Abstract
Problem
To determine if altered trophoblast CD200 and CD200R expression promotes inflammatory cytokine production in preeclamptic placentas.
Methods of study
Placental tissue CD200 and CD200R expression was determined by immunostaining. Tissue sections from first-, second-, and third-trimester, normal term, and preeclamptic placentas were used. CD200 and CD200R expression and cytokine production of TNFα, sTNFR1, INFγ, IL-4, IL-6, IL-8, and IL-10 were determined in trophoblasts from normal and preeclamptic placentas, and in normal trophoblasts transfected with CD200 siRNA.
Results
CD200, but not CD200R, expression was significantly reduced in trophoblasts from preeclamptic compared to normal placentas. Trophoblast from preeclamptic placentas and trophoblast transfected with CD200 siRNA produced significantly more TNFα, sTNFR1, IL-6, and IL-8, but significantly less IL-10, than trophoblasts from normal control placentas.
Conclusion
Downregulation of CD200 expression resulted in an imbalance of increased Th1 cytokine and decreased Th2 cytokine production in placental trophoblasts in preeclampsia.
Keywords: CD200, inflammatory cytokines, trophoblasts, placenta, preeclampsia
Introduction
CD200 is a type-I transmembrane glycoprotein with potent immunosuppressive function through interaction with its receptor, CD200R1. CD200 is expressed in many cell types, including lymphoid cells, neurons, and placental trophoblasts. It was reported that CD200 not only inhibits natural killer cell and mast cell activities [1], promotes regulatory T cell generation [1, 2], but also suppresses macrophage activation by inducing indoleamine 2,3-dioxygenase (IDO) production [2]. IDO is an immune checkpoint molecule produced by immune regulatory cells and has been implicated in immune modulation through its ability to engage mechanisms of immune tolerance [3]. Altered CD200 and its receptor function have been found in several autoimmune/inflammatory associated disorders and diseases in both animals and humans. For example, CD200-deficient mice developed constitutively greater levels of inflammation, including enhanced CD11b and CD45 immunoreactive microglia in the brain [4]. In contrast, in neuropathic pain rats, intrathecal administration of CD200 fusion protein could attenuate glial activation, inflammatory reaction, and hypersensitivity of inflammatory reactions [5]. In humans, CD200R expression was significantly decreased in CD11c+ myeloid dendritic cells (DCs) and in CD123+ plasmacytoid DCs in children with inflammatory bowel diseases (IBD) [6]. In Alzheimer’s disease, decreased CD200 and CD200R expression were detected in brain tissues which was associated with increased chronic inflammatory reactions [7].
In humans, expression of CD200 and its receptor CD200R1 could be detected in placental villous trophoblasts as early as 5 weeks of gestation [8] and both CD200 and CD200R1 expression was significantly reduced in syncytiotrophoblasts in spontaneous abortion placentas [8, 9]. In mice, increase in CD200 expression in placenta trophoblasts could prevent spontaneous abortions induced by TNFα+INFγ [10]. Moreover, upregulation of CD200 expression could also prevent lipopolysaccharides (LPS) - induced abortion in C3(−/−) mice [11]. Therefore, it is believed that CD200 and its receptor play important roles in regulating immune tolerance and inflammatory responses during pregnancy [8, 12]. Darmochwal-Kolarz et al. examined expression of CD200 and CD200R on peripheral blood dendritic cells from normotensive and preeclamptic pregnant women [13] and they found that expression of CD200 molecule on CD1c+ myeloid and BDCA-2+ lymphoid DCs were significantly higher in preeclampsia than in normal pregnant women [13]. They speculated that increased CD200 expression on CD1c+ myeloid and BDCA-2+ lymphoid DCs may constitute the tolerogenic mechanism secondary to the pro-inflammatory response in preeclampsia [13]. Placental trophoblasts function as an immunological barrier between the mother and the fetus during pregnancy. However, little is known about CD200 and CD200R expression and its regulation associated with increased inflammatory response in placental trophoblasts in preeclampsia. The present work was undertaken to determine if altered CD200 and CD200R expression occurs in preeclamptic placentas and to test our hypothesis that downregulation of trophoblast CD200 expression is associated with increased inflammatory cytokine production in preeclampsia.
