 ± 0.05 (95% CI: 1.41–1.59) | 1.59 ± 0.02 (95% CI: 1.55–1.63) | .085 |
| Precuneus | 1.42 ± 0.05 (95% CI: 1.33–1.52) | 1.54 ± 0.02 (95% CI: 1.50–1.59) | .021 |
| Temporal lobe | 1.43 ± 0.04 (95% CI: 1.35–1.51) | 1.52 ± 0.02 (95% CI: 1.48–1.56) | .04 |
Abbreviations: Aβ, amyloid β; T2DM, type 2 diabetes mellitus.
4. Discussion
Amyloid plaque is one of the classical pathological biomarkers for AD. Aβ, the major protein component of amyloid plaque, is generated from a sequential cleavage of amyloid precursor protein by β- and γ-secretase [15]. The amyloid deposited in neuritic plaques exists predominantly as the Aβ1-42 form, which is less soluble and more likely to aggregate than the Aβ1-40 form. More importantly, CSF Aβ1-42 has been shown as a useful pathological biomarker for AD [16], [17].
CSF Aβ1-42 is higher in participants with T2DM than those without T2DM. However, the increased concentration of CSF Aβ1-42 is not due to CSF volume changes, as the CSF volume is comparable between participants with T2DM and nondiabetic controls (Supplementary Fig. 1). It is noteworthy that CSF Aβ1-42 has also been reported to be higher in type 1 diabetes mellitus [18]. Therefore, increased CSF Aβ1-42 level is more likely associated with diabetes-related pathological changes (e.g. hyperglycemia) common in both types of diabetes.
It is known that microvascular lesions associated with T2DM can contribute to an increased blood–brain barrier permeability [19], [20], which can change the distribution of biomarkers including Aβ1-42 as well as the cognitive functions. At the same time, subjects with cognitive impairments caused by the vascular defects might be misdiagnosed as early stage AD [19]. For the ADNI participants with T2DM, the prevalence of AD is low, but they still have a higher AD risk than the same measure from the nondiabetic participants.
Mean CSF Aβ1-42 level was previously shown to be significantly lower in the mild AD group or amnestic MCI group than the control group [21]. Not surprisingly, a higher CSF Aβ1-42 was observed in the HC group than the MCI group and the AD group in the present study (Supplementary Fig. 2). However, CSF Aβ1-42 was comparable between the MCI and AD groups, and the data suggest that measuring CSF Aβ1-42 is more useful for detecting a cognitive deterioration from HC to MCI than differentiating MCI from AD.
Although a lower CSF Aβ1-42 was seen in participants with either MCI or AD than in the HC group, comorbid T2DM is related to a reversion of CSF Aβ1-42 in those with cognitive impairments (MCI or AD). However, a similar relation between T2DM and cerebral cortical Aβ was not observed. More interestingly, CSF Aβ1-42 is inversely correlated with cerebral cortical Aβ, and the relationship is independent of T2DM status.
Our results showed that T2DM is associated with a unique pattern of changes in CSF Aβ1-42 as well as a preferential distribution of decreased cerebral cortical Aβ in certain brain regions. Although the exact mechanism is not clear, immune function might play an important role in leading to these changes as the Aβ autoantibody increases for more than 45% in patients with T2DM in comparison with controls [22].
The present study is limited by its cross-sectional design. A longitudinal study design may be more powerful for evaluating CSF Aβ1-42 as a biomarker of early cognitive impairment in people with or without T2DM. In addition, data on CSF Aβ1-42 and type 1 diabetes mellitus will also be useful for defining CSF Aβ1-42 as an AD biomarker.
In conclusion, CSF Aβ1-42 is positively associated with a T2DM status. The pattern of CSF Aβ1-42 and cerebral cortical Aβ changes in people with T2DM suggests that T2DM may increase AD risk through a disease-associated pathological mechanism. However, the underlying pathological mechanism for CSF Aβ1-42 and cerebral cortical Aβ warrants investigation in subjects with and without T2DM in the future.
Research in Context.
-
1.
Systematic review: Although type 2 diabetes mellitus (T2DM) is a risk factor for Alzheimer's disease, it is controversial on how T2DM relates to Alzheimer's disease biomarker cerebrospinal fluid (CSF) amyloid β (Aβ) 1-42. The authors reviewed available scarce evidence on how T2DM is associated with CSF Aβ1-42 using PubMed as the main literature source. The relevant citations are appropriately cited.
-
2.
Interpretation: T2DM is positively associated with the CSF Aβ1-42 but negatively associated with the cortical Aβ. The decreased cortical Aβ associated with T2DM is preferentially located in anterior cingulate, precuneus, and temporal lobe.
-
3.
