Abstract
Optimal functioning in everyday life requires the ability to override reflexive emotional responses and prevent affective spillover to situations or people unrelated to the source of emotion. In the current study, we investigated whether the lateral prefrontal cortex (lPFC) causally regulates the influence of emotional information on subsequent judgments. We disrupted left lPFC function using transcranial magnetic stimulation (TMS) and recorded electroencephalography (EEG) before and after. Subjects evaluated the likeability of novel neutral faces after a brief exposure to a happy or fearful face. We found that lPFC inhibition biased evaluations of novel faces according to the previously processed emotional expression. Greater frontal EEG alpha power, reflecting increased inhibition by TMS, predicted increased behavioral bias. TMS-induced affective misattribution was long-lasting: Emotionally biased first impressions formed during lPFC inhibition were still detectable outside of the laboratory 3 days later. These findings indicate that lPFC serves an important emotion-regulation function by preventing incidental emotional encoding from automatically biasing subsequent appraisals.
Keywords: emotional control, facial expressions, priming, frontal lobe, causality, open materials
Everyday life is filled with an extraordinary range of affective experiences. To maintain context-appropriate behavior, the brain must continually monitor the extent and scope of any emotional response and constrain the spillover of affect from one context where it might be appropriate into the next for which it may not—for instance, when keeping emotional responses provoked by a disagreement with a coworker from negatively coloring impressions of and interactions with another person moments later. Such constraint of affective spillover is a ubiquitous form of emotion regulation that is fundamental to successful adaptation, yet its neural bases are poorly understood.
Research on the neural bases of other forms of self-regulation affords insight into plausible mechanisms underlying the constraint of affective spillover. In particular, neurons in the lateral prefrontal cortex (lPFC) are known to dynamically represent context- and goal-relevant rules and behavioral repertoires, which can bias processing in distal regions to promote context-appropriate behavior (Miller & Cohen, 2001; Sakai & Passingham, 2006; Wise, 2008). The ability to instantiate abstract goals is particularly important when overriding prepotent responses. Accordingly, lPFC function has been implicated in regulating impulsive urges, as exemplified by healthier dieting choices (Hare, Camerer, & Rangel, 2009). Likewise, temporary disruption of lPFC function leads to stimulus-driven behavior (as opposed to goal-based behavior) in neuroeconomic tasks (Figner et al., 2010; Knoch, Schneider, Schunk, Hohmann, & Fehr, 2009). In the motor domain, lPFC lesions can provoke environmental-dependency syndrome, characterized by context-insensitive motor actions automatically triggered by external cues (Knight & D’Esposito, 2003; Lhermitte, Pillon, & Serdaru, 1986).
These observations pertain to regulation of behavior in nonemotional settings; whether lPFC-dependent computations play a causal role in overriding automatic responses to emotional stimuli is unknown and is the subject of this report. Determining the processes that lPFC causally supports in emotion regulation has significant clinical relevance. Extant work largely focuses on contributions from medial PFC (mPFC) regions (for a review, see Kim, Gee, Loucks, Davis, & Whalen, 2011), which, unlike lPFC, are more directly and robustly connected to emotion-encoding structures such as the amygdala (Ray & Zald, 2012). Yet there are indications of an important but little-understood role for lPFC in adaptive emotional responding, including altered lPFC connectivity and recruitment during emotional challenges in anxiety and mood disorders (Birn et al., 2014; Bishop, Duncan, Brett, & Lawrence, 2004; Mayberg et al., 2005), associations between lPFC lesions and depression (Koenigs et al., 2008), and use of excitatory stimulation to lPFC to effectively treat depression (Fox, Buckner, White, Greicius, & Pascual-Leone, 2012). However, a precise account of emotion-regulatory processes causally supported by lPFC function is lacking.
In the current study, we used a method that allows strong causal inference—transcranial magnetic stimulation (TMS)—to test the hypothesis that lPFC function is required for constraining the unwarranted spillover of affect and permitting the context-appropriate forms of emotional responding that are the stuff of everyday life. We measured individuals’ evaluations of novel faces after brief presentations of emotional faces. We hypothesized that lPFC inhibition would produce affective misattribution, resulting in evaluations biased by the valence of previously processed emotional stimuli.
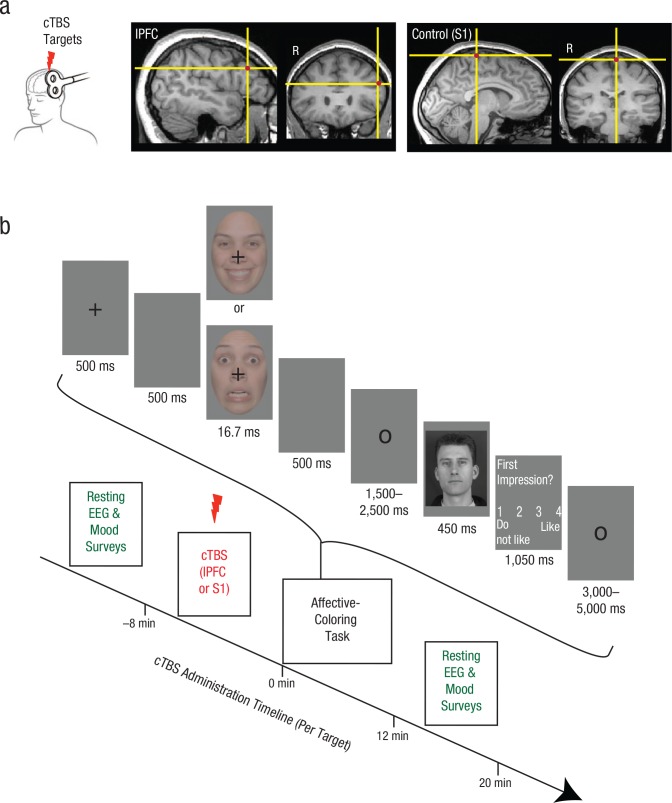
In a within-subjects design, we temporarily altered left-lPFC function in 27 individuals using continuous theta-burst stimulation (cTBS), an inhibitory TMS protocol. We targeted a region of left lPFC previously implicated in affective misattribution via functional connectivity with the amygdala (Lapate et al., 2016; Fig. 1a). Our TMS control region was the left medial primary somatosensory cortex (S1), which allowed us to control for the scalp sensation and nonspecific effects of brain-tissue stimulation (Hamidi, Slagter, Tononi, & Postle, 2009). We accounted for possible interindividual heterogeneity in inhibitory effects of cTBS to PFC (Gratton, Lee, Nomura, & D’Esposito, 2014) by obtaining an electrophysiological index of cortical excitability—power in the alpha-frequency band (8–12 Hz) during resting electroencephalography (EEG; Fig. 1b), which is associated with reduced excitability (Pfurtscheller, Stancák, & Neuper, 1996) and has been found to correlate with TMS-induced behavioral changes (Hamidi et al., 2009).