Materials and Methods
Chemicals and reagents
Antibody for CD200 (AF2724) and DuoSet ELISA kits for human TNFα (DY210), soluble TNFR1 (sTNFR1, DY225), INFγ (DY285), IL-4 (DY204), IL-6 (DY206), IL-8 (DY208), and IL-10 (DY217B) were purchased from R&D Systems (Minneapolis, MN). Antibody for CD200R (S-19, sc-323725) was purchased from Santa Cruz Biotechnology (San Diego, CA). A human CD200 siRNA SMARTpool (L-012181-00-0005) was purchased from GE Dharmacon (Lafayette, CO). Lipofectamine®RNAiMAX Transfection Reagent (Cat: 13778100) was from Invitrogen (Carlsbad, CA). Opti-MEM® I Reduced Serum Medium (Cat: 31985070) was purchased from Gibco Thermo Fisher Scientific (Grand Island, NY). Dulbecco’s modified Eagle’s medium (DMEM), Percoll, and protease inhibitors were from Sigma (St. Louis, MO). All other chemicals were from Sigma unless otherwise noted.
Placenta tissue collection
A total of 34 placentas were used in the study, 16 from third trimester and normal term pregnancies, 12 from preeclampsia, 3 from first-trimester and 3 from second-trimester pregnancies. Diagnosis of normal pregnancy and preeclampsia are based on ACOG guidelines [14, 15]. Third trimester/term placentas from normotensive pregnant women (37–38 weeks) and from preeclamptic pregnancies were collected from University Health Hospital in Shreveport, Louisiana. First trimester (6–8 weeks) and second trimester (16–18 weeks) placental tissues were collected as medical waste from selective pregnancy termination at the Department of Obstetrics and Gynecology, the First Hospital of Harbin Medical University, China. None of the first and second trimester placentas were from women with medical and obstetrical complications. Tissue collection was approved by the Institutional Review Board (IRB) for human research in both institutions. Normal pregnancy was defined as maternal blood pressure <140/90 mmHg without obstetrical and medical complications. Preeclampsia was defined as blood pressure >140/90 mmHg with at least two separate readings with proteinuria (>1+) at dipstick or >300 mg protein/24-hour urine. None of the patients had signs of infection, nor were they smokers. The patient demographic data from normal and preeclamptic are summarized in Table 1.
Table 1.
Demographic data from whom placental was used in the study
| Variables | Normal (n=16) | Preeclampsia (n=12) | P value |
|---|---|---|---|
| Maternal age (years) | 26 ± 2 (18–41) | 22 ± 1(15–34) | 0.101 |
| Racial Status | |||
| White | 5 | 1 | ND |
| Black | 10 | 11 | ND |
| Other | 1 | 0 | ND |
| BMI | 32 ± 2 (21–45) | 37 ± 3 (26–60) | 0.082 |
| Blood Pressure (mmHg) | |||
| Systolic | 117 ± 4 (96–140) | 160 ± 5 (143–193) | <0.001 |
| Diastolic | 68 ± 2 (51–85) | 97 ±5 (73–133) | <0.001 |
| Primigavida | 4 | 6 | ND |
| Serum creatinine (mg/dL) | 0.63 ± 0.05 (0.47–0.9) | 0.80 ± 0.11 (0.5–1.8) | 0.08 |
| Gestational Age (weeks+days) at delivery | 39+0± l+0 (36+6–40+0) | 36+6±2+0(31+6–39+5) | <0.001 |
| Delivery mode | |||
| Vaginal delivery | 10 | 6 | ND |
| C-section | 6 | 6 | ND |
Data are expressed as mean ± SD (range). ND: not determined.
Trophoblast isolation and culture
Placental trophoblasts from normotensive at term and preeclamptic deliveries were isolated by trypsin digestion and isolated trophoblasts were purified by Percoll gradient centrifugation as previously described [16]. Freshly isolated trophoblasts were seeded into 6-well plates (5×106cells/well) and incubated with DMEM containing 10% fetal bovine serum (FBS) and antibiotics. At the end of each experiment, total cellular protein was collected for determination of CD200 and CD200R expression and culture medium were collected for measurement of cytokine production.
Immunohistochemistry
Placental tissue pieces were fixed with 10% formalin and embedded in paraffin. Expression of CD200 and CD200R were examined by immunohistochemistry (IHC) staining of paraffin-embedded tissue sections. A standard IHC staining procedure was performed as previously described [16]. Slides stained with the same antibody were all processed at the same time. Stained slides were reviewed under an Olympus microscope and images were captured with PictureFrame computer software (Uptronics, Sunnyvale, CA).
CD200 siRNA transfection
Transfection assay was conducted using Lipofectamine®RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA) according to the manufacture’s instruction. Briefly, 18h after seeding, trophoblasts were starved with serum free DMEM for 2 hours and then incubated with Opti-MEM I medium for 6 hours, which contains 50nM CD200 siRNA (GE Dharmacon, Lafayette, CO) mixed with Lipofectamine®RNAiMAX Transfection Reagent. Cellular protein was collected 48h after transfection and protein expression was then determined by Western blot.
