Abstract
High-precision measurements of radiocarbon (14C ) near or below a fraction modern 14C of one (F14C ≤ 1) are challenging and costly. An accurate, ultra-sensitive linear absorption approach to detecting 14C would provide a simple and robust bench-top alternative to off-site accelerator mass spectrometry facilities. Here we report the quantitative measurement of 14C in gas-phase samples of CO2 with F14C < 1 using cavity ring-down spectroscopy in the linear absorption regime. Repeated analysis of CO2 derived from the combustion of either biogenic or petrogenic sources revealed a robust ability to differentiate samples with F14C < 1. With a combined uncertainty of 14C /12C = 130 fmol/mol (F14C = 0.11), initial performance of the calibration-free instrument is sufficient to investigate a variety of applications in radiocarbon measurement science including the study of biofuels and bioplastics, illicitly traded specimens, bomb dating, and atmospheric transport.
Keywords: Radiocarbon, Laser spectroscopy, Cavity ring-down spectroscopy, Gas metrology
Graphical Abstract

Quantifying radiocarbon (14C ) can unambiguously apportion carbon sources, yield the age of ancient artifacts, elucidate the global carbon cycle, and pinpoint disruptive events in paleoclimatology.1,2 High-precision measurements also have applications in forensic science and illicit trade.3 These applications in quantitative 14C science require world-wide intercomparisons between measurement facilities, specifically for facilities performing liquid scintillation counting and accelerator mass spectrometer (AMS).4 Recent AMS intercomparisons have focused on high-precision (≤1 %) applications in atmospheric 14C science, where agreed-upon standards for gas handling, sample preparation, and calibration are needed to realize ultimate sensitivities.5,6 For many applications, however, a precision approaching 0.1 % of modern 14C (F14C ) is not required. In this context, the emergence of alternative bench-top instruments could drive rapidly evolving interdisciplinary research and measurement applications, as well as reduce the number of samples processed by high-precision AMS laboratories, thus freeing more state-of-the-art laboratory time for the most demanding 14C applications.
Optical methods for the detection of 14C are one such bench-top alternative. However, optical detection of 14C presents several challenges for precise molecular spectroscopy and gas metrology. In addition to the apparent need for ultra-sensitive detection, quantitative spectroscopy is susceptible to the presence of interfering absorbers at wavelengths near that of the target transition. Thus, just as in AMS, sample separation by chemical or physical means is necessary. The seminal demonstrations of saturated absorption cavity ring-down (SCAR) of 14C 16O2 in the mid-infrared addressed these challenges by implementing a novel non-linear cavity-enhanced approach to achieve a sensitivity of 14C /12C < 10−12 utilizing a targeted rotationally resolved vibrational 14C 16O2 transition with minimum spectroscopic interference.7 Further improvements to the sample cell as well as the addition of a high-powered probe laser recently yielded SCAR precision approaching that of AMS facilities.8 While elegant in theory, in practice complexities, limitations, and potential biases associated with its non-linear nature have proven to be a barrier to broad implementation despite SCAR’s clear potential for ultra-sensitive spectroscopy.9–11
Several groups have demonstrated prototype mid-infrared 14C 16O2 spectrometers based on the principles of linear-absorption cavity ring-down spectroscopy (CRDS) that are appropriate for applications involving enriched levels of radioactivity, such as generating precision line lists,12 studying decommissioned nuclear power plants,13 or for biology tracer experiments.14 The recent publication by McCartt et al. achieved high sensitivity for 14C -enriched biological samples while operating in the presence of significant spectroscopic interferences.14 These interferences limited spectrometer sensitivity, and therefore restricted their calibrated spectrometer to the high-precision study of 14C -enriched samples with F14C > 1. Interferences also amplify the need for a stable, high-precision frequency axis because frequency fluctuations in the presence of large intracavity loss spectral derivatives (i.e., strong molecular absorption) translate into reduced sensitivity. McCartt et al. reported a method to leverage the presence of strong intra-sample absorption for iterative frequency axis calibration.15 Their demonstration of 14C 16O2 CRDS therefore requires an intra-sample frequency reference as well as 14C -enriched samples to achieve the reported precision. This is also limiting, as the relative frequency axis calibration will only yield an optimum fractional stability of ν/Δν of 2×106 (where ν = 66.2 THz is the probe laser frequency and Δν = 30 MHz is the uncertainty in the reference transition frequency). The requirement for a strongly absorbing intracavity reference also precludes the removal of interferences altogether by reducing the sample cell temperature to T < 250 K.