Fig. 1.
Experimental design of the transcranial magnetic stimulation (TMS) and electroencephalography (EEG) session. The brain images (a) show the lateral prefrontal cortex (lPFC) and control (primary somatosensory cortex, or S1) regions targeted during the administration of continuous theta-burst stimulation (cTBS). The target regions are overlaid on a representative subject’s T1-weighted image in native space. Each subject received TMS at both sites during the session. The timeline for each cTBS administration is shown in (b). After cTBS was administered to either left lPFC or to left S1 for 20 s, subjects completed the affective-coloring task. EEG recordings, which provided a neurophysiological index of cortical excitability, were conducted twice for each cTBS administration, before and after completion of the affective-coloring task. The trial structure for the affective-coloring task is shown in the bracketed area above the timeline. Subjects were informed that the task measured the formation of first impressions in the presence of emotional distractors. After the brief presentation of a happy or fearful face, subjects evaluated novel individuals for their likeability.
To test whether lPFC function regulates the influence of incidental emotional encoding on subsequent evaluative behavior, we employed an affect-misattribution procedure (Fig. 1b; Murphy & Zajonc, 1993; Payne, Cheng, Govorun, & Stewart, 2005). Subjects were informed that this task measured the formation of first impressions in the presence of emotional distractors. Distractors were faint, briefly presented happy and fearful expressions, and were followed by novel neutral faces. Subjects were told that their goal was to indicate how much they liked the neutral faces on the basis of their first impression. We hypothesized that lPFC inhibition would be associated with emotion-congruent biases in evaluative ratings (i.e., neutral-face likeability would be lower after the presentation of fearful faces than after presentation of happy faces), which we refer to as affective coloring. In addition, we explored whether lPFC inhibition gave rise to merely momentary or long-lasting changes in affective coloring by acquiring neutral-face likeability ratings again 3 days later, outside of the laboratory.
Method
Subjects
Twenty-seven right-handed subjects (16 men; mean age = 24.19 years, age range = 18–32 years) recruited from the University of Wisconsin–Madison completed the experimental procedures reported here. Our target sample size was based on previous TMS studies of self-regulation targeting lPFC (Figner et al., 2010; Knoch et al., 2009). Before their participation, subjects were screened for neurological and psychiatric conditions, as well as for TMS and MRI safety criteria, during a clinical interview. The study protocol was approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board. All subjects gave written informed consent.
Procedure
Overview
After clinical screening, the experiment consisted of two separate sessions. First, subjects underwent an MRI session in which T1-weighted scans were obtained to enable subject-specific neuronavigation for accurate TMS targeting. Then, the TMS-EEG session took place on a separate day. Continuous theta-burst TMS (cTBS) was delivered to subjects’ left lPFC and a control site (left S1) on that day, with TMS site order counterbalanced across subjects. Between TMS administrations to lPFC and to control sites, subjects took a 15-min break (for details, see Supplemental Method in the Supplemental Material available online).
EEG data were recorded before and after each cTBS administration to provide a metric of the magnitude of depression of lPFC cortical excitability for each subject, as reflected by the power in the alpha frequency (8–12 Hz) band (Pfurtscheller et al., 1996). The TMS-EEG session started with a 3-min recording of subjects’ resting EEG with their eyes closed. A 4-min recording of subjects’ spontaneous eyeblinks followed. Then, mood surveys—the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988), the Implicit Positive and Negative Affect Test (IPANAT; Quirin & Bode, 2014), and the State-Trait Anxiety Inventory (STAI; Spielberger, 1989)—were administered. After the administration of 20 s of cTBS, subjects completed the affective-coloring task. Then, the recordings of resting EEG and spontaneous eyeblinks were acquired again, and mood surveys were administered to examine possible cTBS-induced changes in mood. As part of a larger study, subjects underwent another set of cTBS application to each cortical site followed by a task aimed at measuring visual awareness (those data are not reported here). For information about the MRI acquisition parameters, see the Supplemental Material.
TMS sites
The left mid-lPFC site targeted in this study (Fig. 1a; Montreal Neurological Institute coordinates: x = −48, y = 24, z = 20) was chosen because (a) the magnitude of affective-coloring behavior correlated with the functional connectivity of this region with the amygdala in a prior functional MRI study (Lapate et al., 2016) and (b) prior neuroimaging studies of self-regulation and instructed emotion regulation have reported activation of left mid-lPFC containing this coordinate (e.g., Buhle et al., 2014; Hare et al., 2009). This lPFC region, near the inferior frontal sulcus, is estimated to be in Brodmann’s area 9/46v (Petrides & Pandya, 1999), and is highly interconnected with the frontoparietal network, multimodal temporal cortex, and cingulate cortex (Petrides & Pandya, 1999).
To demarcate the lPFC region for precise TMS targeting on a subject-by-subject basis, we performed a 12-degrees-of-freedom affine registration between each subject’s T1-weighted scan and the Montreal Neurological Institute template. Then, the registration matrix was inverted, and the lPFC target region was registered to each subject’s native space. Next, the native-space target was visually inspected for every subject to ensure satisfactory registration and peak placement on gray matter.