Protein expression by Western blot
Trophoblast protein expression for CD200 and CD200R were examined by Western blot. Briefly, an aliquot of total cellular protein (10μg of each sample) was subjected to electrophoresis and then transferred to nitrocellulose membranes. The membranes were probed with antibody against CD200 or CD200R and then secondary antibody. The bound antibodies were visualized with an enhanced chemiluminescence detection Kit (Amersham, Arlington Heights, IL). β-actin expression was determined and used to normalize CD200 and CD200R expression for each sample. The band densities were scanned and analyzed by NIH Image J software.
Measurement of cytokine production
Trophoblast cytokine production of TNFα, sTNFR1, INFγ, IL-4, IL-6, IL-8, and IL-10 were measured by enzyme-linked immunosorbent assay (ELISA). An aliquot of 100μl of each sample was used for TNFα, sTNFR1, INFγ, IL-4, and IL-10 assays. Samples for measuring IL-6 and IL-8 were diluted with a ratio of 1:5 with dilution buffer recommended by the ELISA kit. All samples were measured in duplicate in each assay. All assays were carried out according to the manufacturer’s instructions. After reaction, plates were read at 492 nm by an autoplate reader (Molecular Devices, Sunnyvale, CA). Within assay variations were <7% for all the assays.
Statistical analysis
Data are present as mean ± SE and analyzed by unpaired and paired t-test using Prism 5 computer software (GraphPad Software, La Jolla, CA). A probability level of less than 0.05 was considered statistically significant.
Results
Expression of CD200 and CD200R in uncomplicated and preeclamptic placentas
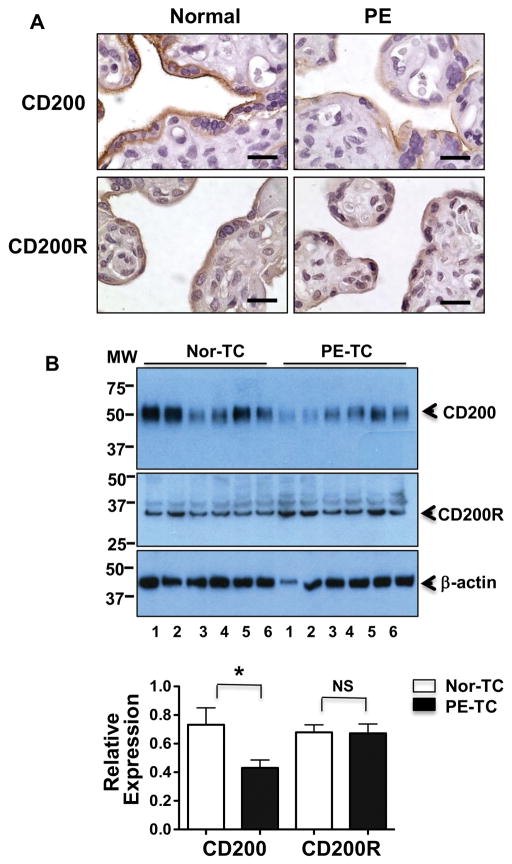
To determine whether altered CD200-CD200R signaling molecules are present in preeclamptic placentas, we first examined CD200 and CD200R expression by immunostaining of paraffin embedded villous tissue sections from normal and preeclamptic pregnancies. Representative images for CD200 and CD200R expression are shown in Figure 1A. Our results showed that expression of CD200 and CD200R was mainly localized in syncytiotrophoblasts layer in placental villous tissues. We also noticed that CD200 expression was reduced in trophoblasts in tissue section from preeclamptic placentas compared to normal placentas. There was no obviously difference in CD200R expression between normal and preeclamptic placentas. To further determine downregulation of CD200 expression in trophoblasts in preeclamptic placentas, CD200 and CD200R expression was determined in trophoblasts isolated from normal and preeclamptic placentas. Results are shown in Figure 1B. Consistent with immunostaining results, relative CD200, but not CD200R, expression was significantly downregulated in trophoblasts from preeclamptic placentas compared to those from normal placentas, p<0.05.
Figure 1. Expression of CD200 and CD200R in normal and preeclamptic placentas.
A: Representative immunostaining images of CD200 and CD200R expressions in tissue sections from normal and preeclamptic (PE) placentas. Bar = 25micron. B: Expression of CD200 and CD200R detected by Western blots in placental trophoblasts (TC) isolated from normal (Nor) and PE placentas. Molecular weight marker is shown on the left of the gel scan for CD200, CD200R and β-actin. The bar graph shows the relative density of protein expression for CD200 and CD200R after normalization with β-actin expression in each sample: CD200: 0.430±0.055 vs. 0.732±0.118, p<0.05; and CD200R: 0.672±0.065 vs. 0.679±0.052, p=0.936, PE vs. normal, respectively. Data are mean ± SE from 6 normal and 6 PE placentas. * p<0.05, PE-TC vs. Nor-TC.
