Abstract
Bile acids are central signals in enterohepatic communication and also integrate microbiota-derived signals into this signaling axis. Discovery of the tissue distribution and signaling pathways activated by the natural receptors for bile acids, farnesoid X receptor and G protein-coupled bile acid receptor 1 (GPBAR1) also known as TGR5, and bile acid transporters has led to the development of therapeutic agents that target these molecules. Obeticholic acid, a selective FXR agonist, and NGM282, a non-mitogenic FGF-19 analog, are two of the agents in this pipeline. Obeticholic acid has been approved by regulatory agencies for use in patients with primary biliary cholangitis.
INTRODUCTION
Enterohepatic Circulation of Bile Acids: The Gut-Liver-Microbiome Axis
The rate limiting enzyme in the classical pathway of bile acid synthesis from cholesterol in the liver is 7α-hydroxylase (cytochrome P450 7A1, CYP7A1). The primary bile acids produced in the liver are cholic acid (CA) and chenodeoxycholic acid (CDCA), which are conjugated with taurine and glycine and are excreted into bile and stored in the gallbladder (Figure 1) (1).
Figure 1. Synthesis, secretion and enterohepatic circulation of bile acids in humans.
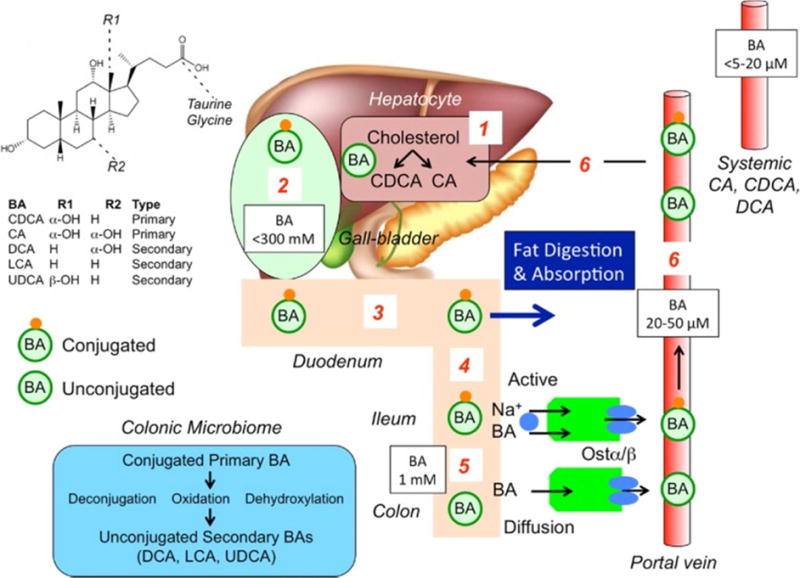
(1) Primary bile acids (BAs) are synthesized in hepatocytes from cholesterol. (2) BAs are conjugated to glycine and taurine and are stored in the gallbladder at high concentrations. (3) After feeding, conjugated BAs are secreted in the intestine where they emulsify dietary fats and form mixed micelles that facilitate digestion and absorption of the products of triglyceride digestion. (4) Conjugated BAs are actively absorbed by the apical sodium BA co-transporter (ASBT [IBAT]) at the apical membrane of enterocytes of the terminal ileum. (5) In the colon, bacteria deconjugate and dehydroxylate primary BAs to form secondary BAs, which are passively absorbed. (6) Conjugated and unconjugated BAs enter the portal vein and recirculate to the liver for re-use.
Reproduced with permission from ref. 1, Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol 2014; 592:2943–2950.
After food ingestion, the gallbladder contracts, delivering bile acids into the small bowel and facilitating digestion and absorption of fat. Most of the conjugated bile acids (~95%) are absorbed in the terminal ileum by active transporters [apical sodium bile acid transporter (ASBT) also called ileal BA transporter (IBAT)] and transported via the portal circulation to the liver to be recycled. The unabsorbed (~5%) CA and CDCA reaching the colon are deconjugated by bacterial bile salt hydrolases and 7α-dehydroxylated by bacteria to secondary bile acids, predominantly deoxycholic acid (DCA) and lithocholic acid (LCA). Thus, colonic microbiota are an integral part of the enterohepatic bile acid axis. In the colon, CDCA and DCA stimulate fluid secretion (2), increase mucosal permeability, and induce high amplitude propagated contractions (3,4). The colon reabsorbs, by diffusion, ~75% of the bile acids reaching the colon.