Here we report a laser-based mid-infrared CRDS instrument capable of measuring 14C in samples with F14C < 1. The laser spectrometer operates on the principles of CRDS in the linear absorption regime, and is capable of a detection limit bounded by quantum noise.16,17 A high-precision frequency axis is defined by the transmission spectrum of a temperature-stabilized high-finesse (F = 52 000) optical resonator containing the gas-phase CO2 sample under investigation, thus achieving a fractional frequency stability (ν/Δν) approaching 1 × 108 over 1 h of continuous operation. By discretely jumping the laser frequency from mode-to-mode of the ring-down cavity about a center wave number near 2 209.107 679 cm−1, we repeatedly record the spectrum of the 00011 ← 00001 P 20e 14C 16O2 transition at a constant sample temperature of 220 K and sample pressure of 0.9 kPa. The experimentally measured line area of the 14C 16O2 transition is related to the mole fraction of 14C (χ14C) by the transition intensity,18 a quantity calculated using ab initio quantum chemistry methods19 that have been rigorously benchmarked against accurate, high-precision measurements of CO2 spectra.20–23 This combination of a high-fidelity stabilized frequency axis, an ultra-sensitive CRDS detection scheme, and the use of an ab initio transition intensity resulted in the quantitative optical detection of 14C at F14C < 1 on an absolute scale using linear absorption spectroscopy.
A block diagram of the experimental apparatus is shown in Fig. 1A. While no effort was made to minimize the physical footprint of this first-generation instrument, it easily fit onto a standard 1.2 m × 3.7 m (4 ft × 12 ft) optical table. A continuous-wave distributed-feedback quantum cascade laser (DFB QCL) was used as the probe laser to excite the lowest-order transverse mode of the high-finesse optical resonator (false-color image of the TEM00 mode transmission is shown in the optical cavity call-out box in Fig. 1A). Once the intra-cavity laser power increased to a predefined level, the laser was switched off-resonance using an acousto-optic modulator and a laser frequency adjustment. The resulting time-dependent decay signals were measured by an ultra-low-noise InSb photoreceiver (PR) placed at an etalon-immune distance24–26 from the output of the optical resonator and digitized. The model function I = I0 exp(−t/τ) relating the time-dependent intensity of the light exiting the cavity to the decay time constant, τ, was fit to each decay signal to yield τ and recorded on a personal computer (PC). The empty-cavity time constant was τ0 = 82 μs. The PC also controlled the laser triggering and maintained laser resonance with a given lowest order transverse mode (TEM00) through a low-bandwidth (4 Hz) servo controller which adjusted the laser current. Course laser wave number adjustments, which were performed by tuning the QCL temperature and monitored by the wavelength meter (wavemeter), also are illustrated in Fig. 1A.
Fig. 1.
A. Illustration of the linear absorption spectrometer for the optical detection of radiocarbon dioxide (14C 16O2). The instrument comprises a distributed feedback quantum cascade laser (DFB-QCL), an acousto-optic modulator (AOM), a wavelength meter (wavemeter), lenses (L1-L3), a half-wave plate (λ/2), the optical cavity, an InSb photoreceiver (PR), and a personal computer (PC). In addition to digitizing and fitting cavity decays, the PC controls the laser servo, frequency adjustment, and fast optical switch triggering. The TEM00 mode of the optical resonator is visible in the false-color image above the optical cavity (image recorded using a mid-infrared InSb camera). B. Partial technical drawing of the optical resonator and cold sample cell (“Optical Cavity” in A). The Invar rods, connected to stainless steel (SS) angle plates, stabilize the cavity length. The SS outer shell of the cylindrical optical resonator separates the CO2 gas sample and inner cold finger from the laboratory environment. Ring-down mirrors are mounted inside the gas sample environment, and CaF2 optical windows transmit the probe laser beam into and out of (not shown) the optical resonator. Shown in the bottom right of B is a two-dimensional cut-through taken at the knife-edge flange of B, further illustrating the SS outer shell, cold finger, and CO2 gas sample.