We targeted left medial somatosensory cortex as a control TMS region; this region is the approximate location of the cortex dedicated to the sensory representation of the right foot (approximate Montreal Neurological Institute coordinates: x = −10, y = −38, z = 78). We thereby avoided inadvertently stimulating lateral face-representation areas. The S1 target was located on each subject’s native-space T1-weighted image on the basis of anatomy. This region was chosen as an active TMS control region because of its circumscribed functional connectivity and because this approach permitted us to rigorously control for nonspecific effects of stimulation of brain tissue (Hamidi et al., 2009), which many prior studies (adopting sham or vertex stimulation) have not. (For additional information about the TMS stimulation protocol and TMS session procedures, see the Supplemental Material.)
Stimuli
Emotional facial expressions (happy and fearful) consisted of 24 identities (12 female and 12 male) selected from the Macbrain Face Stimulus Set (http://www.macbrain.org/resources.htm) and the Karolinska Institute Set (http://www.emotionlab.se/resources/kdef). Faces were cropped to remove hair and neck. Root-mean-square contrast and average luminance was matched across all faces. Emotional faces were presented at a size of 6° × 6°.
Neutral faces used to assess affective coloring consisted of 144 faces chosen from the set of faces at the Extended Multimodal Verification for Teleservices and Security Applications face database project (Messer, Matas, Kittler, Luettin, and Maitre, 1999; http://www.ee.surrey.ac.uk/Research/VSSP/xm2vtsdb), the Max Planck Institute FACES database (http://faces.mpib-berlin.mpg.de/), and the Karolinska Institute Set (http://www.emotionlab.se/resources/kdef). Face images were cropped to remove clothes, converted to gray scale, and matched for average luminance and root-mean-square contrast. Neutral faces were presented at 5° × 6°. (For stimuli and Python scripts used for stimulus presentation, see https://osf.io/j9gct/.)
Affective-coloring task
The affective-coloring task (Fig. 1b) used negative (fearful expressions) and positive (happy expressions) emotional stimuli, consistent with the stimulus type in prior affect-misattribution studies (Gawronski & Ye, 2014; Payne et al., 2005; Ruys, Aarts, Papies, Oikawa, & Oikawa, 2012). This task design was chosen because (a) the primary statistical test of interest in this study (interactive effect of valence and TMS site on neutral-face likeability ratings) did not depend on valence-specific comparisons against emotionally neutral conditions and (b) previous affective-coloring effects have been found most robustly when comparing positive and negative emotional valence conditions, as opposed to positive and neutral conditions (Almeida, Pajtas, Mahon, Nakayama, & Caramazza, 2013; Rotteveel, de Groot, Geutskens, & Phaf, 2001) or negative and neutral conditions (Li, Moallem, Paller, & Gottfried, 2007; Rotteveel et al., 2001). Therefore, this design maximized the number of trials in each emotional valence and TMS condition, thereby enhancing the statistical power to detect emotional-valence-driven shifts in likeability ratings as a function of TMS site.
The experiment was created using PsychoPy (Version 1.79.01; Peirce, 2007) and was presented on an LCD monitor (refresh rate = 60 Hz; screen width = 53 cm; resolution = 1,920 × 1,080 pixels). Emotional facial expressions were shown for 16.7 ms at a low but visible contrast. A test of visual awareness of such stimuli showed that participants were able to detect inversion of the faces with a mean accuracy of .8 (SD = .14) and to detect facial expressions with a mean accuracy of .69 (SD = .15); both of these means highly exceeded chance performance ( ps < .00005). Between 2 and 3 s after the presentation of emotional faces, a novel neutral face was shown for 450 ms, and subjects used a scale from 1 (do not like at all) to 4 (like very much) to indicate their preference for that novel face. Subjects were encouraged to focus on the trustworthiness of neutral faces (rather than on facial attractiveness) when making their likeability judgments. They were given 1.5 s to respond.
A total of 24 unique actors making happy emotional expressions and fearful emotional expressions were used; 12 actors’ expressions were used in the lPFC TMS condition and 12 were used in the control TMS condition (assignment of specific actors’ expressions to each TMS condition was counterbalanced across subjects). Within each TMS condition, each unique emotional face was shown three times. Thus, the affective-coloring task comprised 72 trials (36 with fearful faces and 36 with happy faces) and took 12 min to complete. Each neutral face was presented once and was randomly assigned to a TMS condition and an emotional-expression condition.
To examine whether altered lPFC function was associated with changes in affective-coloring behavior, a repeated measures analysis of variance (ANOVA) was conducted on neutral-face likeability ratings with emotional valence (positive, negative) and cTBS site (lPFC, S1) as within-subjects factors. Data analysis was conducted using IBM SPSS (Version 22). (For EEG data-acquisition parameters, see the Supplemental Material.)
EEG data processing
We extracted power in the alpha frequency band (8–12 Hz) to obtain an objective index of cortical excitability. To that end, each artifact-free 1-s epoch of data was multiplied by a Hanning window and zero-padded to 4 s, and then a fast Fourier transform was computed. Mean power (the square of the absolute value of complex Fourier results) in the alpha frequency band was computed during the eyes-closed recordings after cTBS to lPFC and after cTBS to S1; subjects’ respective pre-cTBS alpha-power baseline values was subtracted from the lPFC and S1 mean power values.
To examine whether cTBS-induced changes in lPFC cortical excitability were associated with affective-coloring behavior, we examined the correlation between affective coloring and changes in EEG alpha power from baseline at the left-lPFC electrode nearest TMS stimulation (channel F7) across subjects (see Fig. 2b). We also explored the scalp topography of this across-subjects correlation to confirm that it reflected a robust frontal alpha-behavioral association not exclusively restricted to one a priori electrode (see Fig. S2 in the Supplemental Material).