Farnesoid X Receptor
The farnesoid X receptor (FXR) is highly expressed in the intestine and liver. It is a natural receptor for bile acids. Of the natural bile acids, it is most potently activated by CDCA. The bile acids absorbed by the ileal enterocytes bind to the nuclear farnesoid X-receptor (FXR) which stimulates synthesis of the hormone, fibroblast growth factor-19, FGF-19, which enters the hepatocyte through fibroblast growth factor-receptor 4 (FGF-R4) with interaction with a surface protein, klotho β. In the hepatocytes, FGF-19 inhibits bile acid synthesis through inhibition of CYP7A1. Direct bile acid activation of hepatocyte FXR leads to the induction of small heterodimer protein (SHP) which inhibits the transcription of CYP7A1. These two pathways constitute the major negative regulators of bile acid synthesis (Figure 2).
Figure 2. Bile acid induced FXR and TGR5 signaling in hepatocytes and cholangiocytes.
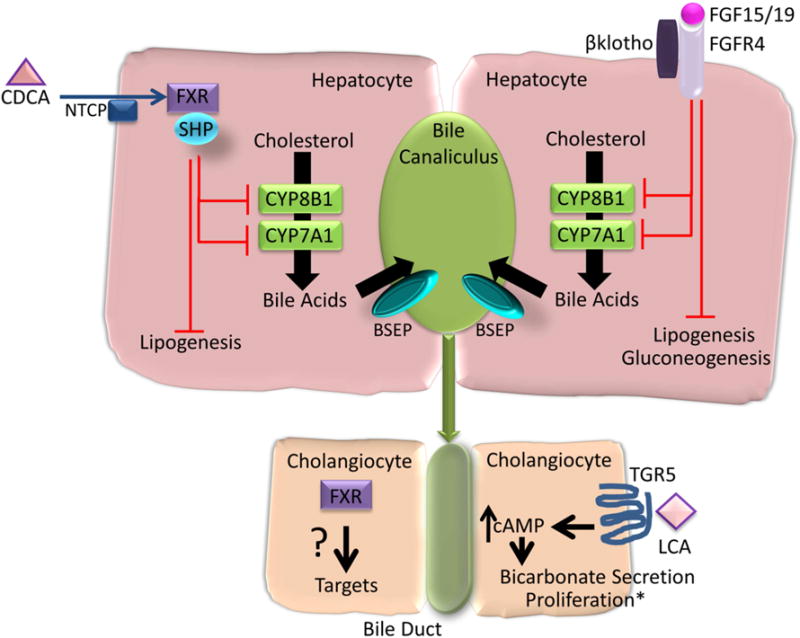
The farnesoid X receptor (FXR) is expressed in hepatocytes and cholangiocytes. Of the natural bile acids, chenodeoxycholic acid (CDCA), most potently activates FXR leading to the induction of small heterodimer protein (SHP) and inhibition of cytochrome P450 (CYP) 7A1 and CYP8B1 and negative feedback regulation of bile acid synthesis from cholesterol. FXR stimulated FGF-19 (Fgf15 in mice) from enterocytes activates fibroblast growth factor 4 (FGFR4) and β klotho leading to inhibition of CYP7A1 and CYP8B1. Bile acids activation of FXR also inhibits lipogenesis and FGF19 inhibits lipogenesis and gluconeogenesis. Though FXR is expressed on cholangiocytes its biologic relevance is less well defined. Lithocholic acid (LCA) activates TGR5 which is expressed on cholangiocytes, and not on hepatocytes. TGR5 activation leads to an increase in intracellular cyclic AMP (cAMP) and bicarbonate secretion. *TGR5 activation leads to cholangiocyte proliferation, except in the case of ciliated cholangiocytes, where proliferation is inhibited. NTCP, sodium taurocholate cotransporting polypeptide. BSEP, bile salt export pump.