A detailed illustration of the high-finesse optical resonator and cold sample cell is shown in Fig. 1B. In the cross-sectional view (bottom right), the central stainless steel cold finger comprises an annular region (illustrated in blue) which continuously circulated a cooling liquid throughout the center of the sample cell. The cooling liquid recirculator has an operating range of 183 K to 373 K and a cooling capacity of 0.6 kW at 213 K. The total gas sample volume is estimated from technical drawings to be 2.94 L. Optical interrogation of the sample occurred at the center of the cold finger. Sample temperature variations were measured by three platinum resistance thermometers (PRTs) in thermal contact with the cold finger to be ΔT = 49 mK over 1 hour. The nominal optical cavity length L = 144.7 cm was passively stabilized by four Invar rods with thermal expansion coefficient β(T) = 9.0 × 10−7 K−1 at a temperature of T = 295 K.27 Along with the Invar rods, approximately 9% of the total optical resonator length extended beyond the angle plates (one of which is depicted in Fig. 1B), and therefore the thermal expansion of the stainless-steel mirror mounts (β(T) = −2.1 × 10−6 K−1 at T = 295 K)27 was also significant. The temperature stability of the ambient laboratory air was ΔT = 16 mK over 1 h, resulting in an estimate of the fractional frequency stability of the optical resonator of ν/Δν = L/ΔL = |−1/Σ(qβ(T)ΔT)| = 9.8 × 107, where q is the fractional contribution of each material to the total length (q = 0.91 for Invar and q = 0.09 for stainless steel). Over 24 h, measured temperature variations increased only marginally to ΔT = 25 mK. Note that this fractional stability is achieved without an internal reference gas, and that the linearity of the detuning axis could be further improved by several orders of magnitude using a monolithic resonator constructed from a low-thermal-expansion material28 and/or by the addition of one or more optical reference lasers.29
Individual spectra of 14C 16O2, along with the spectra of nearby 16O2 and 14N216O transitions, were recorded by step-scanning the QCL frequency via the laser current over a bandwidth of 1.14 GHz at a sampling interval of 103.61 MHz (equal to the cavity free spectral range, with a standard uncertainty of 0.11 MHz). Including the dead-time associated with frequency stepping and relocking, a single 11-point spectrum was completed in 70 s. To maintain approximately constant scan speeds, both the number of cavity time constants averaged per point and the associated single-frequency acquisition rate were adjusted. Typical cavity decay acquisition rates ranged between 30 Hz and 50 Hz, whereas 30 to 200 successive time constants were typically averaged per spectral point prior to laser tuning. A total of 10 scans was recorded at the initial optical resonator length with alternating scan directions. Using a piezoelectric transducer (PZT) to displace one of the optical resonator mirrors and thereby change the ring-down cavity length, the entire frequency sampling grid was shifted by approximately 25 MHz and another 10 scans were recorded. This cavity mode shifting procedure was repeated a total of 3 times, resulting in 40 interleaved spectra with an approximately 25 MHz sampling interval spanning 1.24 GHz recorded in 47 minutes.
Plotted in Fig. 2 are representative interleaved spectra of CO2 originating from the combustion of either biogenic (i.e., purified bioethanol combustion products, Fig. 2A) or petrogenic (i.e., purified fossil fuel combustion products, Fig. 2B) carbon, respectively. Both CO2 samples were purchased as commercial gas cylinders with nominal purity of 99.8%, and no additional filtration and/or purification was necessary before sending the gas samples into the CRDS cell. A model was fit to each individual spectrum by floating the following parameters: ν0, χ14C, T, and a quadratic baseline function. Here, ν0 is the absolute laser frequency at the initial position, χ14C = 1.004 76 × χ646 where the factor of 1.004 76 corrects the measured mole fraction of 14C 16O2 (χ646) for the presence of 18O and 17O isotopologues,22 and T is the sample temperature. The relative frequencies of all interfering transitions from 12C16O2, 13C16O2, and 14N216O were fixed to known literature values. 10,22,30 In addition to the target 14C 16O2 transition, we included in our model two nearby overlapping 13C16O2 transitions (labeled in Fig. 2), one nearby 14N216O transition (labeled in Fig. 2B, and overlapping with the 13C16O2 transitions), and one 12C16O2 transition which is responsible for the underlying baseline (see Table 1). Each transition was modeled by a Voigt line profile with Doppler- and pressure-broadening contributions calculated from the experimentally observed temperature and pressure using known coefficients and intensities.22 The standard deviation of the fitted residuals (observed minus calculated for all 40 spectra) in Fig. 2 were identical, with values of 5.4×10−11 cm−1. For these representative spectra, the observed residuals are less than a factor of 2 from the fundamental quantum-noise-limited theoretical value of 2.9×10−11 cm−1.16
Fig. 2.
Interleaved spectra of biogenic (A) and petrogenic (B) CO2 recorded at T = 220 K and P = 0.9 kPa. In each panel, the black dots are the experimental data points comprising 40 interleaved spectra recorded in a total acquisition time of approximately 47 minutes. Each of the individual spectra was normalized by a quadratic baseline function prior to interleaving. In A, the fitted model (green line) reveals a significant amount of 14C 16O2, centered at a detuning frequency of approximately −250 MHz whereas the fitted model in B (magenta line) reveals no measurable 14C 16O2 spectroscopic signature. Measured χ14C were 0.95 pmol/mol ± 0.08 pmol/mol and 0.05 pmol/mol ± 0.08 pmol for the biogenic (A) and petrogenic (B) samples, respectively. The standard deviation of the fitted residuals in each lower panel is 5.4×10−11 cm−1.
