Abstract
Background and Aims
The DSM-IV Personality Disorders (PDs) are comorbid with Alcohol Use Disorder (AUD) and with each other. It remains unclear which PD criteria are most likely to drive onset and recurrence of AUD and which are merely confounded with those criteria. We determine which individual PD criteria predict AUD and the degree of underlying genetic and/or environmental etiology.
Design
A prospective observational twin study.
Setting
Norway 1999–2011.
Participants
Altogether 2528 and 2275 Norwegian adult twins in wave 1 and 2 variable-selection analyses, and 2785 in biometric analyses.
Measurements
DSM-IV PDs and their 80 criteria were assessed using a structured personal interview, and AUD using WHO’s Composite International Diagnostic Interview.
Findings
In a variable-selection analysis, two PD criteria were associated with AUD even after taking all the other criteria into account: criterion #8 of antisocial PD (childhood conduct disorder) and criterion #4 of borderline PD (self-damaging impulsive behaviors). Adjusting for each other, their respective odds ratios were 3.4 (CI = 2.1–5.4) and 5.0 (CI = 3.3–7.7). Endorsement strength of the criteria was associated with AUD in a dose-response manner and they explained 5.5% of variation in AUD risk—more than the full diagnoses of antisocial and borderline PDs together (0.5%). The association between borderline criterion #4 and AUD 10 years later derived mainly from their overlapping genetic factors, whereas the association between antisocial criterion #8 and AUD 10 years later was due to both genetic and non-genetic factors.
Conclusions
Conduct disorder and self-harming impulsivity are the foremost risk traits for alcohol use disorder (AUD) among the 80 personality disorder criteria of DSM-IV, predicting AUD better than personality disorder diagnoses. The twin-study analysis suggested that conduct disorder represents a joint genetic and developmental risk for alcohol use disorder and that impulsivity is a genetic risk.
Introduction
Many DSM-IV and −5 personality disorders (PDs) reflect problems of emotion regulation and/or impulse control, which may make individuals with PD diagnoses particularly vulnerable to over-use alcohol, and consequently, to develop an alcohol use disorder (AUD). Indeed, all the ten PDs are associated with alcohol dependency, and 42% of all PD patients meet the criteria for lifetime alcohol dependency [1]. However, the co-occurrence of PDs themselves is high, implying that all the PDs might not have an independent association with AUD [2]. In epidemiologic surveys, a PD might just indicate an increased likelihood of having another PD which could be ‘causally’ linked with AUD. Furthermore, many researchers argue that PDs should be conceptualized differently than in the DSM-IV [3–6]. Therefore, it is possible that any PDs associated with AUD merely reflect an increased likelihood of having specific symptoms or behaviors associated with AUD. To date, it has not been clarified which of the 80 criteria used to diagnose the 10 categorical PDs are the most robust correlates of AUD. Two factors complicate the analysis of PD traits in relation to AUD: “curse of dimensionality” and statistical confounding. In this study, we simultaneously address both these problems.
The curse of dimensionality refers to the fact that difficulties in statistical analysis increase exponentially rather than linearly as a function of the number of variables, often precluding use of classic methods in multivariate settings [7]. The fact that there are many more individual criteria than there are composite PDs they combine to (80 vs. 10) exposes the analyst to the “curse of dimensionality”. Recent modifications of the classic methods have attempted to bring high-dimensional datasets into a more manageable and workable framework. Here we specifically make use of the Elastic Net (EN) regression method that modifies the more commonly applied regression methods [8–10]. In addition to its good predictive performance, EN regression offers a competitive alternative to the widely criticized stepwise-regression variable selection methods [11,12]. While EN regression allows us to take in account potential confounding among the PD criteria, a twin design is used to assess possible confounded etiologic mechanisms.
A meta-analysis of family and adoption studies reported an “upper limit” of 30–36% for the heritability of alcohol misuse, whereas another meta-analysis reported a heritability point estimate of 50% for AUD, and a study correcting for measurement errors reported an estimate of 71% heritability for men [13–15]. Despite these varying estimates, it seems that most would agree there is substantial heritability in AUD. PDs have also been shown to be influenced by genetic factors, with heritability estimates ranging from 21% to 77% [16], meaning that associations between AUD and PDs may be confounded by overlapping genetic background. Especially Antisocial (ASPD) and Borderline (BPD) Personality Disorders have been found to be associated with alcohol use problems [17–20]. The heritable variation of ASPD and BPD overlap with each other and also with alcohol use problems [21–24]. The heritable variation in these PDs also overlap with broad dimensions of personality disorders in other emerging models [25]. However, broad personality dimensions typically fail to capture all genetic and outcome-predictive variance available at the criterion level [26–30].
While broad personality dimensions are very useful for many purposes, they are not a complete solution to the curse of dimensionality. Some previous analyses have been statistically biased in favor of broad dimensions [30], but currently the psychopathology field is moving toward broad-to-narrow hierarchical representations [6]. Proponents of these emerging models point out that “complete symptom-level data are rarely available” and that this lack of data is the primary reason for scarcity of criterion-/symptom-level studies [6]. Thus, although the present study does not specifically concentrate on old versus emerging models of psychiatric nosology, it may be useful for research both on alcohol and on nosology as we analyze “complete symptom-level data” on all PDs currently in clinical use.
The aims of this study are to establish (i) which individual PD criteria predict AUD even after the other (correlated) criteria have been taken into account and (ii) whether these criteria predict AUD better or worse than the composite PDs they belong to. Finally, to point towards directions for research on causation and treatment, it is established (iii) whether the detected associations between the selected criteria and AUD are mediated by genetic or environmental factors.
Methods
Design
A two-wave follow up of a population-based sample of twins was studied. To answer aims i-iii, we first conducted a cross-sectional variable-selection analysis to determine which individual PD criteria predict AUD, while using the second wave as a partial replication. Second, we compared the predictive performance of the selected variables in the larger wave 1 sample against DSM-IV diagnostic definitions. Third, we used both longitudinal and genetic information to study whether or not the selected variables predict future AUD, and for what reasons.