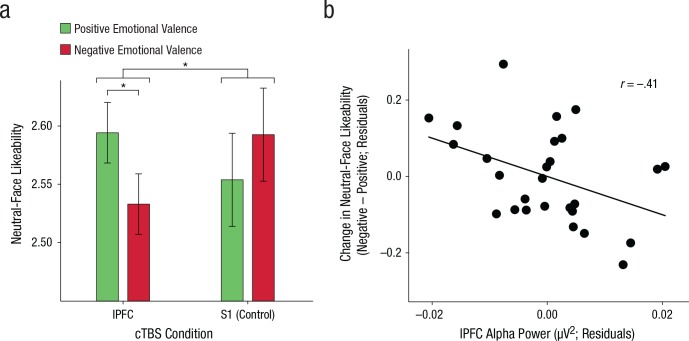
Fig. 2.
Impact of inhibition of lateral prefrontal cortex (lPFC) by continuous theta-burst stimulation (cTBS) on evaluative behavior after emotional processing. The bar graph (a) shows neutral-face likeability separately for faces presented after cTBS to the lPFC and S1, following the presentation of positively and negatively valenced emotional expressions. The error bars represent ±1 SEM of the within-subjects difference between valence conditions. The asterisks indicate significant differences (p < .05). The scatterplot (with best-fitting regression line; b) shows the relationship between change in neutral-face likeability after cTBS administration to lPFC and power in the alpha band (controlling for alpha and behavioral responses in the control condition). Power was measured over the electrode (F7) closest to the location of the transcranial magnetic stimulation coil on lPFC. Plotted on both axes are the residuals saved from linear regressions in which responses recorded in the control condition were regressed out of responses in the lPFC condition. Each data point denotes results for a single subject. (For the scalp topography of this correlation, see Fig. S2 in the Supplemental Material.)
To maximize the reliability of the estimation of the correlation between changes in EEG data and affective-coloring behavior, as well as to avoid the complexity of interpreting results from double difference scores, we used a linear regression and residualization approach that has been demonstrated to have superior psychometric properties compared with change scores for examining correlations between variables (Tucker, Damarin, & Messick, 1966). Specifically, alpha power after cTBS to lPFC was correlated with affective-coloring behavior (i.e., likeability of neutral faces following fearful faces – likeability of neutral faces following happy faces) across subjects, after we controlled for responses in the control condition. To do so, residuals were saved after a linear regression of alpha power in the control cTBS condition on alpha power in the lPFC cTBS condition. Likewise, residuals were saved after a regression of affective-coloring behavior in the control cTBS condition on affective-coloring behavior in the lPFC cTBS condition. Thus, these residuals reflected lPFC alpha power and affective-coloring behavior in the lPFC condition after we accounted for the existing variance in the control condition. Then, those residuals were correlated with one another at the lPFC electrode nearest the TMS coil (channel F7), as well as at all electrodes, using Pearson’s r and a two-tailed alpha of p < .05. We computed the correlation coefficient basic confidence interval for the electrode nearest the TMS coil (100,000 bootstraps) using the boot package (Canty & Ripley, 2016) in the R software environment (Version 3.1.1; R Development Core Team, 2014).
Rating task after the TMS session
We asked participants to complete online rating tasks at home 3 days after the TMS session. Twenty-four subjects (of the original 27) complied with our request and logged in from their computers to complete online assessments that probed their recollection of and likeability for all 144 neutral faces presented during the TMS session after stimulation of a specific site (lPFC or S1) and the presentation of a specific emotional expression (fearful or happy). The previously seen neutral faces were intermixed with 36 foils. The participants responded by moving a slider along a line and placing it on a tick mark. We measured the mouse responses with centesimal precision, and the tick marks were labeled as follows: For the likeability task (“How much do you like this person?”), the leftmost tick mark (corresponding to −100) was labeled really dislike, the middle tick mark (0) was labeled unsure, and the rightmost tick mark (100) was labeled really like. For the memory task (“Have you seen this person before?”), the leftmost tick mark (−100) was labeled no (confident), the middle tick mark (0) was labeled unsure, and the rightmost tick mark (100) was labeled yes (confident). Note that the likeability-rating task for neutral faces performed at home differed from the in-session task in that it was continuous and unanchored by numbers (as opposed to a four-alternative forced choice), used a mouse and sliding scale instead of button presses, and did not impose a time limit for responses (as opposed to 1.5 s). (For data pertaining to the correspondence between neutral-face likeability ratings given on the day of the TMS session and 3 days later as a function of TMS site and emotional-valence condition, see Table S1 in the Supplemental Material.)
Results
Main analyses
By altering lPFC function, we produced affective spillover (Fig. 2a): A repeated measures ANOVA revealed a Valence × TMS Site interaction, F(1, 26) = 7.24, p = .012, ηp2 = .218, which indicates that cTBS to left lPFC produced a bias in neutral-face judgments according to the valence of previously processed emotional expressions. Specifically, when inhibitory cTBS was administered to left lPFC, neutral faces were rated as less likeable when they followed fearful faces rather than happy faces, t(26) = −2.36, p = .026, 95% CI for the difference = [−0.115, −0.008], Cohen’s dz = 0.45. In contrast, affective coloring was not present in the control condition, when lPFC was not inhibited, t(26) = 0.98, p > .337, 95% CI for the difference = [−0.043, 0.12], dz = 0.18. The absence of affective coloring in the TMS control condition is consistent with baseline data (i.e., in the absence of TMS) that we obtained previously in a nearly identical affect-misattribution paradigm with an age-matched sample (n = 33; see Fig. S1 in the Supplemental Material), which indicates that S1 stimulation served as an appropriate control. Collectively, these results provide evidence that lPFC function plays a causal role in appropriately limiting the scope of affective responses by preventing emotional processing from reflexively influencing appraisals of unrelated, novel stimuli.