Table 1.
Molecular transitions comprising the radiocarbon (14C ) spectroscopic model.
| Molecule | Wave number (cm−1) | Upper Statea | Lower Statea | Rotationa | Reference |
|---|---|---|---|---|---|
| 12C16O2 | 2 209.011 379 | 21101 | 10002 | P 8e | 22, 30 b |
| 14C 16O2 | 2 209.107 679 | 00011 | 00001 | P 20e | 10, 18, 22c |
| 14N216O | 2 209.114 440 | 0111 | 0110 | Q 12e | 22 |
| 13C16O2 | 2 209.115 876 | 05511 | 05501 | P 19e | 22, 30 b |
| 13C16O2 | 2 209.117 470 | 21111 | 21101 | P 23e | 22, 30 b |
Mole fractions of 13C16O2 and N2O were independently measured for each CO2 gas sample and then fixed in the models. The 13C16O2 mole fractions were measured by Fourier-transform infrared (FTIR) spectroscopy to be 1.093 % ± 0.002 % and 1.071 % ± 0.002 % for the biogenic and petrogenic samples, respectively (relative to standard reference material 1730-A-17, Northern Continental Air, with 13C16O2 isotopic mole fraction of 1.097 %). By tuning the QCL frequency by ≈900 MHz, we used the low-temperature CRDS spectrometer reported here to measure the mole fraction of N2O via the 0201 ← 0200 R 16e 14N216O transition at ν̃0 = 2 209.085 430 cm−1. Representative spectra plotted in Fig. 3 were recorded at two different sample temperatures (244.6 K and 231.2 K) and fitted using known database parameters for both N2O and CO2.30 The sample temperatures were determined spectroscopically by fitting the 13C16O2 transitions while fixing their known mole fraction (as measured by FTIR spectroscopy). The 14N216O transition was chosen for its proximity to the target 14C 16O2 transition and its relative isolation from spectroscopic interferences. For biogenic CO2, the N2O mole fraction was below our detection limit (2 nmol/mol), and therefore was not included in that fitted model. For petrogenic CO2, a significant amount of N2O was present, with a spectroscopically measured mole fraction of 28.5 nmol/mol ± 4.2 nmol/mol.
Fig. 3.
Spectrum of the petrogenic CO2 sample in the proximity of the 0201 ← 0200 R 16e N2O transition at ν̃0 = 2 209.085 430 cm−1. Spectra were recorded at a constant sample pressure of P = 0.93 kPa and at two different sample temperatures of T = 244.6 K (black open circles) and T = 231.2 K (black dots). The respective fitted models30 are plotted as solid blue and solid red lines.
Fitting the interleaved spectra plotted in Fig. 2 using the spectroscopic model described above resulted in 14C mole fractions of χ14C = 0.95 pmol/mol ± 0.08 pmol/mol and χ14C = 0.05 pmol/mol ± 0.08 pmol/mol for the biogenic and petrogenic samples, respectively. The fitted quantity χ14C is related to the fitted frequency integral (peak area) of the measured absorption coefficient for the 14C 16O2 isotopologue. In units of cm−1 the modeled absorption coefficient is: α14C(ν) = χ14CnSTcg(ν) where ST is the 14C 16O2 transition intensity at temperature T, n = P/kBT is the total number density, P is the total sample pressure, c is the speed of light in a vacuum, and g(ν) is the area-normalized Voigt line profile. The 14C 16O2 P 20e transition intensity ST = 3.079×10−18 cm/molecule at T = 220 K was calculated by Zak et al. using ab initio quantum chemistry methods with a relative uncertainty of 1 %.18,22 Sample pressure and temperature were measured at the beginning of each set of 40 spectra using a calibrated pressure gauge and three PRTs in thermal contact with the cold finger, respectively. For example, the fitted spectroscopic temperature of 216.61 K ± 0.19 K for the spectrum in Fig. 2A was in good agreement with the cold finger temperature of 220 K ± 10 K, estimated from the average of the three PRT readouts recorded at the beginning of the scan.