G-Protein Coupled Bile Acid Target Receptor
This receptor, also called Takeda G-coupled receptor 5 (TGR5), is located on cholangiocytes, the epithelial surface of gallbladder and intestinal cells, the basolateral surface of smooth muscle, neural cells, brown adipose tissue, immune cells including dendritic cells and macrophages, and enteroendocrine cells that produce glucagon-like peptide 1 (GLP-1) (5). Of the natural bile acids, TGR5 is most potently activated by LCA, and is an important receptor for mediating effects of bile acids on motility, directly by action on neurons and indirectly by stimulating serotonin release (1).
PHARMACOTHERAPEUTICS OF BILE ACIDS AND RECEPTORS
I. Intestinal Tract
A. Treatment options for constipation
When used for gallstone dissolution and cholestatic liver disease, CDCA was associated with diarrhea and, in patients with chronic constipation, it increased stool frequency and loosened stool consistency compared with placebo (6). On the other hand, ursodeoxycholic acid (UDCA) did not cause diarrhea, consistent with studies of bile acids on epithelial functions in animal models and in vitro (7,8). Fluid secretion results from stimulation of intracellular messengers (e.g. cAMP and calcium) and activation of chloride secretion through the cystic fibrosis transmembrane regulator (9–11). Bile acids also induce high amplitude propagated contractions of the colon (4), at least partly mediated through TGR5 receptors on enteric cholinergic and nitrergic neurons and smooth muscle cells (12) and resulting in prokinetic effects (13).
A minority of patients with constipation-predominant irritable bowel syndrome (IBS-C) has evidence of bile acid deficiency (14). CDCA accelerates colonic transit, increases stool frequency, loosens stool consistency, and eases passage of stool in patients with IBS-C (15). Similar pharmacodynamics (16) and clinical effects to those of ileocolonic delivery of CDC were observed with an IBAT inhibitor, elobixibat, in chronic idiopathic constipation (17,18).
A preliminary study of NGM282, a non-mitogenic variant of FGF19, reported acceleration of colonic transit and relief of constipation in a single-center, phase 1 trial in patients with functional constipation (19).
B. Treatment options for bile acid diarrhea
Bile acid sequestrants
About 25–33% of patients with diarrhea-predominant irritable bowel syndrome (IBS-D) or functional diarrhea have evidence of bile acid diarrhea (20). Bile acid sequestrants bind bile acids and decrease diarrhea in bile acid diarrhea. There are three currently available bile acid sequestrants: cholestyramine (powder form), colestipol and colesevelam (both available in tablet form).
The only randomized trial of cholestyramine efficacy in bile acid diarrhea showed response rates of 40% and 53.8% in patients with 75SeHCAT (selenium homocholic acid taurine) retention <10% or 20% respectively. Less than 15% retention signifies excessive bile acid loss. In comparison to the effects of cholestyramine, treatment with hydroxypropyl cellulose (which also binds bile salts in the colon without affecting hepatic bile acid synthesis) showed response rates of 25% and 38.5% respectively (no statistical difference between the two treatments) (21). Cholestyramine is unpalatable and associated with bloating; hence, compliance is low (22).
In an open-label trial in patients with bile acid diarrhea with 75SeHCAT retention <20%, colestipol reduced stool frequency and IBS severity score (23).
In another open-label study in patients with high 48-hour stool bile acid excretion, colesevelam, 1875 mg twice daily for 10 days, decreased stool consistency and increased stool excretion of sequestered bile acids (24). Colesevelam also slowed emptying of the ascending colon compared with placebo in IBS-D; the treatment effect was associated with baseline serum C4, which reflects the hepatic bile acid synthesis rate (25).
Further controlled trials are necessary to assess the effects of bile acid sequestrants for diarrhea. Patients will likely need long-term therapy with bile acid sequestrants for symptom relief. In a long-term, follow-up study of patients with a median time from bile acid diarrhea diagnosis of 6.8 years, 38% were still on bile acid sequestrants, with adequate relief of their symptoms, while 24% discontinued therapy, most commonly due to poor tolerability (26).