We next examined whether the observed affective spillover was indeed because of reduced cortical excitability in lPFC after cTBS administration (Huang, Edwards, Rounis, Bhatia, & Rothwell, 2005). Across participants, after cTBS administration to left lPFC, higher left-lPFC alpha power correlated with lower liking of neutral faces following fearful faces relative to neutral faces following happy faces, r = −.41, 95% CI = [−.737, −.141], p = .033 (Fig. 2b). This result suggests that the affective coloring produced by cTBS to left lPFC was due to cTBS-induced reductions in cortical excitability in left lPFC (as well as in its interconnected network; see Fig. S2 in the Supplemental Material).
Note that affective-coloring behavior produced after cTBS administration to left lPFC was not explained by a direct influence of cTBS administration on subjects’ emotional state, because the site of stimulation (lPFC or S1) did not differentially affect subjects’ anxiety or positive or negative mood ( ps > .32), and the interaction between cTBS site and valence on neutral-face likeability ratings remained significant after we controlled for changes in mood or anxiety ( ps < .018; see the Control Analyses section).
Finally, we assessed the possible longevity of emotionally colored first impressions observed after lPFC inhibition to explore whether such affective misattribution was short-lived, or whether lPFC inhibition before emotional processing may have resulted in long-lasting biased associations (Fig. 3a). To that end, we used the data from the online rating task that the subjects completed at home 3 days after the TMS session. It is noteworthy that the structural differences between the online and in-lab rating tasks (i.e., no numbers on the rating scale, no common motor component, and no response time limit) reduce the likelihood that the later ratings would be driven by subjects’ specific memories of their prior in-lab response.
Fig. 3.
Setup for online ratings task and longevity of first impressions formed during the transcranial magnetic stimulation (TMS) session. Three days after the TMS session in the lab, subjects were asked to complete an online rating task (a) in which they evaluated the likeability of the 144 neutral faces (first encountered during the TMS session). These faces were intermixed with 36 foils. Subjects used a continuous scale unanchored by numbers and were given unlimited time to respond. The bar graph (b) shows the neutral-face likeability ratings outside of the lab, separately for faces that had been presented in the TMS session after continuous theta-burst stimulation (cTBS) to the lateral prefrontal cortex (lPFC) or to S1, following the presentation of positively or negatively valenced emotional expressions. The error bars represent ±1 SEM of the within-subjects difference between valence conditions. The asterisk indicates a significant difference (p < .05).
The Valence × cTBS Site interaction on neutral-face likeability ratings first observed in the laboratory persisted 3 days after the TMS session, F(1, 23) = 4.97, p = .036, ηp2 = .178 (Fig. 3b). The primary driver of the persistent interaction of emotional valence with cTBS site was the encoding of negative emotion: Neutral faces shown after negative expressions when left-lPFC function was disrupted remained significantly less liked than when lPFC was not inhibited, t(23) = −2.12, p = .045, 95% CI for the difference = [−6.14, −0.074], dz = 0.43. This suggests that diminished lPFC function may have a long-lasting impact on emotionally biased judgments. Other pairwise comparisons were nonsignificant, ps > .14. Administration of cTBS to left lPFC did not alter recollection of neutral face identities (see the next section), which suggests a dissociation of lPFC function during learning of emotional associations as opposed to learning of face identities. In sum, these findings reinforce the real-world import of our results by suggesting that the affective spillover that occurs when lPFC function is compromised can produce emotionally biased associations that persist days beyond the initial emotional-processing episode.
Control analyses
Order of cTBS administration
Order of cTBS administration (lPFC first or S1 first) did not interact with the impact of cTBS on affective-coloring behavior (p > .68). Critically, the interactive effect of cTBS site and valence on neutral-face likeability ratings during the TMS session remained significant after we controlled for order of cTBS administration by entering it as a between-subjects factor in the repeated measures ANOVA model (p = .014).
Mood
Site of cTBS administration (lPFC or S1) did not differentially affect positive or negative mood, whether measured explicitly (PANAS) or implicitly (IPANAT)—PANAS positive affect: F(1, 26) = 0.54, p > .46; PANAS negative affect: F(1, 26) = 0.98, p > .32; IPANAT positive affect: F(1, 26) = 0.43, p > .51; IPANAT negative affect: F(1, 26) = 0.05, p > .82. cTBS site also did not affect anxiety, state anxiety STAI, F(1, 26) = 0.33, p > .56. Moreover, affective coloring after cTBS to lPFC did not correlate with concurrent changes in mood, as indicated by the correlation of the relevant difference scores as well as the correlation between the residualized scores (of anxiety and positive and negative mood measured implicitly and explicitly after cTBS stimulation of lPFC relative to S1), all ps > .24. In addition, changes in mood after cTBS to lPFC did not interact with the impact of cTBS on affective-coloring behavior (all ps > .45). Critically, the interaction of cTBS site and valence on neutral-face likeability ratings during the TMS session remained significant after we controlled for changes in mood by including the relevant difference or residualized scores as covariates in individual ANOVA models (all ps < .018), as well as in a simultaneous model (ps < .024). Therefore, the effects reported here are not explained by possible differences in mood depending on whether TMS was administered to lPFC or S1.
Reaction times
Analysis of reaction times (RTs) revealed a trend of slightly faster responding to neutral faces after fearful (M = 0.92 s, 95% CI = [0.849, 0.994]) compared with happy faces (M = 0.93 s, 95% CI = [0.856, 1.00]), 95% CI for the difference = [−0.019, 0.001], p = .066, and a trend of slower responding to neutral faces after cTBS was administered to lPFC (M = 0.94 s, 95% CI = [0.873, 1.02]) compared with S1 (M = 0.91 s, 95% CI = [0.826, 0.989]), p = .095, 95% CI for the difference = [−0.007, 0.082]. There was no interactive effect of cTBS site and valence on RTs (p > .29). Further, across individuals, changes in RT by valence (fearful or happy) or by cTBS site (lPFC or S1) were uncorrelated with changes in affective coloring (all ps > .29); the interactive effect of cTBS site and valence on neutral-face likeability ratings during the TMS session remained significant (p < .012) after we controlled for changes in RT.
