Abstract
Objective
Cognitive impairment associated with late-life depression can persist after remission of mood symptoms. Apathy, a common symptom of late-life depression, often leads to worse clinical outcomes. We examined if severity of apathy mediates cognitive difficulties in a cohort of older adults with major depression.
Methods
One hundred and thirty-eight older adults with depression (54.4% female; mean age=69.7(7.4) years; mean education=15.6(2.7) years) were recruited to participate in a treatment study, and only baseline data were analyzed. All participants received a comprehensive evaluation of depression, apathy, and cognition. We examined whether apathy mediated the relationship between depression and cognition, focusing our attention on memory and cognitive control. We then explored whether the mediation effects differed across females and males.
Results
Increased apathy was significantly associated with worse depression and lower performance in the cognitive control domain but not in memory. Higher depressive scores were significantly associated with worse cognitive control but not memory. Mediation analyses revealed a significant indirect effect on cognitive control by depression through increased apathy scores with the mediator accounting for 21% of the total effect. Stratifying by sex, we found that females exhibited a significant indirect effect, with the mediator accounting for 47% of the total effect, while there was no mediation by apathy in males.
Conclusion
The findings imply that increased apathy mediates the relationship between cognition and depression. The identification of mediating effects may inform future treatment strategies and preventive interventions that can focus on decreasing apathy to improve cognition in late-life depression.
Keywords: Apathy, cognition, sex differences, late life depression
OBJECTIVE
Cognitive impairment in late-life depression has been well-documented in the literature1,2, with patients often demonstrating difficulties in subjective memory3 and cognitive control4. The impact of cognitive impairment on the quality of life of older adults is high, including increased loss of independence in activities of daily living5, poorer health self-care6, and even increased frailty and fall risk in patients with reduced executive abilities7. While not well understood, variability in the presentation, quality and severity of cognitive dysfunction is present in older adults with depression2,8. Research to determine attributes of depression that may directly relate to cognitive dysfunction would help providers deliver more targeted interventions, and reduce the impact of cognitive impairment on daily function of older adults.
Apathy is a prominent symptom in late-life depression in older adults and has been associated with poorer response to antidepressant medications9,10. Apathy has also been linked to higher rates of disability11, particularly among the oldest old12. In clinical studies with patients with Alzheimer’s disease and Parkinson’s disease, apathy has been proposed to be a frontally-mediated symptom, and has been observed to act as a mediator for cognitive impairment in these populations13. Emerging evidence supports apathy as a separate construct from depression despite frequent comorbidity.14,15 In addition, neuroimaging studies have found differential functional and structural correlates between depressed patients with and without apathy, for example in white matter microstructural changes16 and altered structural and functional connectivity10. This research further suggests that apathy may play a distinct role in cognitive functioning in late-life depression.
To our knowledge, no previous study has examined apathy as a possible mediator of the association between severity of depression and cognitive difficulties in depressed patients and only few studies have investigated the relationship between apathy and cognitive impairment across cognitive domains in depressed older adults10. To address this knowledge gap, we studied the predictive ability of depression and apathy on two of the most commonly reported cognitive impairments in depression, memory and cognitive control3,4. Based on the literature, we predicted that apathy would mediate memory and executive control difficulties in older adults with major depressive disorder. Further, we examined if apathy played a different role in male and female participants with late-life depression, as gender differences in apathy prevalence have been observed in other clinical samples such as Parkinson’s disease,17 and multiple sclerosis18.
METHODS
Participants
A total sample of 138 elderly depressed participants who had complete mood, apathy and neuropsychological testing at baseline was drawn from a larger, clinical trial examining the use of methylphenidate to improve antidepressant response to citalopram9. The measure outcomes utilized in this study were all baseline measures, prior to randomization into a study condition. Briefly, from August 2008 to September 2012, 510 subjects were screened by phone, and 203 individuals were recruited for a diagnostic interview. After describing the details of the study to interested and eligible subjects, written informed consent was obtained in accordance with the procedures set by the UCLA Institutional Review Board (IRB). Of these 203 subjects, 143 were randomized into the study. Five subjects had some missing cognitive data, so baseline data from 138 subjects were analyzed for the present study. Further details on recruitment are described in the original study9.
Inclusion criteria to the clinical trial were: 1) current episode of unipolar MDD according to DSM-IV-TR criteria19; 2) Hamilton Depression Rating Scale (HDRS-24)20 score ≥ 16; and 3) Mini-Mental State Exam (MMSE)21 score ≥ 26. Exclusion criteria were: 1) history of any other psychiatric disorders (other than unipolar MDD with or without comorbid anxiety symptoms); 2) severe or acute unstable medical illness, including the presence of either atrial or ventricular arrhythmia, or acute ischemic changes on the baseline electrocardiogram; 3) acute suicidal or violent behavior or history of suicide attempt within the last year; or 4) any other central nervous system diseases. Patients were free of psychotropic medications for at least two weeks before starting the trial.