FXR agonist
Obeticholic acid (OCA), a potent FXR agonist that stimulates FGF-19 production and decreases hepatic bile acid synthesis, was administered at 25 mg orally, daily for two weeks; it decreased stool frequency, improved stool consistency, increased FGF-19 levels, and decreased serum C4 and serum bile acids in patients with primary and secondary bile acid diarrhea, but not in patients with diarrhea and normal 75SeHCAT levels (27).
C. UDCA reduces Clostridium difficile sporulation, infection and pouchitis
It has been suggested that restoration of secondary bile acid metabolism may be a key mechanism for the beneficial effects of fecal microbiota transplantation in treating recurrent C. difficile infection. Thus, bile acids at concentrations found in patients after fecal microbiota transplantation did not induce germination of C. difficile strains and actually inhibited vegetative growth (28). Moreover, administration of UDCA eradicated C. difficile infection in a patient with recurrent pouchitis (29). Indeed, it has been shown in experimental animal studies that complete microbial engraftment following fecal microbiota transplantation is not required to recover from recurrent C. difficile infection, and bile acid metabolism with formation of secondary bile acids (who typically harbored greater relative abundance of members of the Clostridium XIVa clade or Holdemania in the family Erysipelotrichaceae) could potentially provide resistance to the infection (30).
II. Liver
In the liver, FXR is predominantly expressed on hepatocytes and cholangiocytes, whereas TGR5 is expressed on non-parenchymal cells including hepatic macrophages (Kupffer cells), sinusoidal endothelial cells, and on the apical surface and primary cilium of cholangiocytes (31). In contrast, both receptors are poorly expressed on hepatic stellate cells. The importance of FXR and TGR5 in hepatic pathophysiology has led to the development of potent steroidal and non-steroidal agonists, with variable specificity for each of these receptors. These pharmacological agents are being tested for their anti-inflammatory and anti-fibrotic actions in diverse liver diseases including nonalcoholic steatohepatitis (NASH), primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), and portal hypertension.
A. Primary Biliary Cholangitis
A (i). OCA
The POISE trial was a 12-month, double-blind, phase 3 trial followed by a 12-month, open-label extension examining the efficacy of 5 mg and 10 mg OCA, a selective FXR agonist, in PBC patients with inadequate response or intolerance to ursodeoxycholic acid (UDCA) (32). The trial met its primary endpoint, which was biochemical, and was approved for marketing; the long-term efficacy of OCA on patient survival and disease-related outcomes such as the need for liver transplantation is unknown, though improved outcome would be predicted based on previous studies that have associated improved prognosis in PBC patients with normalization of serum alkaline phosphatase (33). This study has entered a 5-year extension phase that will provide information on long-term outcomes. Pruritus, a significant symptom in PBC patients, is also the most common and dose-dependent adverse effect of OCA. In the POISE trial, only 4% of patients discontinued OCA due to pruritus, most in the 10 mg dosage group.
(Aii). IBAT inhibitors
In a Phase 2a, 14-day trial in PBC patients, the IBAT inhibitor, GSK2330672, reduced pruritus and the serum total bile acid by 50%s and increased serum C4 about 3-fold, consistent with impaired reabsorption and increased fecal bile acid losses. The most common and predictable adverse effect was diarrhea, which might limit its use (34).
The IBAT inhibitor, LUM001 (maralixibat), has been tested in combination with UDCA for treating pruritus in 66 patients with PBC (NCT01904058); the study results presented in Clinical Trials.gov show that changes from baseline in pruritus weekly sum score or serum alkaline phosphatase after 4, 8 or 13 weeks’ treatment were not significant compared to UDCA alone.
(Aiii) FGF19 analog
NGM282 was tested in PBC patients with incomplete response to UDCA, and showed significant biochemical responses: reduced alkaline phosphatase and serum C4. The most common adverse effect was diarrhea (3–4 fold that of placebo), which might limit its long-term use (35).
B. Primary Sclerosing Cholangitis
In a preclinical mouse model of PSC, the dual FXR and TGR5 agonist, INT-767, improved liver injury, inflammation and fibrosis (36).