Memory
Recollection of neutral faces 3 days after the TMS session was not modulated by cTBS site, F(1, 23) = 0.93, p > .34, or by valence, F(1, 23) = 0.03, p > .86, and there was no significant cTBS Site × Valence interaction, F(1, 23) = 0.03, p > .86.
Discussion
By employing a technique that enables causal inference to perturb lPFC function, we demonstrated a causal contribution of this region in constraining the spillover of emotional information from one context to the next. After left-lPFC disruption by TMS, mild emotional cues influenced subsequent novel-face evaluations in a stimulus-driven (i.e., valence-congruent) manner. The extent of TMS-induced inhibition in left lPFC, as indicated by electrophysiological recordings, correlated with greater emotional-stimulus-congruent influence on evaluations. It is noteworthy that the behavioral consequences of emotional-cue processing after lPFC inhibition were still detectable outside of the laboratory 3 days later. Thus, our findings unveil an important neural substrate underlying a ubiquitous and little-studied form of context-appropriate emotion regulation.
The lPFC region we targeted is intimately connected with the frontoparietal network, which has been frequently interrogated during “cold” cognitive processing, such as the control of attention and working memory (D’Esposito & Postle, 2015). In contrast, the specific causal contributions of lPFC function to adaptive emotional responding have been neglected. Compared with other prefrontal regions, lPFC is weakly connected with emotion-encoding structures such as the amygdala (Ray & Zald, 2012). Nonetheless, lesions to lPFC, compared with lesions to other frontal sites, increase risk for a depressive episode (Koenigs et al., 2008), and individuals with anxiety and depression often show reduced lPFC activation at rest and during processing of negative emotion (Bishop et al., 2004; Mayberg et al., 2005; Siegle, Thompson, Carter, Steinhauer, & Thase, 2007), which suggests that function of lPFC or of its interconnected network (or of both) plays an important yet poorly understood role in emotion regulation.
Prior work detailing lPFC’s involvement in emotion-regulation processes has focused on explicitly cued forms of regulation, such as cognitive reappraisal, during which ventral and dorsal aspects of lPFC are engaged (Buhle et al., 2014). However, because of the high rate at which people are exposed to affective information, together with the virtually continuous pressure of foreground tasks that constrain their resources, most emotion regulation has to be initiated implicitly (i.e., noncued). We recently found that inverse coupling between lPFC and amygdala during processing of fearful faces correlated negatively with subsequent affective misattribution (Lapate et al., 2016), providing a hint that lPFC may also participate in implicit forms of emotion regulation. Further, lPFC responses to negative facial expressions have been associated with successful self-regulation after interpersonal conflict (Hooker, Gyurak, Verosky, Miyakawa, & Ayduk, 2010). Although compelling, this evidence remained largely correlational: Whether and how lPFC function is causally implicated in adaptive emotional responding in healthy individuals was previously unclear.
The present experiment causally supports an important role in emotion regulation for lPFC—that of preventing emotional encoding from automatically and unwarrantedly influencing subsequent appraisals of unrelated stimuli. The finding that left-lPFC inhibition led participants to evaluate novel neutral stimuli according to the valence of previously processed, irrelevant emotional information is reminiscent of “environmental-dependency syndrome,” wherein the behavior of patients with frontal lesions is overly guided by salient external stimuli irrespective of context (Knight & D’Esposito, 2003). Collectively, these results are consistent with evidence suggesting that task-structure representation and monitoring in lPFC promotes goal-oriented (as opposed to stimulus-driven) behavior across a variety of tasks and stimulus domains (Figner et al., 2010; Miller & Cohen, 2001; Sakai & Passingham, 2006).
Although our results indicate that inhibition of left lPFC produced affective misattribution, it is worth noting that TMS can affect not only the excitability of the region under the coil, but also that region’s interconnected network (Gratton, Lee, Nomura, & D’Esposito, 2013). Examination of the topography of correlation between affective coloring and alpha power following cTBS administration to left lPFC reveals a distributed rather than local pattern (see Fig. S2 in the Supplemental Material), which is consistent with the known frontoparietal connectivity of the mid-lPFC region we targeted. Therefore, examining whether the observed effects result from computations originating in lPFC or depend on a frontoparietal network-level reconfiguration (or both) warrants future research employing, for example, simultaneous TMS and functional MRI. In addition, lPFC is interconnected with medial PFC structures previously implicated in (a) the direct modulation of emotional processing (via amygdala projections) and (b) the efficacy of TMS stimulation of lPFC as a treatment for depression (Fox et al., 2012). Therefore, elucidating whether the present results are dependent on amygdala function and are mediated via intermediate amygdala projections from lPFC to medial PFC is another important direction for future work.
In the control condition (cTBS to S1), when lPFC function was not inhibited, we did not observe affective coloring. This concurs with results from prior studies that used similar affective-priming tasks and comparably mild emotional cues (e.g., facial expressions or scents; Li et al., 2007; Murphy & Zajonc, 1993; Rotteveel et al., 2001) and with our work employing a similar affect-misattribution procedure without TMS (see Fig. S1 in the Supplemental Material). Collectively, these results suggest an effective discounting of prior emotional processing while evaluating unrelated stimuli in normative circumstances (Li et al., 2007). In everyday life, the magnitude of affective spillover probably depends not only on an individual’s regulatory capacity and lPFC intactness but also on the intensity of the emotional event (Payne et al., 2005), the awareness of its influence (Jones, Fazio, & Olson, 2009; Lapate, Rokers, Li, & Davidson, 2014; Li et al., 2007; Murphy & Zajonc, 1993), and the interval between emotional processing and subsequent events (Payne et al., 2005).