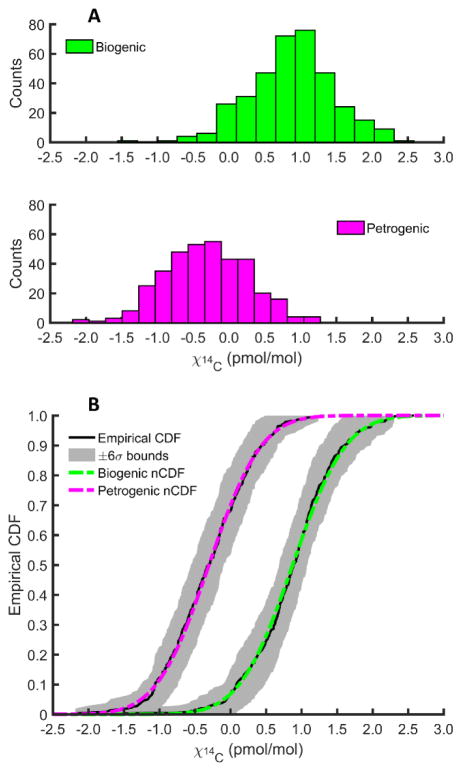
Histograms of repeated measurements of χ14C are plotted in Fig. 4A. Spectra were recorded for different aliquots of the same gas sample (either biogenic or petrogenic) over a period of 14 days. Over that time, we recorded 9 unique data sets (360 measurements of χ14C) each for the biogenic and petrogenic samples. For the biogenic sample, the mean value of all 360 measurements was χ14C = 0.88 pmol/mol with a standard deviation of σ14C = 0.59 pmol/mol. In contrast, the petrogenic sample mean value for 360 measurements was χ14C = −0.30 pmol/mol with σ14C = 0.58 pmol/mol. The reported standard deviations for each large data set represent the statistical precision with which the current instrument can measure χ14C in 70 s of laboratory time.
Fig. 4.
A. Histograms (15 bins each) of all fitted values of the 14C mole fraction (χ14C) for biogenic (green, top panel) and petrogenic (magenta, bottom panel) samples. Each histogram comprises 360 individual fits. B. Empirical cumulative distribution functions (CDFs) are plotted for each sample as solid black lines. The shaded regions capture the ±6σ bounds for each CDF. Assuming a normal distribution of fitted values, the normal CDFs (nCDFs) plotted as dash-dot lines for each sample (biogenic in green, petrogenic in magenta) are in excellent agreement with the empirical CDFs. The normal distributions have characteristic mean values and standard deviations of χ14C = 0.88 pmol/mol ± 0.59 pmol/mol and χ14C = −0.30 pmol/mol ± 0.58 pmol/mol for the biogenic and petrogenic samples, respectively.
The certainty with which repeated measurements can distinguish the two samples from one another is illustrated by the empirical cumulative distribution functions (CDFs) plotted in Fig. 4B. For each sample, an empirical CDF is plotted as a solid black line along with gray shaded regions which represent ±6σ bounds. Overlaid upon each empirical CDF in Fig. 4B is the calculated CDF for a normal distribution with mean and standard deviation values for the biogenic (green dash-dot line) and petrogenic (magenta dash-dot line) samples stated in the preceding paragraph. Given that the measured distributions of χ14C for each sample are well-represented by a normal distribution, we estimate the precision for N scans to equal . For N = 40 scans (≈47 min of laboratory time), σN = 0.09 pmol/mol, in excellent agreement with the fitted uncertainties of 0.08 pmol/mol measured for the representative interleaved spectra plotted in Fig. 2.The total uncertainty budget is presented in Table 2 where all represented quantities are relative standard uncertainties. The statistical uncertainty associated with fitting χ14C (Type A) is tabulated in units of F14C = χ14C/χ14C,mod, where χ14C,mod = 1.176 pmol/mol ± 0.010 pmol/mol.2 The Type B standard uncertainty in χ14C,mod is estimated from the relative uncertainty in the half-life of radiocarbon (0.7 %)31 and in the specific radioactivity of the original standard (oxalic acid I, 0.5 %).32–34 The largest contributors to the total uncertainty budget are the statistical fit uncertainty in χ14C (8.1 %) followed by the uncertainty in the absolute wave numbers of the interfering transitions (7.5 %). The latter was estimated by a weighted variance analysis of changes in χ14C resulting from random shifts in transition frequencies within ±30 MHz of the nominal values listed in Table 1. The remaining sources of uncertainty are relatively small.
Table 2.
Radiocarbon (14C ) uncertainty budget. A sample with F14C = 1 has an absolute 14C mole fraction of χ14C,mod = 1.176 pmol/mol ± 0.010 pmol/mol.
| Parameter | 1σ (%) | Description |
|---|---|---|
| Type A | ||
| F14C | 8.1 | fit uncertainty, σN, N = 40 |
| Type B | ||
| ν̃0 | 7.5 | wave number of spectral interferencesa |
| χN2O | 1.0 | low-T CRDS measurement |
| ST | 1.0 | ab initio transition intensityb |
| t1/2 | 0.7 | half-life of radiocarbonc |
| AOA-I | 0.5 | radioactivity of oxalic acid I (OA-I)d |
| FSR | 0.11 | free spectral range |
| ρ | 0.11 | sample density (T and P) |
| XCO2 | 0.06 | commercial gas cylinder puritye |
| χ18O | 0.04 | 18O mole fraction in CO2f |
| χ13C | 0.03 | FTIR measurement |
|
| ||
| Combined F14C | 11.2 | |
Estimated from the effect of random shifts in transition frequencies on the fitted value of χ14C.