Based on the results of OCA observed in other liver diseases, OCA is being tested in PSC patients (NCT02177136) in a Phase 2 trial expected to be completed in November 2018. Similarly, NGM282 is being tested in a phase 2 trial (NCT02704364).
An open-label, dose-finding, Phase 2 study of LUM001 (maralixibat) (NCT02061540) has been completed in 27 PSC patients, and there was significant reduction in fasting serum bile acids and numerical reduction (nonsignificant) in circulating liver enzymes, and no effect on pruritus after 14 weeks’ treatment. A larger, randomized, placebo-controlled, double-blind trial is ongoing.
C. Nonalcoholic Steatohepatitis
Glucose homoeostasis, lipid and lipoprotein metabolism, inflammatory responses and intestinal dysbiosis are all components of the pathogenesis of NASH that are benefited by bile acid signaling (Figure 3). TGR5 activation is anti-inflammatory and increases energy expenditure in brown adipose tissue. Furthermore, it induces secretion of GLP-1 from enteroendocrine L cells. However, the wide tissue distribution of TGR5 limits the use of systemic agonists which can increase gallbladder volume and pruritus.
Figure 3. Role of BAs in the control of metabolic and immune homeostasis via activation of their receptors FXR and TGR5.
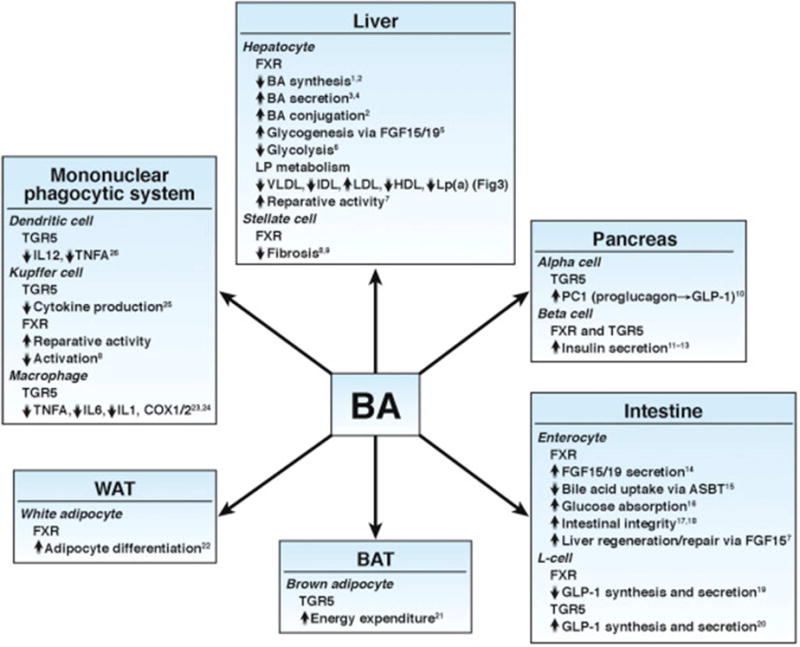
COX, cyclooxygenase; IDL, intermediary density lipoprotein; IL, interleukin; Lp, lipoprotein; Lp(a), lipoprotein (a); VLDL, very-low-density lipoprotein.
Reproduced with permission from ref. 31, Chavez-Talavera O, et al. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia and nonalcoholic fatty liver disease. Gastroenterology 2017;152:1679–1694.
The FXR agonist, OCA, has shown clinical efficacy in the treatment of NASH. In the NIH-sponsored FLINT trial (37), OCA was evaluated in patients with biopsy-proven NASH. OCA, 25 mg, showed histologic improvement as assessed by the NAFLD activity score and alanine aminotransferase (ALT). This was associated with a modest weight loss, an increase in plasma low density lipoprotein (LDL) cholesterol, and a decrease in plasma high density lipoprotein cholesterol which reversed upon discontinuation of drug at study completion.
Results from a Phase 2a trial of the FGF19 analog, NGM282, in NASH patients showed significant reduction in liver fat, ALT and serum C4, and increase in serum LDL (38).