The following limitations of the present study warrant additional investigation. First, we examined affective spillover as revealed by contrasting negative and positive emotional stimuli, consistent with the approach of prior work (e.g., Ruys et al., 2012). Future studies, ideally employing longer (e.g., 40 s) cTBS protocols, should additionally include a neutral-stimulus condition to disentangle whether lPFC function contributes similarly to regulation of affective spillover provoked by processing of both negative and positive emotion, or whether its regulatory function is valence-specific. Second, we targeted left lPFC because of its previously documented involvement in the modulation of affective misattribution (Lapate et al., 2016). Future work should therefore determine whether these findings generalize to right lPFC, as suggested by the bilateral pattern of brain-behavior correlations in the EEG data. Last, the TMS control condition adopted in this study was an active control, in which we targeted a left medial somatosensory region. Using an active TMS control condition presents clear advantages over sham stimulation (e.g., controlling for nonspecific cortical stimulation effects). However, the additional inclusion of a sham TMS condition in future work would unambiguously ascertain whether medial somatosensory active controls (as well as those in other areas) yield negligible effects on the construct of interest.
In this study, the impact of lPFC disruption on biased first impressions formed in the laboratory was still detectable 3 days later, which suggests that rapid, one-trial emotional learning can take place when lPFC function is perturbed, even in the absence of alterations to mood or explicit memory. Moving forward, it will be interesting to examine the extent to which these effects extend to overt behavior in navigating a complex social world, one in which people are often exposed to a rapid succession of stimuli of varying valences and challenged to react in a context-sensitive manner. Moreover, it is intriguing to consider whether a similar lPFC-dependent mechanism governs responses to emotional cues during stress-induced PFC impairments (Arnsten, 2009) and whether affect-misattribution phenomena are involved in the pathophysiology of mood disorders in which lPFC dysfunction has been implicated, such as anxiety and depression (Bishop et al., 2004; Koenigs et al., 2008).
Supplementary Material
Acknowledgments
We thank A. Austermuehle and N. Vack for assistance with data collection; D. W. Grupe, R. Goldman, S. Schaefer, O. Gosseries, A. Sheldon, M. Starrett, and A. Haun for discussions; and Waisman Center staff for their support.
Footnotes
Action Editor: Ralph Adolphs served as action editor for this article.
Declaration of Conflicting Interests: R. J. Davidson serves on the board of directors for two nonprofit organizations: The Mind and Life Institute and Healthy Minds Innovations. The authors declared that they had no other conflicts of interest with respect to their authorship or the publication of this article.
Funding: This study was supported by National Institute of Mental Health Grants R01-MH43454 (to R. J. Davidson), P50-MH069315 (to R. J. Davidson), F32-MH113347 (to R. C. Lapate), and MH095984 (to B. R. Postle).
Supplemental Material: Additional supporting information can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797617699837
Open Practices:

All materials have been made publicly available via Open Science Framework and can be accessed at https://osf.io/m9jch/. Institutional Review Board constraints precluded the authors from publicly sharing the data. The complete Open Practices Disclosure for this article can be found at http://journals.sagepub.com/doi/suppl/10.1177/0956797617699837. This article has received the badge for Open Materials. More information about the Open Practices badges can be found at https://www.psychologicalscience.org/publications/badges.
References
- Almeida J., Pajtas P. E., Mahon B. Z., Nakayama K., Caramazza A. (2013). Affect of the unconscious: Visually suppressed angry faces modulate our decisions. Cognitive, Affective, & Behavioral Neuroscience, 13, 94–101. doi: 10.3758/s13415-012-0133-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10, 410–422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R. M., Shackman A. J., Oler J. A., Williams L. E., McFarlin D. R., Rogers G. M., . . . Kalin N. H. (2014). Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Molecular Psychiatry, 19, 915–922. doi: 10.1038/mp.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S., Duncan J., Brett M., Lawrence A. D. (2004). Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience, 7, 184–188. doi: 10.1038/nn1173 [DOI] [PubMed] [Google Scholar]
- Buhle J. T., Silvers J. A., Wager T. D., Lopez R., Onyemekwu C., Kober H., . . . Ochsner K. N. (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. doi: 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canty A., Ripley B. (2016). boot: Bootstrap functions (Version 1.3-18) [Software]. Retrieved from http://cran.r-project.org/package=boot
- D’Esposito M., Postle B. R. (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66, 115–142. doi: 10.1146/annurev-psych-010814-015031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B., Knoch D., Johnson E. J., Krosch A. R., Lisanby S. H., Fehr E., Weber E. U. (2010). Lateral prefrontal cortex and self-control in intertemporal choice. Nature Neu-roscience, 13, 538–539. doi: 10.1038/nn.2516 [DOI] [PubMed] [Google Scholar]
- Fox M. D., Buckner R. L., White M. P., Greicius M. D., Pascual-Leone A. (2012). Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry, 72, 595–603. doi: 10.1016/j.biopsych.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawronski B., Ye Y. (2014). What drives priming effects in the affect misattribution procedure? Personality and Social Psychology Bulletin, 40, 3–15. doi: 10.1177/0146167213502548 [DOI] [PubMed] [Google Scholar]
- Gratton C., Lee T. G., Nomura E. M., D’Esposito M. (2013). The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Frontiers in Systems Neuroscience, 7, Article 124. doi: 10.3389/fnsys.2013.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C., Lee T. G., Nomura E. M., D’Esposito M. (2014). Perfusion MRI indexes variability in the functional brain effects of theta-burst transcranial magnetic stimulation. PLoS ONE, 9(7), Article e101430. doi: 10.1371/journal.pone.0101430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M., Slagter H. A., Tononi G., Postle B. R. (2009). Repetitive transcranial magnetic stimulation affects behavior by biasing endogenous cortical oscillations. Frontiers in Integrative Neuroscience, 3, Article 14. doi: 10.3389/neuro.07.014.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T. A., Camerer C. F., Rangel A. (2009). Self-control in decision-making involves modulation of the vmPFC valu-ation system. Science, 324, 646–648. doi: 10.1126/science.1168450 [DOI] [PubMed] [Google Scholar]