Sample
We analyze a population-based sample of Norwegian twins recruited from the Norwegian Institute of Health Twin Panel [31]. Approval for this study was received from The Norwegian Data Inspectorate and the Regional Committee for Medical and Health Research Ethics, and written informed consent was obtained from all participants after a complete description of the study. The sample has been used in many previous investigations [e.g., 24,32]. In wave 1, lifetime history of major DSM-IV Axis I disorders and all the 10 Axis II PDs in past 5 years were assessed at interview in 2801 twins (43.5% of those who were eligible; 1390 complete twin pairs and 21 single twins), between the years 1999 and 2004. Their mean age was 28.2 years and age range 19 to 36. A previous analysis of attrition bias indicated that, despite moderate selection towards good mental and somatic health, attrition does not appear to affect twin analyses of mental health [33]. In wave 2, altogether 2284 twins (987 complete pairs and 310 single twins) were re-interviewed approximately 10 years later. Attrition from 1st to 2nd wave was low (82.2% were retained), and a complete breakdown of twin pairs indicated that attrition is unlikely to affect analyses on pertinent PDs [24].
Procedures
The interviewers were senior graduate students in their final part of training or experienced psychiatric nurses or psychologists, all trained and closely followed by professionals who had extensive previous experience with the instrument. Most of the PD interviews in wave 1 were conducted face to face, but for practical reasons, 231 (8.3%) were obtained by telephone. All wave 2 interviews were conducted by telephone. Each twin in a pair was interviewed by a different interviewer. Only few Axis I disorders (major depression, anxiety, and alcohol use) and 6 out of the 10 PDs were assessed in the second wave (paranoid, schizotypal, antisocial, borderline, avoidant, and obsessive-compulsive PDs).
Measures
Mono- (MZ) versus dizygosity (DZ) of twins was determined by a combination of questionnaire items and genotyping, resulting in a less than 1% miss-classification rate, which is unlikely to substantially bias results [34]. Our biometric analyses used data from the both waves in the same analysis. Only 16 twin pairs lacked all data relevant to biometric analyses and were excluded, plus one case with a recorded negative age of alcohol use onset. The 433 pairs with some information missing from either wave were retained in full-information maximum likelihood analyses [35], totaling in 1394 available pairs (Table 1 footnote for breakdown by zygosity and sex).
Table 1.
Descriptive frequencies and prevalence of key variables in wave 1 (approx. ages 19–36) and wave 2 (approx. ages 30–46).
| Variable (Wave 1) | Not present/No | Sub-threshold | Present/Yes | Strongly present |
|---|---|---|---|---|
| Male | 1603 (63.4%) | – | 925 (36.6%) | – |
| Alcohol use disorder (AUD) | 2281 (90.2%) | – | 247 (9.8%) | – |
| AUD within past 5 years† | 2603 (93.8%) | – | 173 (6.2%) | – |
| ASPD‡ | 2770 (99.7%) | – | 9 (0.3%) | – |
| BPD‡ | 2768 (99.6%) | – | 11 (0.3%) | – |
| ASPD CD (conduct disorder)* | 2141 (84.7%) | 283 (11.2%) | 77 (3.0%) | 27 (1.1%) |
| BPD 4 (impulsivity)* | 2048 (81.0%) | 362 (14.3%) | 90 (3.6%) | 28 (1.1%) |
| ASPD 1 (non-conforming)* | 2315 (91.6%) | 163 (6.4%) | 33 (1.3%) | 17 (0.7%) |
| Variable (Wave 2) | Not present/No | Sub-threshold | Present/Yes | Strongly present |
| Male | 1476 (64.9%) | – | 799 (35.1%) | – |
| Alcohol use disorder (AUD) | 1985 (87.2%) | – | 291 (12.8%) | – |
| AUD within past 5 years† | 2169 (95.4%) | – | 105 (4.6%) | – |
| ASPD CD (conduct disorder)* | 2022 (88.8%) | 202 (8.9%) | 36 (1.6%) | 16 (0.7%) |
| BPD 4 (impulsivity)* | 2050 (90.1%) | 153 (6.7%) | 57 (2.5%) | 16 (0.7%) |
| ASPD 1 (non-conforming)* | 2182 (95.9%) | 66 (2.9%) | 17 (0.7%) | 11 (0.5%) |
= variable used only in aim iii (biometric analyses). Full-information maximum likelihood allowed use of partially missing vectors of observations. Complete breakdown of twin pairs in wave 1 by zygosity and gender was: 224 male and 447 female monozygotic (MZ) twin pairs, 119 male and 263 female dizygotic (DZ) twins, and 340 separate-sex DZ twins.
= Antisocial (ASPD) and borderline personality disorder (BPD) diagnoses used in aims ii and iii
= Individual criteria used in all aims i–iii. These criteria are further discussed in the text, but see Supplementary Table S1 for descriptive data on all the 80 PD criteria. Altogether 2528 participants had full PD and AUD data in wave 1 and 2275 in wave 2.
PDs were assessed using a Norwegian version of the Structured Interview for DSM-IV Personality [36]. It is a comprehensive semi-structured interview of all DSM-IV Axis II diagnoses, rating the specific DSM-IV criteria according to following coding: 0 = not present or limited to rare isolated examples; 1 = subthreshold (some evidence of the trait, but not sufficiently pervasive for the criterion to be considered present); 2 = present (criterion clearly present for most of the time during last 5 years); 3 = strongly present (associated with subjective distress or some impairment in social or occupational functioning or intimate relationships). All 80 criteria were assessed without skip rules. Inter-rater reliability was assessed based on 2 raters’ scoring of 70 audiotaped wave 2 interviews: intra-class correlations for the number of endorsed PD criteria within disorder ranged from 0.81 to 0.96.