Reference 18.
Reference 32.
Commercial gas cylinder purity was XCO2 ≥99.8 %. For a uniform distribution, we approximate an uncertainy of , where 0.1 % is the distribution half-width.
Estimated assuming a maximum deviation in the 18O mole fraction of 10 %.
Taking the quadrature sum of the individual uncertainties in Table 2 along with the population means from the distributions plotted in Fig. 4, we report optical measurements of F14C for biogenic and petrogenic fuel combustion products of 0.75 ± 0.11 and −0.26 ± 0.11, respectively, in a measurement time of 47 min. An AMS facility provided independent measurements of F14C = 0.86 and F14C = 0.00, respectively. These values have a quoted precision of F14C = 0.03, and are in good agreement with the optical measurements reported here.
Significant improvements to the first-generation instrument will further validate the use of higher-precision linear absorption spectrometers in radiocarbon science. Our current cold cell requires 18 mg C to fill at T = 220 K and P = 0.93 kPa. By replacing the sample volume outside of the cold finger (see Fig. 1B) with an isolating vacuum jacket, we can reduce the sample size approximately tenfold while also reducing heat transfer to the laboratory environment, lowering the sample temperature to T ≤ 180 K and improving overall stability and uniformity. This approach will dramatically reduce spectroscopic interferences8 without sacrificing fractional frequency stability. Fractional frequency stability, already at least a factor of 40 better than previous implementations of 14C -enriched CRDS,14 could be further improved by more than an order-of-magnitude via the inclusion of an optical reference.29 Finally, because the spectroscopic sensitivity of the first-generation instrument is limited by photon shot noise at the photoreceiver,16 high-reflectivity low-loss mirrors made from emerging crystalline coating technology would increase cavity throughput,35 thus further improving instrument performance for the optical detection of 14C .
The quantitative optical detection of radiocarbon (14C ) using linear absorption spectroscopy requires precision measurements of time, frequency, pressure, and temperature. Each of these quantities can be measured relative to the International System of Units (SI), and are therefore traceable to the SI regardless of where, when, or how they were measured.36 As outlined here, the remaining quantity required to report a 14C mole fraction using optical spectroscopy is the line strength of the molecular transition used as a proxy for 14C . Recent ab initio quantum chemistry calculations of CO2 line strengths are in excellent agreement with accurate experiments using gravimetrically prepared standard samples of known CO2 concentration.20–23 The remarkable relative precision of ab initio CO2 line intensities when compared to accurate experiments has motived their inclusion for weak isotopologues of CO2 in the most recent updated to the HITRAN database.22 We therefore argue that optical spectrometers are capable of measuring radiocarbon on an absolute scale, and thus potentially capable of replacing the current artifact-based scale with one that is entirely SI-traceable. The validity of an absolute 14C scale will be determined by further evaluation and intercomparison between high-precision optical instruments and other methods of routine radiocarbon analysis, the results of which could potentially usher in a new era of accurate and traceable radiocarbon metrology.
Acknowledgments
We acknowledge funding from a National Institute of Standards and Technology (NIST) Innovation in Measurement Science award. We also acknowledge comments on the manuscript from Christina Liaskos (NIST) and R. Michael Verkouteren (NIST).
Footnotes
Notes
A.J.F., D.A.L., and J.T.H. are co-inventors on a U.S. Patent Application entitled “Linear Absorption Spectrometer to Optically Determine an Absolute Mole Fraction of Radiocarbon in a Sample” that, in part, includes the scientific results reported in this paper.