Accumulated empirical data have shown that loss of intestinal FXR protected high fat-fed mice from hepatic steatosis (39). Inhibition of the IBAT (ASBT) led to increased fecal bile acid loss and altered the bile acid pool to increase bile acids, which are agonists to FXR and enhance resistance to high fat diet-induced hepatic steatosis and glucose intolerance (40). Conversely, fexaramine, an intestinal FXR agonist, also ameliorated high fat diet-induced weight gain, insulin resistance and hepatic steatosis (41). Fexaramine treatment induced expression of intestinal Fgf15 (murine equivalent of human FGF19) with changes in bile acid composition. Therefore, larger and longer comparative studies will be needed to determine which of these approaches is efficacious in the long term. It is likely that both approaches, i.e., intestinal FXR agonism and antagonism, will ameliorate fatty liver, albeit via different signaling pathways; one of these involves enteric FGF19 (agonist), whereas the antagonist may lead to primary changes in bile acid composition and the microbiome.
D. Pruritus, Diarrhea and Dyslipidemia
Pruritus (itch) is the most common symptom in PBC and is frequent in PSC and other cholestatic diseases such as intrahepatic cholestasis of pregnancy. Recent advances have identified bile acid activated neuronal TGR5 as one mechanism of cholestasis-induced pruritus (42,43). Though complete understanding of endogenous pruritogens remains obscure, pruritus is a frequent (~60% prevalence on OCA compared to 38% on placebo in 12-month trial in PBC patients) and dose-related adverse effect of OCA and may potentially be observed with TGR5 agonists (32,37,44). Bile acid diarrhea remains a potential side effect of intestinal FXR antagonists and IBAT antagonists. Lastly, increase in plasma LDL has been observed with the FXR agonist, OCA, and the FGF19 agonist, NGM282. Given the high prevalence of cardiovascular disease in obese patients, either a more selective agent without this adverse effect, or concurrent pharmacotherapy to lower LDL cholesterol may be necessary.
III. Conclusions
Bile acid signaling is the endogenous circuit for communication between the microbiome, intestine, liver and also other organ systems such as adipose tissue. Therapeutic manipulation of bile acid signaling has proven to be efficacious in several intestinal and hepatic diseases. This field has seen rapid advances, including the identification of receptors, FXR and TGR5, the development of candidate drugs, and the FDA approval of OCA for PBC patients.
HIGHLIGHTS.
-
*
Bile acids are central signals in enterohepatic communication.
-
*
The natural receptors for bile acids [farnesoid X receptor and G protein-coupled bile acid receptor 1 (GPBAR1) also known as TGR5] and transporters are therapeutic targets.
-
*
Obeticholic acid (a selective FXR agonist) and NGM282 (a non-mitogenic FGF-19 analog) are two of the agents in this pipeline.
Acknowledgments
Funding support: Dr. Camilleri is supported by NIH RO1-DK92179. Dr. Malhi is supported by NIH R03-DK107402, NIH R01-DK111378, by Gilead Sciences and the Palumbo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Camilleri has conducted sponsored research on elobixibat and NGM282.