The outcome variable was DSM-IV alcohol dependence and/or alcohol use disorder, here jointly referred to as “alcohol use disorder” (AUD; notice that these two diagnoses have been merged in DSM-5). AUD was assessed using computerized Norwegian version of World Health Organization’s Composite International Diagnostic Interview [37]. The Munich version that it is based on has an excellent test-retest reliability for AUD [38]. The interviewers attended a standardized CIDI training programme administered by teachers certified by the World Health Organization, and were closely followed-up individually during the whole data collection period. Exclusion criteria were not used. As specific outcomes, we study presence of a lifetime AUD diagnosis (failures to recall wave 1 AUD in wave 2 were corrected for), age of AUD onset, and presence of AUD within 5-year recency (i.e., current age minus the age at most recent episode was no more than five years).
Data analyses
The applied statistical approach was designed to answer the study aims (i–iii). First (i), Elastic Net (EN) regression models were used to identify and select the specific PD criteria that were the best predictors of lifetime AUD (Logistic model) over and above the other criteria [8–10]. The EN method is able to use many predictor variables and set regression coefficients of unnecessary variables to zero, automatically selecting them out from the model. It was designed to improve the performance of classic regression methods when the number of predictor variables is large. To examine the robustness of the findings, the variable-selection analysis was replicated using age of AUD onset (Cox’s proportional-hazards model) as the outcome, and both the outcomes were further analyzed in the wave 2 data using all the available PD information (totaling 4 partial replications). EN models were estimated using “glmnet” R package, version 2.0–5. The supplementary material provides more details on EN regression.
After selecting the PD criteria most predictive for AUD, we (ii) compared their performance to that of the composite PDs they are a part of (i.e., diagnoses) to demonstrate their specific relevance. To this end, we determined which one is more strongly associated with AUD, the broad DSM-IV disorders (Dx model) or their individual criteria (Sx model) in terms of generalized coefficient of determination (R2) and area under the receiver-operating characteristics curve (AUC) [7,39]. Standard (non-EN) Logistic regression models were used as implemented in the statistical R software, version 3.2.2. [40].
A PD criterion may predict cross-sectional AUD variance because of shared genetic factors or because of correlated environmental influences, and may or may not be predictive of future changes in individual differences. To better understand the underlying causal factors, (iii) we partitioned both cross-sectional and longitudinal associations between the selected PD criteria and AUD into their (additive) genetic and (shared and non-shared) environmental components using the biometric “ACE” model [41]. This is a structural equation model that partitions a between- and within-twin covariance matrix into additive genetic covariance (A), shared environmental covariance (C) that makes the twins similar with each other, and non-shared environmental covariance (E) that makes the twins different (E variance includes measurement error).
Biometric models were fit using “Open Mx” package, version 3.2.2, for R software [42], and estimated using full information maximum likelihood [35] and a threshold-liability model, which models ordinal categories as arising from estimated thresholds on an underlying normal distribution [41]. For this purpose, the second and third categories of PD criteria were collapsed to avoid empty or rarely endorsed cells in the three-way tables; similarly, criterion counts of 3 or more were collapsed when studying liabilities for composite PDs. We then estimated associations among a wave 1 PD-related variable, wave 1 AUD (5-year recency), and wave 2 AUD (5-year recency) for all the covariance components, A, C, and E using Cholesky decompositions (see Figure 1a for a path diagram of one component) [41]. It is often found that the C component is not statistically significant, leading to an “AE” model. We selected between ACE and AE models using Akaike’s Information Criterion [43]. For individual biometric parameter estimates, likelihood-ratio tests and likelihood-based 95% Confidence Intervals (CIs) were assessed [44].
Figure 1.
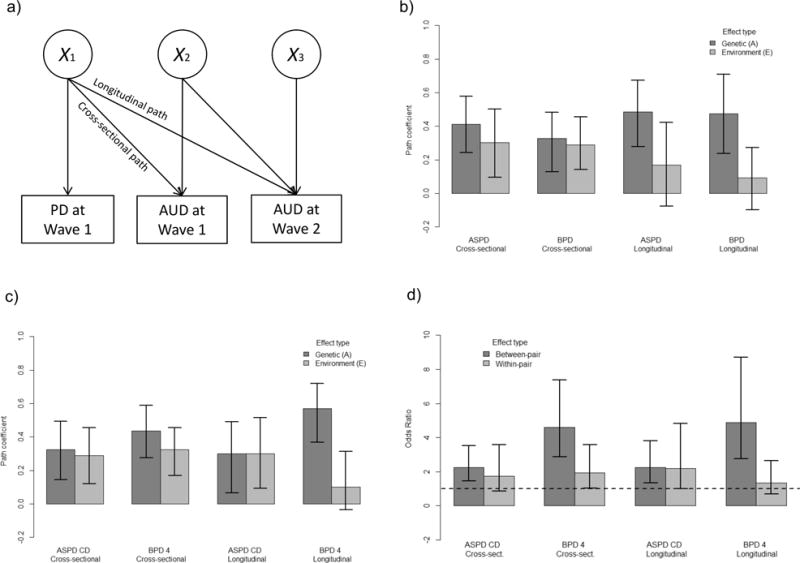
Biometric analyses. a) Illustration of a Cholesky decomposition. Variance in right-hand observed variables (boxes) is partitioned to that due to the left-hand variables plus unique variance due to unobserved causes (circles). For example, a ‘path’ (or regression) coefficient between Personality Disorder (PD), or its criterion, at wave 1 and Alcohol Use Disorder (AUD) at wave 2 is estimated (“longitudinal path”), while also estimating and controlling for the baseline association between the PD and AUD at wave 1 (“cross-sectional path”). Twin design allows estimating the decomposition simultaneously for both additive genetic (A) and environmental (E) covariance. b) Biometric path coefficients and 95% confidence intervals for composite PD criterion counts. c) Biometric path coefficients and 95% confidence intervals for selected PD criteria. d) Between- and within-pair odds ratios in genetically identical individuals (i.e., monozygotic twins).