Future directions: The underlying pathological mechanism for CSF Aβ1-42 and cortical Aβ changes is to be investigated in patients with and without T2DM in the future.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904), DOD ADNI (Department of Defense award number W81XWH-12-2-0012), and IADC grant (P30 AG10133). This publication was made possible, in part, with support from the Indiana Clinical and Translational Sciences Institute funded, in part by grant number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical, and Translational Sciences Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2017.11.002.
Supplementary data
References
- 1.Ott A., Stolk R.P., van Harskamp F., Pols H.A., Hofman A., Breteler M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 2.Cheng D., Noble J., Tang M.X., Schupf N., Mayeux R., Luchsinger J.A. Type 2 diabetes and late-onset Alzheimer's disease. Dement Geriatr Cogn Disord. 2011;31:424–430. doi: 10.1159/000324134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janson J., Laedtke T., Parisi J.E., O'Brien P., Petersen R.C., Butler P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen N., Hesse C., Davidsson P., Minthon L., Wallin A., Winblad B. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999;56:673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 5.van Harten A.C., Visser P.J., Pijnenburg Y.A., Teunissen C.E., Blankenstein M.A., Scheltens P. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–487. doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Moran C., Beare R., Phan T.G., Bruce D.G., Callisaya M.L., Srikanth V. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology. 2015;85:1123–1130. doi: 10.1212/WNL.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack C.R., Jr., Bernstein M.A., Borowski B.J., Gunter J.L., Fox N.C., Thompson P.M. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2010;6:212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagust W.J., Bandy D., Chen K., Foster N.L., Landau S.M., Mathis C.A. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6:221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen R.C., Aisen P.S., Beckett L.A., Donohue M.C., Gamst A.C., Harvey D.J. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saykin A.J., Shen L., Foroud T.M., Potkin S.G., Swaminathan S., Kim S. Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement. 2010;6:265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trojanowski J.Q., Vandeerstichele H., Korecka M., Clark C.M., Aisen P.S., Petersen R.C. Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6:230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner M.W., Aisen P.S., Jack C.R., Jr., Jagust W.J., Trojanowski J.Q., Shaw L. The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6:202-211.e7. doi: 10.1016/j.jalz.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi A., Koeppe R.A., Fessler J.A. Reducing between scanner differences in multi-center PET studies. Neuroimage. 2009;46:154–159. doi: 10.1016/j.neuroimage.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jovanovic K., Loos B., Da Costa Dias B., Penny C., Weiss S.F. High resolution imaging study of interactions between the 37 kDa/67 kDa laminin receptor and APP, beta-secretase and gamma-secretase in Alzheimer's disease. PLoS One. 2014;9:e100373. doi: 10.1371/journal.pone.0100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faull M., Ching S.Y., Jarmolowicz A.I., Beilby J., Panegyres P.K. Comparison of two methods for the analysis of CSF Aβ and tau in the diagnosis of Alzheimer's disease. Am J Neurodegener Dis. 2014;3:143–151. [PMC free article] [PubMed] [Google Scholar]
- 17.Frisoni G.B., Prestia A., Zanetti O., Galluzzi S., Romano M., Cotelli M. Markers of Alzheimer's disease in a population attending a memory clinic. Alzheimers Dement. 2009;5:307–317. doi: 10.1016/j.jalz.2009.04.1235. [DOI] [PubMed] [Google Scholar]
- 18.Ouwens D.M., van Duinkerken E., Schoonenboom S.N., Herzfeld de Wiza D., Klein M., van Golen L. Cerebrospinal fluid levels of Alzheimer's disease biomarkers in middle-aged patients with type 1 diabetes. Diabetologia. 2014;57:2208–2214. doi: 10.1007/s00125-014-3333-6. [DOI] [PubMed] [Google Scholar]
- 19.Serlin Y., Levy J., Shalev H. Vascular pathology and blood-brain barrier disruption in cognitive and psychiatric complications of type 2 diabetes mellitus. Cardiovasc Psychiatry Neurol. 2011;2011:609202. doi: 10.1155/2011/609202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z., Zeng W., Sun J., Chen W., Zhang R., Yang Z. The quantification of blood-brain barrier disruption using dynamic contrast-enhanced magnetic resonance imaging in aging rhesus monkeys with spontaneous type 2 diabetes mellitus. Neuroimage. 2017;158:480–487. doi: 10.1016/j.neuroimage.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Kuo H.C., Yen H.C., Huang C.C., Hsu W.C., Wei H.J., Lin C.L. Cerebrospinal fluid biomarkers for neuropsychological symptoms in early stage of late-onset Alzheimer's disease. Int J Neurosci. 2015;125:747–754. doi: 10.3109/00207454.2014.971787. [DOI] [PubMed] [Google Scholar]
- 22.Kim I., Lee J., Hong H.J., Jung E.S., Ku Y.H., Jeong I.K. A relationship between Alzheimer's disease and type 2 diabetes mellitus through the measurement of serum amyloid-beta autoantibodies. J Alzheimers Dis. 2010;19:1371–1376. doi: 10.3233/JAD-2010-1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.