- Hooker C. I., Gyurak A., Verosky S. C., Miyakawa A., Ayduk Ö. (2010). Neural activity to a partner’s facial expression predicts self-regulation after conflict. Biological Psychiatry, 67, 406–413. doi: 10.1016/j.biopsych.2009.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-Z., Edwards M. J., Rounis E., Bhatia K. P., Rothwell J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron, 45, 201–206. doi: 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- Jones C. R., Fazio R. H., Olson M. A. (2009). Implicit misattribution as a mechanism underlying evaluative conditioning. Journal of Personality and Social Psychology, 96, 933–948. doi: 10.1037/a0014747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J., Gee D. G., Loucks R. A., Davis F. C., Whalen P. J. (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cerebral Cortex, 21, 1667–1673. doi: 10.1093/cercor/bhq237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R. T., D’Esposito M. (2003). Lateral prefrontal syndrome: A disorder of executive control. In D’Esposito M. (Ed.), Neurological foundations of cognitive neuroscience (pp. 259–281). Cambridge, MA: MIT Press. [Google Scholar]
- Knoch D., Schneider F., Schunk D., Hohmann M., Fehr E. (2009). Disrupting the prefrontal cortex diminishes the human ability to build a good reputation. Proceedings of the National Academy of Sciences, USA, 106, 20895–20899. doi: 10.1073/pnas.0911619106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Huey E. D., Calamia M., Raymont V., Tranel D., Grafman J. (2008). Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. The Journal of Neuroscience, 28, 12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapate R. C., Rokers B., Li T., Davidson R. J. (2014). Nonconscious emotional activation colors first impressions: A regulatory role for conscious awareness. Psychological Science, 25, 349–357. doi: 10.1177/0956797613503175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapate R. C., Rokers B., Tromp D. P. M., Orfali N. S., Oler J. A., Doran S. T., . . . Davidson R. J. (2016). Awareness of emotional stimuli determines the behavioral consequences of amygdala activation and amygdala-prefrontal connectivity. Scientific Reports, 6, Article 25826. doi: 10.1038/srep25826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhermitte F., Pillon B., Serdaru M. (1986). Human autonomy and the frontal lobes. Part I: Imitation and utilization behavior: A neuropsychological study of 75 patients. Annals of Neurology, 9, 326–334. [DOI] [PubMed] [Google Scholar]
- Li W., Moallem I., Paller K. A., Gottfried J. A. (2007). Subliminal smells can guide social preferences. Psychological Science, 18, 1044–1049. doi: 10.1111/j.1467-9280.2007.02023.x [DOI] [PubMed] [Google Scholar]
- Mayberg H. S., Lozano A. M., Voon V., McNeely H. E., Seminowicz D., Hamani C., . . . Kennedy S. H. (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45, 651–660. doi: 10.1016/j.neuron.2005.02.014 [DOI] [PubMed] [Google Scholar]
- Messer K., Matas J., Kittler J., Luettin J., Maitre G. (1999). XM2VTSbd: The extended M2VTS database. In Proceedings of the 2nd Conference on Audio and Video-Based Biometric Personal Authentication (pp. 72–77). New York, NY: Springer Verlag. [Google Scholar]
- Miller E. K., Cohen J. D. (2001). An integrative theory of prefrontal cortex. Annual Review of Neuroscience, 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Murphy S. T., Zajonc R. B. (1993). Affect, cognition, and awareness: Affective priming with optimal and suboptimal stimulus exposures. Journal of Personality and Social Psychology, 64, 723–739. [DOI] [PubMed] [Google Scholar]
- Payne B. K., Cheng C. M., Govorun O., Stewart B. D. (2005). An inkblot for attitudes: Affect misattribution as implicit measurement. Journal of Personality and Social Psychology, 89, 277–293. doi: 10.1037/0022-3514.89.3.277 [DOI] [PubMed] [Google Scholar]
- Peirce J. W. (2007). PsychoPy–Psychophysics software in Python. Journal of Neuroscience Methods, 162, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Pandya D. N. (1999). Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience, 11, 1011–1036. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Stancák A., Neuper C. (1996). Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: A review. International Journal of Psychophysiology, 24, 39–46. doi: 10.1016/S0167-8760(96)00066-9 [DOI] [PubMed] [Google Scholar]
- Quirin M., Bode R. C. (2014). An alternative to self-reports of trait and state affect: The Implicit Positive and Negative Affect Test (IPANAT). European Journal of Psychological Assessment, 30, 231–237. doi: 10.1027/1015-5759/a000190 [DOI] [Google Scholar]
- Ray R. D., Zald D. H. (2012). Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience & Biobehavioral Reviews, 36, 479–501. doi: 10.1016/j.neubiorev.2011.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. (2014). R: A language and environment for statistical computing (Version 3.0.2) [Computer software]. Retrieved from https://www.r-project.org/index.html
- Rotteveel M., de Groot P., Geutskens A., Phaf R. H. (2001). Stronger suboptimal than optimal affective priming? Emo-tion, 1, 348–364. doi: 10.1037/1528-3542.1.4.348 [DOI] [PubMed] [Google Scholar]
- Ruys K. I., Aarts H., Papies E. K., Oikawa M., Oikawa H. (2012). Perceiving an exclusive cause of affect prevents misattribution. Consciousness and Cognition, 21, 1009–1015. doi: 10.1016/j.concog.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Sakai K., Passingham R. E. (2006). Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. The Journal of Neuroscience, 26, 1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle G. J., Thompson W., Carter C. S., Steinhauer S. R., Thase M. E. (2007). Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry, 61, 198–209. doi: 10.1016/j.biopsych.2006.05.048 [DOI] [PubMed] [Google Scholar]
- Spielberger C. D. (1989). State-trait anxiety inventory: Bibliography (2nd ed.). Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Tucker L. R., Damarin F., Messick S. (1966). A base-free measure of change. Psychometrika, 31, 457–473. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A., Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wise S. P. (2008). Forward frontal fields: Phylogeny and fundamental function. Trends in Neurosciences, 31, 599–608. doi: 10.1016/j.tins.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.