A major motivation for the genetic analysis is to get information about the causal mechanisms behind an observed association between an exposure of interest and its presumed outcome. When designing interventions, it is important to understand whether an association is due to shared genetic factors or due to some sequences of developmental events. Covariation between an exposure and an outcome captured in the “E” component of an ACE model is closely related to results from “co-twin control” analysis, because the additive genetic effects and the family environment have been controlled for [45–47]. The logic of co-twin control is that if an exposure difference between genetically identical MZ twins is associated with a difference in an outcome, then the observed association is likely to be environmentally mediated because there is no genetic variance to explain it. Thus, ACE modeling results carry implications about environmental causation or lack of it. While the ACE model is effective in using both MZ and DZ twins, it is blind for non-additive genetic effects. That is why we also provide the within- and between-MZ-twin pair logistic regression coefficients. In such an analysis, dependent errors across the twin pairs need to be modelled, for example, using generalized estimating equations [46,48]. We implemented Generalized estimating equations using “geepack” R package, version 1.2–0.1. Since the MZ twins share all their genes, this analysis does take into account also the possible non-additive genetic effects.
Results
Table 1 gives sample prevalence of key variables in what follows (Supplementary Table S1 for all the PD criteria).
Associations between the PD criteria on AUD
The main findings from the EN analyses were the following: All the other analyses (3 out of 4) excluding the age of onset analysis of the wave 2 data robustly detected the childhood conduct disorder (criterion #8 from ASPD diagnosis; referred to as ASPD CD) and the criterion #4 from the BPD diagnosis (self-damaging impulsive behaviors; referred to as BPD 4) as significant predictors of AUD over and above the other traits. The other 78 PD criteria were often selected out from the optimal predictive models. The below-reported criterion-level analyses, therefore, concentrated on these two PD criteria (see supplementary section 2.2 for tuning of EN regression models and section 2.3.1 for regression coefficients). Of note, the importance of Obsessive-Compulsive PD criterion #6 (reluctance to delegate tasks unless done in one’s exact way of doing things) may increase in late adulthood (Supplementary Tables S2 and S3). In a sensitivity analysis, we removed BPD 4 from the predictor set due to potential construct overlap with AUD. As shown in Table S3, criterion #1 of ASPD was then the other robust predictor besides the conduct disorder (failure to conform to social norms with respect to lawful behavior, referred to as ASPD 1; see Supplementary section 2.3.2 for more details).
Performance of the selected PD criteria versus diagnoses in predicting AUD
Based on the above results, we next studied predictive regression models with either ‘sex and ASPD and BPD diagnoses (Dx model, Model I in Table 2)’ or ‘sex and the ASPD CD and BPD 4 criteria (Model II for clear presence of criteria, Model III for their dummy-coded ordinal-valued severity levels)’ as the independent/predictive variables. Table 2 shows that the levels of the selected criteria displayed a clear dose-response relationship with AUD risk (less clear when replacing BPD4 with ASPD 1, Supplementary Table S4). A typical interpretation of the model-performance indices would be a “fair” performance for the Sx model and “poor” for the Dx model (Table 2). In part, the Dx model performed badly because of the low endorsement rates of ASPD and BPD in comparison to AUD, but the Sx model also explained more variance than a model using sum scores of ASPD and BPD criteria (ΔR2 = 0.055 vs. 0.039).
Table 2.
Three alternative Logistic multiple regression models predicting alcohol use disorder at Wave 1 (ages 19–36, N = 2779)
| Model performance | Model parameters | ||||
|---|---|---|---|---|---|
| Model | Independent variable | β | OR | 95% CI | |
| Number (name) | I (Dx) | Intercept | −2.863 | – | – |
| Generalized ΔR2 | 0.005 | Male sex | 1.189 | 3.284 | 2.5–4.3 |
| Area under ROC | 0.652 | ASPD | 1.932 | 6.904 | 1.7–29.1 |
| BPD | 2.124 | 8.363 | 2.0–29.3 | ||
| Number (name) | II (Sx present) | Intercept | −2.973 | – | – |
| Generalized ΔR2 | 0.033 | Male sex | 1.008 | 2.741 | 2.0–3.6 |
| Area under ROC | 0.719 | ASPD CD criterion ≥ 2 | 1.233 | 3.432 | 2.1–5.4 |
| BPD4 criterion ≥ 2 | 1.617 | 5.038 | 3.3–7.7 | ||
| Number (name) | III (Sx) | Intercept | −3.253 | – | – |
| Generalized ΔR2 | 0.055 | Male sex | 0.868 | 2.382 | 1.8–3.2 |
| Area under ROC | 0.767 | ASPD CD criterion = 1 | 0.649 | 1.913 | 1.3–2.7 |
| ASPD CD criterion = 2 | 1.014 | 2.755 | 1.6–4.7 | ||
| ASPD CD criterion = 3 | 1.651 | 5.209 | 2.1–12.7 | ||
| BPD4 criterion = 1 | 1.058 | 2.880 | 2.1–4.0 | ||
| BPD4 criterion = 2 | 1.695 | 5.448 | 3.3–8.9 | ||
| BPD4 criterion = 3 | 2.006 | 7.430 | 3.1–17.7 | ||
Abbreviations: “Dx” = diagnoses-based model (Model I); “Sx” = symptom-/criterion-based model (Model II for diagnostic threshold, Model III for dummy-coded ordinal gradations); “ΔR2” = change in coefficient of determination in comparison to model with intercept and sex covariates only; “ROC” = Receiver operating characteristic curve; “β” = regression coefficient; “OR” = odds ratio (natural exponent of β); “95% CI” = confidence interval for the odds ratio; “ASPD” = antisocial personality disorder; “BPD” = borderline personality disorder; “ASPD CD” = conduct disorder criterion of ASPD; “BPD4” = criterion #4 of BPD (impulsivity). Individual DSM-IV criteria rated according to following guidelines: 0 = not present or limited to rare isolated examples (reference category); 1 = subthreshold; 2 = present; 3 = strongly present. Adding ASPD and BPD variables did not lead to significant improvement in Model II (χ2 = 1.75, d.f. = 2, p = 0.418) or Model III (χ2 = 1.07, d.f. = 2, p = 0.586).