Trophoblast expression of CD200 and CD200R at different gestational ages
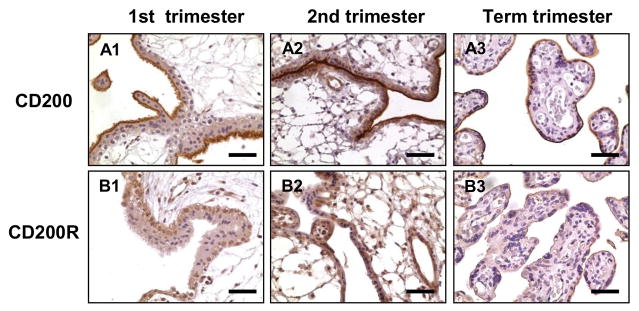
Since most placentas in the preeclamptic group were delivered before term (<37 weeks), we then determined if reduced CD200 expression in preeclamptic placentas was due to the difference in gestational age. We examined CD200 and CD200R expression by immunostaining in nine human placentas: three from 8–10 weeks (first trimester), three from 16–18 weeks (second trimester), and three from 37–38 weeks (term) pregnancy. For each antibody staining, all slides were stained at the same time, and consistent results were obtained. Representative immunostaining of CD200 and CD200R expression at different gestational ages are shown in Figure 2. Both CD200 and CD200R were expressed in trophoblasts throughout gestation. However, CD200 was mainly localized in the apical surface of syncytiotrophoblasts, whereas CD200R was detected in both cytotrophoblasts and syncytiotrophoblasts, and especially in cytotrophoblasts of first and second trimester placentas. In addition, CD200R was also detected in stromal cells and in endothelial cells in the first and second trimester villous tissues (Figure 2, A2 and B2).
Figure 2. Expression and distribution of CD200 and CD200R in placentas from 1st trimester, 2nd trimester, and term pregnancies.
A: CD200; B: CD200R; A1-B1: 1st trimester. A2–B2: 2nd trimester. A3–B3: term placentas. Bar = 50micron.
Trophoblasts from preeclamptic placentas produce more TNFα, sTNFR1, IL-6, and IL-8 and less IL-10 than cells from normal placentas
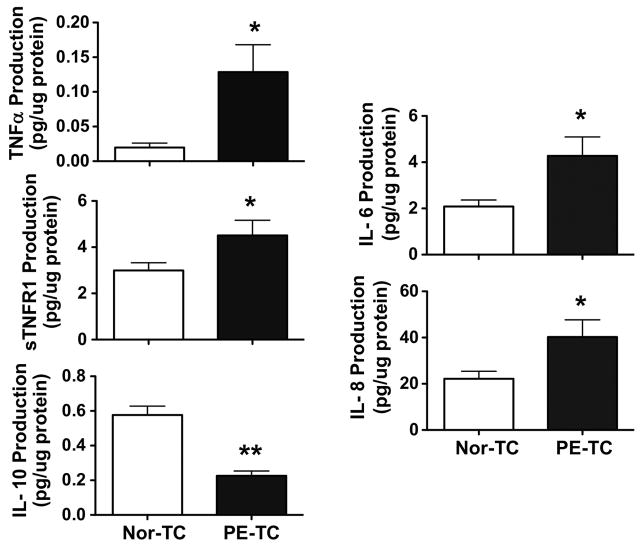
Altered placental cytokine productions have been considered to be a significant feature of aberrant innate and adaptive immune response and increased inflammatory reactions in preeclampsia. To determine if reduced CD200 expression was relevant to altered Th1 and Th2 cytokine production in preeclamptic placentas, we assessed Th1 cytokine, TNFα and sTNFR1 production and Th2 cytokine IL-4 and IL-10 production by placental trophoblast from normal and preeclamptic pregnancies. Trophoblast IL-6 and IL-8 production was also determined. Our results showed that trophoblasts from preeclamptic placentas produced more TNFα and sTNFR1 than that from normal placentas, p<0.05, Figure 3. Similar to sTNFR1, trophoblasts from preeclamptic placentas also produced more IL-6 and IL-8 than cells from normal placentas, p<0.05, respectively, Figure 3. In contrast, trophoblasts from preeclamptic placentas produced less IL-10 than that from normal pregnancies, p<0.01, Figure 3. However, trophoblast production of INFγ and IL-4 were undetectable in our study. These results support the notion that increased inflammatory cytokine production and reduced anti-inflammatory cytokine production is associated with downregulation of CD200 expression in preeclamptic placentas.