References
- 1**.Bunnett NW. Neuro-humoral signalling by bile acids and the TGR5 receptor in the gastrointestinal tract. J Physiol. 2014;592:2943–2950. doi: 10.1113/jphysiol.2014.271155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wingate DL, Krag E, Mekhjian HS, Phillips SF. Relationships between ion and water movement in the human jejunum, ileum and colon during perfusion with bile acids. Clin Sci Mol Med. 1973;45:593–606. doi: 10.1042/cs0450593. [DOI] [PubMed] [Google Scholar]
- 3.Kirwan WO, Smith AN, Mitchell WD, et al. Bile acids and colonic motility in the rabbit and the human. Gut. 1975;16:894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bampton PA, Dinning PG, Kennedy ML, et al. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G443–G449. doi: 10.1152/ajpgi.00194.2001. [DOI] [PubMed] [Google Scholar]
- 5.Brighton CA, Rievaj J, Kuhre RE, et al. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein–coupled bile acid receptors. Endocrinology. 2015;156:3961–3970. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bazzoli F, Malavolti M, Petronelli A, Barbara L, Roda E. Treatment of constipation with chenodeoxycholic acid. J Int Med Res. 1983;11:120–123. doi: 10.1177/030006058301100211. [DOI] [PubMed] [Google Scholar]
- 7.Chadwick VS, Gaginella TS, Carlson GL, Debongnie JC, Phillips SF, Hofmann AF. Effect of molecular structure on bile acid-induced alterations in absorptive function, permeability, and morphology in the perfused rabbit colon. J Lab Clin Med. 1979;94:661–674. [PubMed] [Google Scholar]
- 8.Kelly OB, Mroz MS, Ward JB, Colliva C, Scharl M, Pellicciari R, Gilmer JF, Fallon PG, Hofmann AF, Roda A, Murray FE, Keely SJ. Ursodeoxycholic acid attenuates colonic epithelial secretory function. J Physiol. 2013;591:2307–2318. doi: 10.1113/jphysiol.2013.252544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alrefai WA, Saksena S, Tyagi S, Gill RK, Ramaswamy K, Dudeja PK. Taurodeoxycholate modulates apical Cl−/OH− exchange activity in Caco2 cells. Dig Dis Sci. 2007;52:1270–1278. doi: 10.1007/s10620-006-9090-8. [DOI] [PubMed] [Google Scholar]
- 10.Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl− secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am J Physiol Cell Physiol. 2013;305:C447–C456. doi: 10.1152/ajpcell.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peregrin AT, Ahlman H, Jodal M, Lundgren O. Involvement of serotonin and calcium channels in the intestinal fluid secretion evoked by bile salt and cholera toxin. Br J Pharmacol. 1999;127:887–894. doi: 10.1038/sj.bjp.0702615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, Bunnett NW, Corvera CU. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145–154. doi: 10.1053/j.gastro.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijayvargiya P, Busciglio I, Burton D, Donato L, Lueke A, Camilleri M. Bile acid deficiency in subgroup of irritable bowel syndrome with constipation based on serum and fecal biomarkers. Clin Gastroenterol Hepatology. 2017 Jun 27; doi: 10.1016/j.cgh.2017.06.039. pii: S1542-3565(17)30786-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao AS, Wong B, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R, Zinsmeister AR. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong BS, Camilleri M, McKinzie S, Burton D, Graffner H, Zinsmeister AR. Effects of A3309, an ileal bile acid transporter inhibitor, on colonic transit and symptoms in females with functional constipation. Am J Gastroenterol. 2011;106:2154–2164. doi: 10.1038/ajg.2011.285. [DOI] [PubMed] [Google Scholar]
- 17.Chey WD, Camilleri M, Chang L, Rikner L, Graffner H. A randomized placebo-controlled phase IIb trial of A3309, a bile acid transporter inhibitor, for chronic idiopathic constipation. Am J Gastroenterol. 2011;106:1803–1812. doi: 10.1038/ajg.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima A, Seki M, Taniguchi S. Determining an optimal clinical dose of elobixibat, a novel inhibitor of the ileal bile acid transporter, in Japanese patients with chronic constipation: a phase II, multicenter, double-blind, placebo-controlled randomized clinical trial. J Gastroenterol. 2017 Aug 24; doi: 10.1007/s00535-017-1383-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oduyebo I, Nelson AD, Khemani D, et al. NGM282, variant of FGF19, is a gastric and colonic prokinetic and stimulates bowel function in patients with functional constipation: phase 1B, two-dose, placebo-controlled study. Gastroenterology. 2017;152:S1315. [Google Scholar]