An analysis of genetic and environmental influences
To better understand causation, the associations between PDs and AUD and those between PD traits and AUD were partitioned according to genetic and environmental factors. For this purpose, AE models were favored over ACE models (Supplement, section 4) and are thus presented. Figure 1b shows the estimated cross-sectional and longitudinal biometric path coefficients from modeled liabilities for ASPD and BPD to that of AUD (see Figure 1a for the path diagram). Consistent genetic effects, as well as cross-sectional environmental paths, were found, but the longitudinal environmental paths did not differ from zero statistically. The difference between the genetic and environmental longitudinal paths was more clear for BPD (χ2 = 4.35, d.f. = 1, p = 0.037) than for ASPD (χ2 = 2.12, p = 0.145).
At the criterion level, ASPD CD had equally strong genetic and environmental longitudinal effects (χ2 < 0.001, d.f. = 1, p = 0.999), whereas these differed for BPD 4 (χ2 = 5.39, d.f. = 1, p = 0.020), the environmental component being negligible in the latter case (Figure 1c). While the AE models made use of all the twin data, they neglected possible non-additive genetic effects. Therefore, we re-examined the possibility of genetic confounding by studying within-twin-pair effects in the genetically identical MZ twins. Qualitatively similar conclusions were recovered, indicating that the findings were robust with respect to the assumptions on additivity of genetic effects and threshold-liability model (Figure 1d). The “ASPD 1” criterion that emerged in the sensitivity analysis without BPD 4 shared characteristics from both BPD 4 and ASPD CD (Supplement, section 3).
Discussion
Previous studies have indicated that the 10 DSM-5 PDs are associated with AUD, especially ASPD and BPD [1,17,18,20,23]. Our results add to the literature suggesting that (i) the criterion #8 of ASPD (ASPD CD; i.e., childhood conduct disorder), and the criterion #4 of BPD (BPD 4; self-harming impulsive behaviors) are the foremost correlates of AUD among all the 80 criteria for all the 10 PDs, and (ii) that predictive models using just these two criteria superseded those using the full ASPD and BPD diagnoses. Furthermore, it was found that (iii) the two criteria may have distinct biometric structure as risk factors of future AUD: BPD 4 predicted future AUD over the 5–15 years (10-year follow up and 5-year diagnostic window) mainly due to their shared genetic factors, whereas ASPD CD had both genetic and environmentally mediated effects on future AUD.
According to DSM-5, ASPD diagnosis can be given when “[t]here is evidence of conduct disorder with onset before age 15 years”. Conduct disorder is characterized by minimum of three items related to aggression to people and animals, destruction of property, deceitfulness or theft, and/or serious violations of rules. The BPD diagnosis requires at least five criteria out of nine being fulfilled, one of which can (but does not need to) be the criterion “BPD 4” worded as “[i]mpulsivity in at least two areas that are potentially self-damaging (e.g., spending, sex, substance abuse, reckless driving, binge eating)”, and not including “suicidal or self-mutilating behavior covered in Criterion 5”. However, ~67% of variance in the impulsivity criterion was independent of overall BPD in a previous factor analytic study of the same sample [28], and a psychometric analysis indicated differential age and sex moderation of the impulsivity criterion in comparison to overall BPD [26].
Other investigations have also highlighted childhood conduct disorder and facets of impulsivity as central predictors of future AUD in adolescents, along with other genetic, neuropsychological, and neural predictors [49,50]. In general, impulsive behaviors are well-known risk factors for AUD [51]. However, “impulsivity” is not a simple construct and many different, but widely used, measures of impulsivity are not strongly correlated with each other [51,52]. Also many of the 80 PD criteria describe behaviors that could feasibly be characterized as being “related to impulsivity”, but only two of them stood out from the mass when predicting AUD. For example, criterion #3 of ASPD, titled “impulsivity or failure to plan ahead”, was frequently selected out from the EN models in favor of the other two criteria (Supplement, section 2.3). Similarly, laboratory tasks related to impulsive choice rather than impulsive action are associated with AUD [53]. Behavioral ‘laboratory’ tasks are highly heritable and promising intermediate phenotypes for “genetic dissection of impulsivity and externalizing spectrum” [54], including PDs. Regarding such tasks, impulsivity in BPD patients has been characterized as choice- and reward-related rather than motor/action impulsivity [55]. The “self-harming impulsivity” criterion (BPD 4) also overlaps with failures to conform to social norms as defined in the first ASPD criterion (Supplement, sections 2.3.2 and 4). Conduct disorder, on the other hand, is associated both with choice-/reward-related impulsivity [56] and with poor motor inhibition [57].
We also obtained tentative evidence that reluctance to delegate tasks and works may emerge as a middle-age AUD risk factor. However, despite much work on comorbidity of social anxiety and AUD, anxious or fearful traits were not highlighted by the present explorative analysis. Previous findings have been conflicting, suggesting differing effects across population sub-groups [58]. Thus, it may be possible to find groups of individuals that reveal links between anxious traits and AUD. On the other hand, all PDs have associations with AUD and many studies do not adjust for comorbid PD traits [1], leaving open the possibility that statistical confounding explains the findings. This study did control for possible confounding due to all correlated PD traits.