Figure 3. Production TNFα, sTNFR1, IL-6, IL-8, and IL-10 production in trophoblasts from normal and preeclamptic placentas.
Trophoblasts from preeclamptic placentas produced significantly more TNFα, sTNFR1, IL-6, and IL-8 cytokines and less IL-10 than cells from normal placentas, * p<0.05, ** p<0.01, PE-TC vs. Nor-TC. Data are mean ± SE from 10–12 independent experiments from each group.
Inhibition of CD200 expression resulted in increased TNFα, sTNFR1, IL-6, and IL-8 production, but decreased IL-10 production by placental trophoblasts
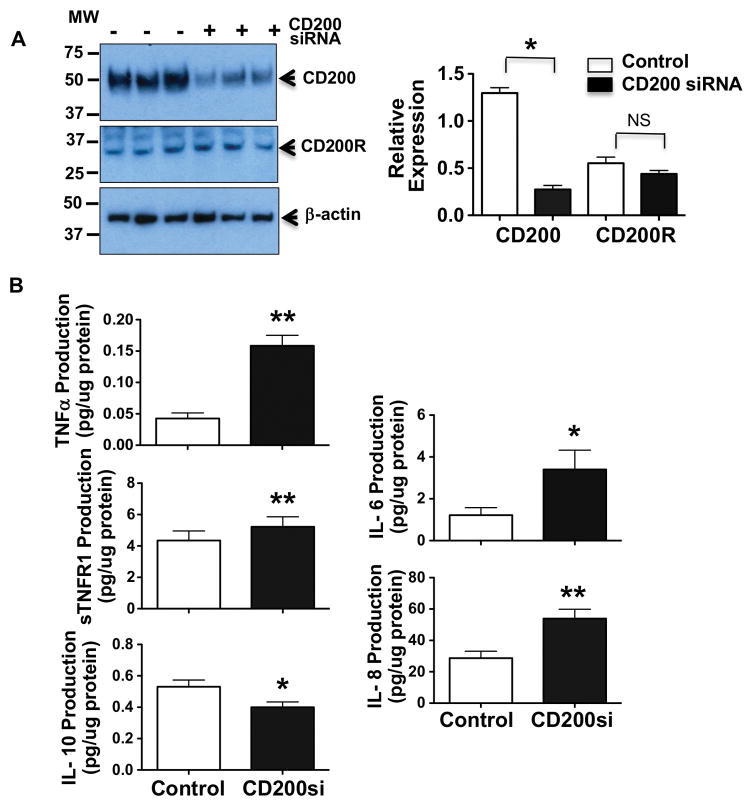
To determine whether down-regulation of CD200 expression was associated with increased inflammatory cytokine release in placental trophoblasts, CD200 siRNA was employed to inhibit CD200 expression in primary isolated trophoblasts from normal term placentas. Production of TNFα, sTNFR1, IL-6, IL-8, and IL-10 were assessed. Our results showed that inhibition of CD200 expression by CD200 siRNA had little effect on CD200R expression in placental trophoblasts, Figure 4A. However, cells transfected with CD200 siRNA produced significantly more TNFα, sTNFR1, IL-8 and IL-6, but significantly less IL-10 than those control cells. These findings suggest that reduced CD200 expression could alter the balance of Th1/Th2 cytokine production by placental trophoblasts.
Figure 4. Effects of CD200 inhibition on TNFα, sTNFR1, IL-6, IL-8, and IL-10 production in trophoblasts form normal placentas.
A: CD200 and CD200R expression in trophoblasts transfected with or without CD200 siRNA (50nM). Molecular weight marker is shown on the left of the gel scan for CD200, CD200R and β-actin. The bar graph shows relative density of protein expression for CD200 and CD200R after normalization with β-actin in each sample. * p<0.05, siCD200 vs. control. B: TNFα, sTNFR1, IL-8, IL-6, and IL-10 production in placental trophoblasts transfected with or without CD200 siRNA, respectively, * p<0.05 and ** p<0.01: siCD200 vs. control. Data are mean ± SE from 5–7 independent experiments. Inhibition of CD200 expression by CD200 siRNA results in increased TNFα, sTNFR1, IL-6, and IL-8 production, and decreased IL-10 production by primary isolated trophoblasts from normotensive placentas.