References
- 1.Libby WF. Radiocarbon Dating. 2. University of Chicago Press; Chicago: 1955. [Google Scholar]
- 2.Schuur EAG, Druffel ERM, Trumbore SE, editors. Radiocarbon and Climate Change. Springer; Switzerland: 2016. [Google Scholar]
- 3.Cerling TE, Barnette JE, Chesson LA, Douglas-Hamilton I, Gobush KS, Uno KT, Wasser SK, Xu X. Radiocarbon Dating of Seized Ivory Confirms Rapid Decline in African Elephant Populations and Provides Insight into Illegal Trade. Proc Nat Acad Sci. 2016;113:13330–13335. doi: 10.1073/pnas.1614938113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott EM, Cook GT, Naysmith P. The Fifth International Radiocarbon Intercomparison (VIRI): an Assessment of Laboratory Performance in Stage 3. Radiocarbon. 2010;52:859–865. [Google Scholar]
- 5.Miller JB, Lehman S, Wolak C, Turnbull J, Dunn G, Graven H, Keeling R, Meijer HAJ, Aerts-Bijma AT, Palstra SWL, et al. Initial Results of an Intercomparison of AMS-Based Atmospheric 14CO2 Measurements. Radiocarbon. 2013;55:1475–1483. [Google Scholar]
- 6.Hammer S, Friedrich R, Kromer B, Cherkinsky A, Lehman SJ, Meijer HAJ, Nakamura T, Palonen V, Reimer RW, Smith AM, et al. Compatibility of Atmospheric 14CO2 Measurements: Comparing the Heidelberg Low-Level Counting Facility to International Accelerator Mass Spectrometry (AMS) Laboratories. Radiocarbon. 2017;59:875–883. [Google Scholar]
- 7.Galli I, Bartalini S, Borri S, Cancio P, Mazzotti D, De Natale P, Giusfredi G. Molecular Gas Sensing Below Parts Per Trillion: Radiocarbon-Dioxide Optical Detection. Phys Rev Lett. 2011;107:270802. doi: 10.1103/PhysRevLett.107.270802. [DOI] [PubMed] [Google Scholar]
- 8.Galli I, Bartalini S, Ballerini R, Barucci M, Cancio P, De Pas M, Giusfredi G, Mazzotti D, Akikusa N, De Natale P. Spectroscopic Detection of Radiocarbon Dioxide at Parts-Per-Quadrillion Sensitivity. Optica. 2016;3:385–388. [Google Scholar]
- 9.Lehmann KK. Theoretical Detection Limit of Saturated Absorption Cavity Ring-Down Spectroscopy (SCAR) and Two-Photon Absorption Cavity Ring-Down Spectroscopy. Appl Phys B. 2014;116:147–155. [Google Scholar]
- 10.Giusfredi G, Galli I, Mazzotti D, Cancio P, De Natale P. Theory of Saturated-Absorption Cavity Ring-Down: Radiocarbon Dioxide Detection, a Case Study. J Opt Soc Am B. 2015;32:2223–2237. [Google Scholar]
- 11.Sadiek I, Friedrichs G. Saturation Dynamics and Working Limits of Saturated Absorption Cavity Ringdown Spectroscopy. Phys Chem Chem Phys. 2016;18:22978–22989. doi: 10.1039/c6cp01966h. [DOI] [PubMed] [Google Scholar]
- 12.Galli I, Pastor PC, Di Lonardo G, Fusina L, Giusfredi G, Mazzotti D, Tamassia F, De Natale P. The ν3 Band of 14C16O2 Molecule Measured by Optical-Frequency-Comb-Assisted Cavity Ring-Down Spectroscopy. Mol Phys. 2011;109:2267–2272. [Google Scholar]
- 13.Genoud G, Vainio M, Phillips H, Dean J, Merimaa M. Radiocarbon Dioxide Detection Based on Cavity Ring-Down Spectroscopy and a Quantum Cascade Laser. Opt Lett. 2015;40:1342–1345. doi: 10.1364/OL.40.001342. [DOI] [PubMed] [Google Scholar]
- 14.McCartt AD, Ognibene TJ, Bench G, Turteltaub KW. Quantifying Carbon-14 for Biology using Cavity Ring-Down Spectroscopy. Anal Chem. 2016;88:8714–8719. doi: 10.1021/acs.analchem.6b02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCartt AD, Ognibene TJ, Bench G, Turteltaub KW. Model-Based, Closed-Loop Control of PZT Creep for Cavity Ring-Down Spectroscopy. Meas Sci Technol. 2014;25:095201. doi: 10.1088/0957-0233/25/9/095201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long DA, Fleisher AJ, Liu Q, Hodges JT. Ultra-Sensitive Cavity Ring-Down Spectroscopy in the Mid-Infrared Spectral Region. Opt Lett. 2016;41:1612–1615. doi: 10.1364/OL.41.001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleisher AJ, Long DA, Liu Q, Hodges JT. Precision Interferometric Measurements of Mirror Birefringence in High-finesse Optical Resonators. Phys Rev A. 2016;93:013833. doi: 10.1103/physreva.93.013833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zak EJ, Tennyson J, Polyansky OL, Lodi L, Zobov NF, Tashkun SA, Perevalov VI. Room Temperature Line Lists for CO2 Symmetric Isotopologues with ab initio Computed Intensities. J Quant Spectrosc Radiat Transfer. 2017;189:267–280. [Google Scholar]