By controlling environmental factors for genetic confounding (and vice versa) and longitudinal effects for baseline variations, our study design also allows certain etiologic inferences. Cross-sectional correlation between BPD 4 and AUD was partly driven by shared environmental factors, but when controlling for baseline value, BPD 4 predicted future AUD only through shared genetic factors. In contrast, childhood conduct disorder implied both current and future risk due to both shared environmental and shared genetic factors. This suggests that while shared environmental factors may link acute AUD with other self-harming impulsive behaviors in short term, their long-term (~10-year) association appears to derive from shared genetic liabilities. In contrast to self-harming impulsive behaviors, environmental factors influencing childhood conduct disorder may predispose to a life course where accumulating life events (environment) keep increasing or sustaining the future risk of AUD.
The robust association between childhood conduct disorder and adulthood AUD is in accordance with findings indicating that those who initiate alcohol drinking at early age are at much-elevated risk for developing AUD [59]. However, a recent study found that this is mainly because early age of initiation is a major indicator of genetic risk [32]. This genetic liability is partially shared with childhood conduct disorder [60], which mediates the effects of attention deficit hyperactivity disorder on AUD, for example [61]. But in addition to genetic factors, environmental factors, such as peer deviance and coercive parent-child interactions, affect the development of conduct disorder [62,63], and the present study suggests that these factors also increase AUD risk in adulthood. Therefore, preventive efforts targeting environmental risk factors of childhood conduct disorder might reduce adulthood AUD by interrupting ‘vicious developmental circles’. In contrast, containment of the genetically influenced tendency towards self-harming impulsive behaviors, including alcohol abuse, may require more systematic, life-long efforts.
Limitations
The following important limitations apply to the present investigation. First, impulsivity as defined by the BPD 4 criterion, requires at least two self-harming and recurring behaviors, one of which can be alcohol intoxications and the other an illicit substance use. When studying substance use, it would help to be able to better distinguish specific “impulsive” behaviors both from substance use and from each other [51]. However, the criterion BPD 4 should not be excluded merely based on potential construct overlap with AUD because it predicted future AUD over and above present AUD (Figure 1c; the online supplement provides a full sensitivity analysis without the BPD 4 criterion). Second, although conduct disorder is a single criterion for adulthood ASPD, it is also a diagnostic composite of multiple conduct problems that may have distinct etiologies; for example, “rule breaking”, “overt aggression”, and “covert delinquency” factors have been reported [64]. Thus, future studies could inquire whether this composite can be further broken down to its constituents or whether the use of multiple indicators is the source of its predictive power. Third, the sample consisted of young adult Norwegians and generalizations to other populations or age groups should take appropriate caution. Fourth, we cannot exclude the possibility that study attrition affects the results to some extent, but attrition analyses indicate it is highly unlikely that the effect is large [24,33]. Of the 10 PDs, only ASPD and narcissistic PD predicted participation in wave 2, with non-participants having 0.09 sub-threshold criteria more than the participants; total number of Axis I disorders or any specific disorder were not predictive [24]. In addition, wave 2 lacked data for some of the PDs and included the same participants as in wave 1, meaning that only partial rather than full replications were attainable in variable selection. Fifth, even larger samples may reveal further AUD-predictive PD criteria with smaller effects. However, we believe the present findings are useful in pinpointing two especially salient criteria among the 80 potentially relevant behavioral criteria. Finally, while we demonstrated the AUD predictive value of the selected criteria in comparison to the PD diagnoses currently in use, future studies could extend the comparison to the emerging PD models [6,25].
Supplementary Material
Acknowledgments
We acknowledge funding from the US National Institutes of Health and National Institute on Drug Abuse (1R01DA037558-01A1), the Research Council of Norway (226985), the Norwegian Foundation for Health and Rehabilitation, the Norwegian Council for Mental Health, and the European Commission under the program “Quality of Life and Management of the Living Resources” of the Fifth Framework Program (QLG2-CT-2002-01254). THR had full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of competing interests: All authors declare no conflict of interest
References
- 1.Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: Gender, prevalence, and comorbidity with substance dependence disorders. J Personal Disord. 2010;24(4):412–26. doi: 10.1521/pedi.2010.24.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ. Co-occurrence of DSM-IV personality disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Compr Psychiatry. 2005;46(1):1–5. doi: 10.1016/j.comppsych.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Krueger RF, Markon KE. The role of the DSM-5 personality trait model in moving toward a quantitative and empirically based approach to classifying personality and psychopathology. Annu Rev Clin Psychol. 2014;10:477–501. doi: 10.1146/annurev-clinpsy-032813-153732. [DOI] [PubMed] [Google Scholar]
- 4.Cramer AOJ, van der Sluis S, Noordhof A, Wichers M, Geschwind N, Aggen SH, et al. Dimensions of normal personality as networks in search of equilibrium: You can’t like parties if you don’t like people. Eur J Personal. 2012;26(4):414–31. [Google Scholar]
- 5.Morey LC, Hopwood CJ, Markowitz JC, Gunderson JG, Grilo CM, McGlashan TH, et al. Comparison of alternative models for personality disorders, II: 6-, 8- and 10-year follow-up. Psychol Med. 2012;42(8):1705–13. doi: 10.1017/S0033291711002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, et al. The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. J Abnorm Psychol. 2017;126(4):454–77. doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- 7.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2nd. New York, USA: Springer-Verlag; 2009. [Google Scholar]
- 8.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B Stat Methodol. 2005;67(2):301–20. [Google Scholar]
- 9.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 10.Simon N, Friedman JH, Hastie T, Tibshirani R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J Stat Softw. 2011;39(5):1–13. doi: 10.18637/jss.v039.i05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mundry R, Nunn CL. Stepwise model fitting and statistical inference: Turning noise into signal pollution. Am Nat. 2009;173(1):119–23. doi: 10.1086/593303. [DOI] [PubMed] [Google Scholar]