Discussion
Expression of CD200 and its receptor CD200R at the maternal-fetal interface has been reported to be associated with successful pregnancy in both murine animals and humans [17]. For example, downregulation of CD200 expression could be detected in trophoblasts and in decidua prior to onset of abortion in mice dependent on the Th1 cytokine TNFα + INFγ [18]. CD200/CD200R axis is considered ‘tolerance signaling molecule’. It modulates innate and adaptive immunity and suppresses inflammatory response through promotion of Th2 cytokines. Increased inflammatory response in trophoblasts is one of the characteristics of placental dysfunction in preeclampsia. In the present study, we examined if increased inflammatory cytokine production was associated with altered CD200 and CD200R expression in placental trophoblasts in preeclampsia. By immunostaining of villous tissue sections and by detection of cellular protein expression in primary isolated placental trophoblasts, we found that CD200, but not CD200R, expression was significantly downregulated in placental trophoblasts from preeclampsia compared to that from normotensive pregnant controls. Trophoblasts from preeclamptic placentas produced significantly more TNFα, sTNFR1, IL-8, and IL-6, but significantly less IL-10, than trophoblasts from normotensive placentas. These results clearly show that downregulation of CD200 expression is associated with increased Th1 cytokine and decreased Th2 cytokine production by trophoblasts from preeclamptic placentas.
To determine if reduced CD200 expression seen in preeclamptic placentas is due to the difference in gestational age from normal placentas, we examined CD200 and CD200R expression in villous tissues from first, second, and third/term placentas. Our results showed that CD200 was strongly expressed in syncytiotrophoblasts, but not in cytotrophoblasts, throughout gestation. More specifically, strong CD200 expression was detected at the apical surface of syncytiotrophoblasts. In contrast, CD200R expression was detected in both syncytiotrophoblasts and cytotrophoblasts in the first and second trimester villous tissues and strong CD200R expression was seen in cytotrophoblasts compared to syncytiotrophoblasts. In addition, CD200R expression was also detected in villous core stromal cells and villous fetal vessel endothelium. The differences between CD200 and CD200R signaling distribution within villous core tissue pointed out the importance of immune suppressive activity of CD200 and CD200R at the maternal-fetal interface. Although we did not specifically test CD200-mediated tolerance signaling in placental TCs, we speculated that CD200 at apical surface of syncytiotrophoblast membrane could produce tolerance signaling that directly interacts with maternal circulating cells such as lymphocytes and neutrophils when they travel through intervillous space, whereas CD200R might have a greater intimate relationship with fetal compartmental cells. Therefore, reduced trophoblast CD200 expression could be a sign of reduced immune tolerance at maternal-fetal interface in preeclampsia.
It is well known that successful pregnancy has been linked to a Th1 to Th2/3 cytokine shift at the maternal-fetal interface. It is also known that impaired immune function is associated with increased inflammatory response. To determine the association of downregulation of CD200 expression with Th1/Th2 and inflammatory cytokine production in placental trophoblasts in preeclampsia, we assessed production of Th1 cytokine TNFα and INFγ and Th2 cytokine IL-4 and IL-10 by placental trophoblasts from normal and preeclamptic placentas. We also measured inflammatory cytokine IL-6 and IL-8 production. Although trophoblast production of INFγ and IL-4 was undetectable, increased TNFα, IL-6, and IL-8 production and decreased IL-10 production were demonstrated in trophoblasts from preeclamptic placentas. We also measured sTNFR1 production. sTNFR1 is a soluble form of membrane bound TNFR1, which is essential for TNFα-signaling. sTNFR1 binds to TNFα and functions simultaneously as both TNF carriers and TNF antagonists. Because sTNFR1 is an important counter regulatory mediator of TNFα and it is more stable and easier to detect, sTNFR1 is considered a surrogate marker of TNFα activity [19, 20]. Similar to TNFα, IL-6, IL-8, trophoblast sTNFR-1 production was also significantly increased in preeclampsia.
To demonstrate if downregulation of CD200 expression contributes to increased inflammatory response in placental trophoblasts in preeclampsia, CD200 siRNA was employed and transfected into primary isolated trophoblasts from normotensive placentas. Our results showed that cells transfected with CD200 siRNA produced significantly more TNFα, sTNFR1, IL-6, and IL-8 and significantly less IL-10 than control cells, similar to what we see in trophoblasts from preeclamptic placentas [21]. These results suggest that downregulation of CD200 expression or impaired CD200/CD200R signaling could reduce immune tolerance signal and increase inflammatory response at maternal-fetal interface and the changes in cytokine production could be due to reduced autocrine/paracrine effects. Although in the present study, we did not examine the outcome of downregulation of CD200 expression mediated immune response in maternal cells, a study made by Clark et al. did show that a subpopulation of CD9-/CD200+ trophoblasts isolated from normal term placentas were able to alter maternal lymphocyte immune responses in a favorable Th1 to Th2 shift [12].