- 19.Tennyson J. Perspective: Accurate Ro-Vibrational Calculations on Small Molecules. J Chem Phys. 2016;145:120901. doi: 10.1063/1.4962907. [DOI] [PubMed] [Google Scholar]
- 20.Polyansky OL, Bielska K, Ghysels M, Lodi L, Zobov NF, Hodges JT, Tennyson J. High-Accuracy CO2 Line Intensities Determined from Theory and Experiment. Phys Rev Lett. 2015;114:243001. doi: 10.1103/PhysRevLett.114.243001. [DOI] [PubMed] [Google Scholar]
- 21.Odintsova TA, Fasci E, Moretti L, Zak EJ, Polyansky OL, Tennyson J, Gianfrani L, Castrillo A. Highly accurate intensity factors of pure CO2 lines near 2 μm. J Chem Phys. 2017;146:244309. doi: 10.1063/1.4989925. [DOI] [PubMed] [Google Scholar]
- 22.Gordon IE, Rothman LS, Hill C, Kochanov RV, Tan Y, Bernath PF, Birk M, Boudon V, Campargue A, Chance KV, et al. The HITRAN2016 Molecular Spectroscopic Database. J Quant Spectrosc Radiat Transfer. 2017 doi: 10.1016/j.jqsrt.2017.06.038. [DOI] [Google Scholar]
- 23.Hongming L, Liu Q, Fleisher AJ, Hodges JT. High-Accuracy 12C16O2 Line Intensities in the 2 μm Wavelength Region Measured by Frequency-Stabilized Cavity Ring-Down Spectroscopy. doi: 10.1016/j.jqsrt.2017.12.008. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foltynowicz A, Silander I, Axner O. Reduction of Background Signals in Fiber-Based NICE-OHMS. J Opt Soc Am B. 2011;28:2797–2805. [Google Scholar]
- 25.Chen Y, Lehmann KK, Kessler J, Lollar BS, Couloume GL, Onstott TC. Measurement of the 13C/12C of Atmospheric CH4 Using Near-Infrared (NIR) Cavity Ring-Down Spectroscopy. Anal Chem. 2013;85:11250–11257. doi: 10.1021/ac401605s. [DOI] [PubMed] [Google Scholar]
- 26.Ehlers P, Johansson AC, Silander I, Foltynowicz A, Axner O. Use of Etalon-Immune Distances to Reduce the Influence of Background Signals in Frequency-Modulation Spectroscopy and Noise-Immune Cavity-Enhanced Optical Heterodyne Molecular Spectroscopy. J Opt Soc Am B. 2014;31:2938–2945. [Google Scholar]
- 27.Mann D, editor. LNG Materials and Fluids. National Bureau of Standards; Boulder, CO: 1977. [Google Scholar]
- 28.Ghysels M, Liu Q, Fleisher AJ, Hodges JT. A Variable-Temperature Cavity Ring-Down Spectrometer with Application to Line Shape Analysis of CO2 Spectra in the 1600 nm Region. Appl Phys B. 2017;123:124. [Google Scholar]
- 29.Long DA, Cygan A, van Zee RD, Okumura M, Miller CE, Lisak D, Hodges JT. Frequency-Stabilized Cavity Ring-Down Spectroscopy. Chem Phys Lett. 2012;536:1–8. [Google Scholar]
- 30.Rothman LS, Gordon IE, Babikov Y, Barbe A, Benner DC, Bernath PF, Birk M, Bizzocchi L, Boudon V, Brown LR, et al. The HITRAN2012 Molecular Spectroscopic Database. J Quant Spectrosc Radiat Transfer. 2013;130:4–50. [Google Scholar]
- 31.Olsson IU. Radiocarbon Dating History: Early Days, Questions, and Problems Met. Radiocarbon. 2009;51:1–43. [Google Scholar]
- 32.Karlén I, Olsson IU, Kållberg P, Kilicci S. Absolute Determination of the Activity of Two C14 Dating Standards. Arkiv Geofysik. 1964;4:465–471. [Google Scholar]
- 33.Mann WB. An International Reference Material for Radiocarbon Dating. Radiocarbon. 1983;25:519–527. [Google Scholar]
- 34.Stuiver M. International Agreements and the Use of the New Oxalic Acid Standard. Radiocarbon. 1983;25:793–795. [Google Scholar]
- 35.Cole G, Zhang W, Bjork BJ, Follman D, Heu P, Deutsch C, Sonderhouse L, Robinson J, Franz C, Alexandrovski A, et al. High-Performance Near- and Mid-Infrared Crystalline Coatings. Optica. 2016;3:647–656. [Google Scholar]
- 36.Sené M, Gilmore I, Janssen JT. Metrology is Key to Reproducing Results. Nature. 2017;547:397–399. doi: 10.1038/547397a. [DOI] [PubMed] [Google Scholar]