- 20*.Valentin N, Camilleri M, Altayar O, Vijayvargiya P, Acosta A, Nelson AD, Murad MH. Biomarkers for bile acid diarrhea in functional bowel disorder with diarrhea: a systematic review and meta-analysis. Gut. 2016;65:1951–1959. doi: 10.1136/gutjnl-2015-309889. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Bañares F, Rosinach M, Piqueras M, et al. Randomised clinical trial: cholestyramine vs. hydroxypropyl cellulose in patients with functional chronic watery diarrhoea. Aliment Pharmacol Ther. 2015;41:1132–1140. doi: 10.1111/apt.13193. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox C, Turner J, Green J. Systematic review: the management of chronic diarrhoea due to bile acid malabsorption. Aliment Pharmacol Ther. 2014;39:923–939. doi: 10.1111/apt.12684. [DOI] [PubMed] [Google Scholar]
- 23.Bajor A, Törnblom H, Rudling M, et al. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut. 2015;64:84–92. doi: 10.1136/gutjnl-2013-305965. [DOI] [PubMed] [Google Scholar]
- 24.Camilleri M, Acosta A, Busciglio I, et al. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:438–448. doi: 10.1111/apt.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol and Hepatol. 2010;8:159–165. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin S, Sanders DS, Gleeson JT, et al. Long-term outcomes in patients diagnosed with bile-acid diarrhoea. Eur J Gastroenterol Hepatol. 2016;28:240–245. doi: 10.1097/MEG.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 27.Walters JR, Johnston IM, Nolan JD, et al. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41:54–64. doi: 10.1111/apt.12999. [DOI] [PubMed] [Google Scholar]
- 28.Weingarden AR, Dosa PI, DeWinter E, et al. Changes in colonic bile acid composition following fecal microbiota transplantation are sufficient to control Clostridium difficile germination and growth. PLoS One. 2016;11:e0147210. doi: 10.1371/journal.pone.0147210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weingarden AR, Chen C, Zhang N, et al. Ursodeoxycholic acid inhibits Clostridium difficile spore germination and vegetative growth, and prevents the recurrence of ileal pouchitis associated with the infection. J Clin Gastroenterol. 2016;50:624–630. doi: 10.1097/MCG.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staley C, Kelly CR, Brandt LJ, et al. Complete microbiota engraftment is not essential for recovery from recurrent Clostridium difficile infection following fecal microbiota transplantation. MBio. 2016;7 doi: 10.1128/mBio.01965-16. pii: e01965-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31**.Chávez-Talavera O, Tailleux A, Lefebvre P, et al. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152:1679–1694 e3. doi: 10.1053/j.gastro.2017.01.055. [DOI] [PubMed] [Google Scholar]
- 32.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary dholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 33.Kuiper EM, Hansen BE, de Vries RA, et al. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281–1287. doi: 10.1053/j.gastro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 34**.Hegade VS, Kendrick SF, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–1123. doi: 10.1016/S0140-6736(17)30319-7. [DOI] [PubMed] [Google Scholar]
- 35.Mayo MJ, Wigg AJ, Roberts SK, et al. NGM282, a novel variant of FGF-19, demonstrates biologic activity in primary biliary cirrhosis patients with an incomplete response to ursodeoxycholic acid: results of a phase 2 multicenter, randomized, double blinded, placebo controlled trial. Hepatology. 2015;62:263A–264A. [Google Scholar]
- 36.Baghdasaryan A, Claudel T, Gumhold J, et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO−3 output. Hepatology. 2011;54:1303–1312. doi: 10.1002/hep.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison SA, Abdelmalek MF, TroRer JF, et al. NGM282, a novel variant of FGF19, significantly reduces hepatic steatosis and key biomarkers of NASH: results of a phase 2, multicenter, randomized, double-blinded, placebo controlled trial in biopsy-confirmed NASH patients. Presented at The International Liver Congress™; Amsterdam, Netherlands. April 22, 2017. [Google Scholar]
- 39*.Jiang C, Xie C, Li F, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao A, Kosters A, Mells JE, et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci Transl Med. 2016;8:357ra122. doi: 10.1126/scitranslmed.aaf4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang S, Suh JM, Reilly SM, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42*.Alemi F, Kwon E, Poole DP, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beuers U, Kremer AE, Bolier R, et al. Pruritus in cholestasis:facts and fiction. Hepatology. 2014;60:399–407. doi: 10.1002/hep.26909. [DOI] [PubMed] [Google Scholar]
- 44.Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–761. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]