IL-6 plays diverse roles in inflammation and it could act as a pro-inflammatory cytokine and an anti-inflammatory cytokine. IL-6 is produced by a number of cell types including macrophages, dendritic cells, lymphocytes, fibroblasts, and endothelial cells in response to a variety of internal and external stimuli. IL-6 induces the synthesis of acute phase response proteins in hepatocytes, and promotes B cell and monocyte differentiation [22]. In CD4+ T cells, IL-6 could shift Th1 to Th2 cytokine production by promoting Th2 cytokine IL-4 production and inhibiting Th1 cytokine INFγ production [22]. Studies also showed that IL-6 plays a role in converting Treg cells into Th17 cells [23, 24] and IL-6 also negatively regulates inflammatory response by induction of suppressor of cytokine signaling-3 (SOCS-3) expression via binding to its membrane receptor IL-6R/gp130 [25]. In our study, IL-6 production was significantly increased, but INFγ and IL-4 production were under detectable, in trophoblasts from preeclamptic placentas. Since IL-6R expression was downregulated in placental trophoblast in preeclampsia [26], we believe that increased trophoblast IL-6 production is a sign of increased inflammatory, not anti-inflammatory, response in preeclampsia. Nonetheless, increased IL-6 production seen in trophoblasts transfected with CD200 siRNA suggests that CD200 could modulate IL-6 production in placental trophoblasts.
Our results also showed that trophoblast CD200R expression was not different in preeclamptic from normal placentas. In addition, inhibition of CD200 expression did not affect CD200R expression in placental trophoblasts. These results suggest that altered inflammatory and Th1/Th2 cytokine production could be mainly linked to downregulation of CD200, but not CD200R, expression in placental trophoblasts in preeclampsia. This notion is supported by the finding made by Gao et al., in which these investigators found that altered CD200R expression was correlated with Th17/Treg imbalance, but not correlated with Th1 (IL-2, IFN-γ) or Th2 (IL-4, IL-10) cytokine responses in monocytes from patients with rheumatoid arthritis [27].
In summary, we found that CD200 expression was significantly downregulated in placental trophoblasts in preeclampsia, which was associated with increased inflammatory cytokine TNFα, sTNFR1, IL-6, and IL-8 production and decreased anti-inflammatory cytokine IL-10 production. By transfection of CD200 siRNA into primary isolated placental trophoblasts, we further demonstrated that inhibition of CD200 could lead to increased TNFα, sTNFR1, IL-6, and IL-8 production and decreased IL-10 production, which recaptures the phenomenon that was seen in trophoblasts from preeclamptic placentas. TNFα and sTNFR1 are Th1 cytokines and IL-10 is Th2 cytokine. IL-6 and IL-8 are markers of increased inflammatory response. CD200 is considered ‘tolerance signaling molecule’ and it is strongly expressed at the apical surface of syncytiotrophoblasts. Since trophoblasts are directly bathed in the maternal blood in the placental intervillous space, cytokines produced by trophoblasts could be released into intervillous space and enter the maternal circulation. Therefore, there is no doubt that our data provides plausible evidence that CD200 might govern homeostasis of inflammatory responses at the maternal-fetal interface during pregnancy. We also speculate that downregulation CD200 expression-induced increase in inflammatory cytokine production may contribute to elevated maternal inflammatory cytokine levels in preeclampsia. Whether altered trophoblast CD200 expression contributes to impaired maternal adaptive immune response warrants further investigation.
Acknowledgments
This study was supported in part by grants from National Institute of Health NICHD R21HD076289 to Yuping Wang and Youth Innovation Foundation of Harbin Medical University 2016JCZX39 to Jie Xu.