- 12.Morozova O, Levina O, Uusküla A, Heimer R. Comparison of subset selection methods in linear regression in the context of health-related quality of life and substance abuse in Russia. BMC Med Res Methodol. 2015;15:71. doi: 10.1186/s12874-015-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walters GD. The heritability of alcohol abuse and dependence: a meta-analysis of behavior genetic research. Am J Drug Alcohol Abuse. 2002;28(3):557–84. doi: 10.1081/ada-120006742. [DOI] [PubMed] [Google Scholar]
- 14.Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med. 2015;45(05):1061–1072. doi: 10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ystrom E, Reichborn-Kjennerud T, Aggen SH, Kendler KS. Alcohol dependence in men: reliability and heritability. Alcohol Clin Exp Res. 2011;35(9):1716–22. doi: 10.1111/j.1530-0277.2011.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichborn-Kjennerud T. The genetic epidemiology of personality disorders. Dialogues Clin Neurosci. 2010;12(1):103–14. doi: 10.31887/DCNS.2010.12.1/trkjennerud. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein RB, Dawson DA, Saha TD, Ruan WJ, Compton WM, Grant BF. Antisocial behavioral syndromes and DSM-IV alcohol use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Clin Exp Res. 2007;31(5):814–28. doi: 10.1111/j.1530-0277.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- 19.Walter M, Gunderson JG, Zanarini MC, Sanislow CA, Grilo CM, McGlashan TH, et al. New onsets of substance use disorders in borderline personality disorder over 7 years of follow-ups: findings from the Collaborative Longitudinal Personality Disorders Study. Addiction. 2009;104(1):97–103. doi: 10.1111/j.1360-0443.2008.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long EC, Aggen SH, Neale MC, Knudsen GP, Krueger RF, South SC, et al. The association between personality disorders with alcohol use and misuse: A population-based twin study. Drug Alcohol Depend. 2017;174:171–80. doi: 10.1016/j.drugalcdep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrieze SI, McGue M, Miller MB, Hicks BM, Iacono WG. Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: Twin biometry, GCTA, and genome-wide scoring. Behav Genet. 2013;43(2):97–107. doi: 10.1007/s10519-013-9584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu Q, Heath AC, Bucholz KK, Nelson E, Goldberg J, Lyons MJ, et al. Shared genetic risk of major depression, alcohol dependence, and marijuana dependence: contribution of antisocial personality disorder in men. Arch Gen Psychiatry. 2002;59(12):1125–32. doi: 10.1001/archpsyc.59.12.1125. [DOI] [PubMed] [Google Scholar]
- 23.Few LR, Grant JD, Trull TJ, Statham DJ, Martin NG, Lynskey MT, et al. Genetic variation in personality traits explains genetic overlap between borderline personality features and substance use disorders. Addiction. 2014;109(12):2118–27. doi: 10.1111/add.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichborn-Kjennerud T, Czajkowski N, Ystrøm E, Ørstavik R, Aggen SH, Tambs K, et al. A longitudinal twin study of borderline and antisocial personality disorder traits in early to middle adulthood. Psychol Med. 2015;45(14):3121–31. doi: 10.1017/S0033291715001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichborn-Kjennerud T, Krueger RF, Ystrom E, Torvik FA, Rosenström TH, Aggen SH, et al. Do DSM-5 Section II personality disorders and Section III personality trait domains reflect the same genetic and environmental risk factors? Psychol Med. 2017 doi: 10.1017/S0033291717000824. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Aggen SH, Neale MC, Røysamb E, Reichborn-Kjennerud T, Kendler KS. A psychometric evaluation of the DSM-IV borderline personality disorder criteria: age and sex moderation of criterion functioning. Psychol Med. 2009;39(12):1967–78. doi: 10.1017/S0033291709005807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenström T, Ystrom E, Torvik FA, Czaijkowski NO, Gillespie NA, Aggen SH, et al. Genetic and environmental structure of DSM-IV criteria for Antisocial Personality Disorder: a twin study. Behav Genet. 2017;47(3):265–77. doi: 10.1007/s10519-016-9833-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichborn-Kjennerud T, Ystrom E, Neale MC, Aggen SH, Mazzeo SE, Knudsen GP, et al. Structure of genetic and environmental risk factors for symptoms of DSM-IV Borderline Personality Disorder. JAMA Psychiatry. 2013;70(11):1206–14. doi: 10.1001/jamapsychiatry.2013.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paunonen SV, Ashton MC. Big Five factors and facets and the prediction of behavior. J Pers Soc Psychol. 2001;81(3):524–39. [PubMed] [Google Scholar]
- 30.Ashton MC, Paunonen SV, Lee K. On the validity of narrow and broad personality traits: A response to Salgado, Moscoso, and Berges (2013) Personal Individ Differ. 2014;56:24–8. [Google Scholar]
- 31.Nilsen TS, Knudsen GP, Gervin K, Brandt I, Røysamb E, Tambs K, et al. The Norwegian Twin Registry from a public health perspective: a research update. Twin Res Hum Genet. 2013;16(1):285–95. doi: 10.1017/thg.2012.117. [DOI] [PubMed] [Google Scholar]
- 32.Ystrom E, Kendler KS, Reichborn-Kjennerud T. Early age of alcohol initiation is not the cause of alcohol use disorders in adulthood, but is a major indicator of genetic risk. A population-based twin study. Addiction. 2014 Nov 1;109(11):1824–32. doi: 10.1111/add.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tambs K, Rønning T, Prescott CA, Kendler KS, Reichborn-Kjennerud T, Torgersen S, et al. The Norwegian Institute of Public Health twin study of mental health: examining recruitment and attrition bias. Twin Res Hum Genet. 2009;12(2):158–68. doi: 10.1375/twin.12.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neale MC. A finite mixture distribution model for data collected from twins. Twin Res Hum Genet. 2003;6(3):235–9. doi: 10.1375/136905203765693898. [DOI] [PubMed] [Google Scholar]
- 35.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equ Model. 2001;8(3):430–57. [Google Scholar]
- 36.Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV) Iowa City: University of Iowa, Department of Psychiatry; 1995. [Google Scholar]
- 37.Wittchen HU, Pfister H. DIA-X Interview (M-CIDI) Frankfurt: Swets & Zeitlinger; 1997. [Google Scholar]
- 38.Wittchen H-U, Lachner G, Wunderlich U, Pfister H. Test-retest reliability of the computerized DSM-IV version of the Munich-Composite International Diagnostic Interview (M-CIDI) Soc Psychiatry Psychiatr Epidemiol. 1998;33(11):568–78. doi: 10.1007/s001270050095. [DOI] [PubMed] [Google Scholar]
- 39.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–2. [Google Scholar]
- 40.R Core Team. R: A Language and Environment for Statistical Computing [Internet] Vienna, Austria: R Foundation for Statistical Computing; 2016. Available from: http://www.R-project.org. [Google Scholar]
- 41.Neale M, Maes H. Methodology for Genetic Studies of Twins and Families (an online version revised from Neale & Cardon; Dordrecht: Kluwer, 1992[Internet] 2002 [cited 2016 Apr 18]. Available from: http://ibgwww.colorado.edu/twins2002/cdrom/HTML/BOOK/book2002c.pdf.