References
- 1.Holmannová D, Kolácková M, Kondélková K, Kunes P, Krejsek J, Andrýs C. CD200/CD200R paired potent inhibitory molecules regulating immune and inflammatory responses; Part I: CD200/CD200R structure, activation, and function. Acta Medica (Hradec Kralove) 2012;55:12–17. doi: 10.14712/18059694.2015.68. [DOI] [PubMed] [Google Scholar]
- 2.Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173:3748–3754. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- 3.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 5.Hernangómez M, Klusáková I, Joukal M, Hradilová-Svíženská I, Guaza C, Dubový P. CD200R1 agonist attenuates glial activation, inflammatory reactions, and hypersensitivity immediately after its intrathecal application in a rat neuropathic pain model. J Neuroinflammation. 2016;13:43. doi: 10.1186/s12974-016-0508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elshal MF, Aldahlawi AM, Saadah OI, McCoy JP. Reduced Dendritic Cells Expressing CD200R1 in Children with Inflammatory Bowel Disease: Correlation with Th17 and Regulatory T Cells. Int J Mol Sci. 2015;16:28998–28990. doi: 10.3390/ijms161226143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark DA, Arredondo JL, Dhesy-Thind S. The CD200 tolerance-signaling molecule and its receptor, CD200R1, are expressed in human placental villus trophoblast and in peri-implant decidua by 5 weeks’ gestation. J Reprod Immunol. 2015;112:20–23. doi: 10.1016/j.jri.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Wang LQ, Yan CF, Zhao Y, Chu J, Yu XW. Reduced CD200 and CD200R1 expression in human chorionic villi contributes to early spontaneous abortion. Acta Obstet Gynecol Scand. 2014;93:1248–1254. doi: 10.1111/aogs.12476. [DOI] [PubMed] [Google Scholar]
- 10.Clark DA, Ding JW, Yu G, Levy GA, Gorczynski RM. Fgl2 prothrombinase expression in mouse trophoblast and decidua triggers abortion but may be countered by OX-2Mol. Hum Reprod. 2001;7:185–194. doi: 10.1093/molehr/7.2.185. [DOI] [PubMed] [Google Scholar]
- 11.Yu G, Sun Y, Foerster K, Manuel J, Molina H, Levy GA, Gorczynski RM, Clark DA. LPS-induced murine abortions require C5 but not C3, and are prevented by upregulating expression of the CD200 tolerance signaling molecule. Am J Reprod Immunol. 2008;60:135–140. doi: 10.1111/j.1600-0897.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- 12.Clark DA, Keil A, Chen Z, Markert U, Manuel J, Gorczynski RM. Placental trophoblast from successful human pregnancies expresses the tolerance signaling molecule, CD200 (OX-2) Am J Reprod Immunol. 2003;50:187–195. doi: 10.1034/j.1600-0897.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 13.Darmochwal-Kolarz DA, Kludka-Sternik M, Chmielewski T, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. The expressions of CD200 and CD200R molecules on myeloid and lymphoid dendritic cells in pre-eclampsia and normal pregnancy. Am J Reprod Immunol. 2012;67:474–481. doi: 10.1111/j.1600-0897.2012.01126.x. [DOI] [PubMed] [Google Scholar]
- 14.Bulletin AP. ACOG Practice Bulletin: Diagnosis and management of preeclampsia and eclampsia. Number 33. Obstet Gynecol. 2002;99(1):159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 15.ACOG Guidelines. Chapter 2: Establishng the diagnosisof preeclampsia and eclampsia. 2013. Hypertension in Pregnancy. [Google Scholar]
- 16.Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2012;303:E928–935. doi: 10.1152/ajpendo.00279.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark DA, Dmetrichuk JM, McCready E, Dhesy-Thind S, Arredondo JL. Changes in expression of the CD200 tolerance-signaling molecule and its receptor (CD200R) by villus trophoblasts during first trimester missed abortion and in chronic histiocytic intervillositis. Am J Reprod Immunol. 2017;78(1) doi: 10.1111/aji.12665. [DOI] [PubMed] [Google Scholar]
- 18.Clark DA, Chaouat G, Wong K, Gorczynski RM, Kinsky R. Tolerance mechanisms in pregnancy: a reappraisal of the role of class I paternal MHC antigens. Am J Reprod Immunol. 2010;63(2):93–103. doi: 10.1111/j.1600-0897.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- 19.Tesch GH. Review: Serum and urine biomarkers of kidney disease: A pathophysiological perspective. Nephrology (Carlton) 2010;15:609–616. doi: 10.1111/j.1440-1797.2010.01361.x. [DOI] [PubMed] [Google Scholar]
- 20.Mohler KM1TD, Smith CA, Goodwin RG, Stremler KE, Fung VP, Madani H, Widmer MB. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993;151:1548–1561. [PubMed] [Google Scholar]
- 21.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 23.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 25.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Förster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 26.Zhao S, Gu Y, Dong Q, Fan R, Wang Y. Altered IL-6 receptor, IL-6R and gp130, production and expression and decreased SOCS-3 expression in placentas from women with preeclampsia. Placenta. 2008;29:1024–1028. doi: 10.1016/j.placenta.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao S, Hao B, Yang XF, Chen WQ. Decreased CD200R expression on monocyte-derived macrophages correlates with Th17/Treg imbalance and disease activity in rheumatoid arthritis patients. Inflamm Res. 2014;63:441–450. doi: 10.1007/s00011-014-0716-6. [DOI] [PubMed] [Google Scholar]