- 42.Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, et al. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306–17. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vrieze SI. Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) Psychol Methods. 2012;17(2):228–43. doi: 10.1037/a0027127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neale MC, Miller MB. The use of likelihood-based confidence intervals in genetic models. Behav Genet. 1997;27(2):113–20. doi: 10.1023/a:1025681223921. [DOI] [PubMed] [Google Scholar]
- 45.McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspect Psychol Sci. 2010;5(5):546–56. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34(5):1089–99. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- 47.Gurrin LC, Carlin JB, Sterne JAC, Dite GS, Hopper JL. Using bivariate models to understand between- and within-cluster regression coefficients, with application to twin data. Biometrics. 2006;62(3):745–51. doi: 10.1111/j.1541-0420.2006.00561.x. [DOI] [PubMed] [Google Scholar]
- 48.Sjölander A, Johansson ALV, Lundholm C, Altman D, Almqvist C, Pawitan Y. Analysis of 1 : 1 matched cohort studies and twin studies, with binary exposures and binary outcomes. Stat Sci. 2012;27(3):395–411. [Google Scholar]
- 49.Squeglia LM, Ball TM, Jacobus J, Brumback T, McKenna BS, Nguyen-Louie TT, et al. Neural predictors of initiating alcohol use during adolescence. Am J Psychiatry. 2017;174(2):172–85. doi: 10.1176/appi.ajp.2016.15121587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, et al. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512(7513):185–9. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, et al. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15(2):217–26. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma L, Markon KE, Clark LA. Toward a theory of distinct types of “impulsive” behaviors: A meta-analysis of self-report and behavioral measures. Psychol Bull. 2014;140(2):374–408. doi: 10.1037/a0034418. [DOI] [PubMed] [Google Scholar]
- 53.MacKillop J, Weafer J, C Gray J, Oshri A, Palmer A, de Wit H. The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology (Berl) 2016;233(18):3361–70. doi: 10.1007/s00213-016-4372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anokhin AP, Grant JD, Mulligan RC, Heath AC. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol Psychiatry. 2015;77(10):887–94. doi: 10.1016/j.biopsych.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barker V, Romaniuk L, Cardinal RN, Pope M, Nicol K, Hall J. Impulsivity in borderline personality disorder. Psychol Med. 2015;45(09):1955–64. doi: 10.1017/S0033291714003079. [DOI] [PubMed] [Google Scholar]
- 56.White SF, Clanton R, Brislin SJ, Meffert H, Hwang S, Sinclair S, et al. Reward: empirical contribution. Temporal discounting and conduct disorder in adolescents. J Personal Disord. 2014;28(1):5–18. doi: 10.1521/pedi.2014.28.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castellanos-Ryan N, Rubia K, Conrod PJ. Response inhibition and reward response bias mediate the predictive relationships between impulsivity and sensation seeking and common and unique variance in conduct disorder and substance misuse. Alcohol Clin Exp Res. 2011;35(1):140–55. doi: 10.1111/j.1530-0277.2010.01331.x. [DOI] [PubMed] [Google Scholar]
- 58.Morris EP, Stewart SH, Ham LS. The relationship between social anxiety disorder and alcohol use disorders: A critical review. Clin Psychol Rev. 2005;25(6):734–60. doi: 10.1016/j.cpr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 59.DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: A risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157(5):745–50. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- 60.Slutske WS, Heath AC, Dinwiddie SH, Madden PA, Bucholz KK, Dunne MP, et al. Common genetic risk factors for conduct disorder and alcohol dependence. J Abnorm Psychol. 1998 Aug;107(3):363–74. doi: 10.1037//0021-843x.107.3.363. [DOI] [PubMed] [Google Scholar]
- 61.Tuithof M, ten Have M, van den Brink W, Vollebergh W, de Graaf R. The role of conduct disorder in the association between ADHD and alcohol use (disorder). Results from the Netherlands Mental Health Survey and Incidence Study-2. Drug Alcohol Depend. 2012;123(1–3):115–21. doi: 10.1016/j.drugalcdep.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 62.Kendler KS, Jacobson K, Myers JM, Eaves LJ. A genetically informative developmental study of the relationship between conduct disorder and peer deviance in males. Psychol Med. 2008;38(7):1001–11. doi: 10.1017/S0033291707001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith JD, Dishion TJ, Shaw DS, Wilson MN, Winter CC, Patterson GR. Coercive family process and early-onset conduct problems from age 2 to school entry. Dev Psychopathol. 2014 Nov;26(4pt1):917–32. doi: 10.1017/S0954579414000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kendler KS, Aggen SH, Patrick CJ. Familial influences on conduct disorder reflect 2 genetic factors and 1 shared environmental factor. JAMA Psychiatry. 2013;70(1):78–86. doi: 10.1001/jamapsychiatry.2013.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