Measures
Participants’ depression severity was evaluated using the HDRS-24,20 a measure that does not contain questions that directly assess prominent symptoms of apathy (e.g., disinterest, indifference, suppression of emotion). Apathy was measured using the Apathy Evaluation Scale (AES)22, which specifically identifies the degree to which the patients display emotional, cognitive and motor symptoms of apathy. All items in AES were coded so that greater scores represent less apathy. Level of depression and apathy (high vs. low) were characterized to identify distinction in symptom presentation (see results). Participants also completed a comprehensive neuropsychological test battery to assess the two most frequently compromised cognitive domains in depression: memory, defined as the ability to recall previously learned information over a delay (California Verbal Learning Test-II23 [long delayed free recall], Rey–Osterrieth Complex Figure Test24 [30-minute delayed recall]), and cognitive control, defined as the ability to quickly bypass automatic processes and identify irrelevant information to execute a complex task25 (the Trail Making Test, parts A and B26, and the Stroop Color Trial and interference task27). We transformed raw scores to z-scores for each participant, and then averaged the z-scores within each domain to produce composite scores. Z-scores were calculated from published normative data28. For variables in which better performance was represented by lower values (e.g., Trail Making Test), z-scores were reversed so that higher z-scores represented better performance for all measures.
Statistical analysis
Data were screened for outliers and normality assumptions. General linear models were used to examine associations between depression and cognitive domain (memory, cognitive control) scores. We then conducted mediation analyses using Andrew Hayes’s PROCESS Procedure29 for SAS, with depression as the predictor, cognitive scores as the outcome and apathy as the mediator. Path coefficients (a, b, c, and c’) representing the linear regression coefficients for each path in the mediation model (Figure 1) were obtained. The a-path represents the association between the predictor and mediator variables. The b-path denotes the relationship between the mediator and outcome variables, while also controlling for the predictor variable. The c’-path (also called “direct effect”) and the c-path (also known as “total effect”) represent the associations between the predictor and outcome variables including and excluding the mediator variable, respectively. A statistically significant difference between c and c’ (or equivalently, significance of a*b, also known as “indirect effect”) is indication of a mediation effect. Bootstrapped 95% confidence interval of the mediation effect was determined using 5000 simulations30. In addition, percent mediation [PM], a measure of effect size interpreted as the percent of the total effect accounted for by the indirect effect was calculated. We then repeated the above mediation analyses stratified by sex to examine whether mediation effects are present in females and males. We also estimated a moderated (by sex) mediation model to test for differential effects in females and males. Age and educational level were used as covariates in all analyses and sex was an additional covariate in the model that included all participants. These demographic factors have been commonly associated with cognitive performance differences in the literature.1,2 Further, the effects of age, sex and education were significant (p-values ranging from .001 to .05) in several of the regression models. We also considered using ethnicity as a covariate, however in all the regression models, the effect of ethnicity was not significant (p-values ranging from 0.3–0.5).
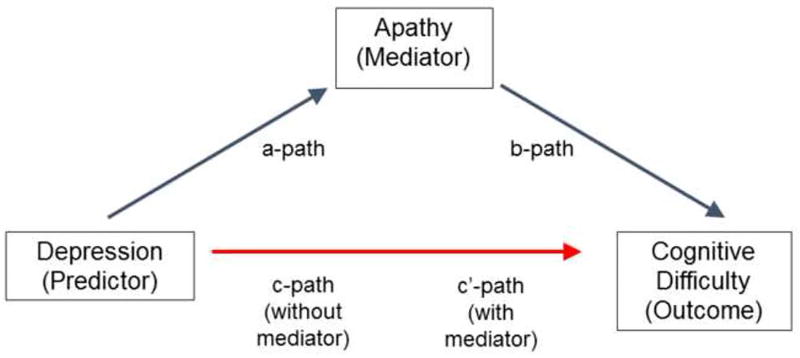
Figure 1.
Mediation Model. The a-path represents the association between depression (HDRS-24) scores and apathy. The b-path denotes the relationship between apathy and cognitive scores, while also controlling for depression. The c-path and the c’-path represent the associations between HDRS-24 and cognitive scores without and with apathy included as a mediator, respectively. Age, sex, educational level used as covariates in all analyses.
RESULTS
Demographic and clinical characteristics
Demographic and clinical characteristics of the participants are shown in Table 1. Table 2 presents the raw neuropsychological test scores as well as the z-scores of the cognitive domains. There were no significant differences between male and female participants with regard to age, education, ethnicity as well as severity of depression, apathy, and cognition. Dividing the participants into 4 groups based on the median values of depression (18) and apathy (30), we found that 28.3% of the sample presented with high levels of depression and high apathy scores (n=39), 26.1% presented with high depression and low apathy scores (n=36), and 18.8% of the same presented with lower depression scores in the presence of high apathy (n=26). Thus 44.9% of the sample has (high depression and low apathy) or (low depression and high apathy), supporting past research that suggests that apathy and depression, while correlated, have independent roles to play.14
Table 1.
Demographic and Clinical Characteristics* of the Sample
| Females (N=75) |
Males (N=63) |
||
|---|---|---|---|
| Age (years) | 68.2 (6.3) | 71.3 (8.1) | |
| Education (years) | 15.7 (2.1) | 15.5 (3.2) | |
| Race/ethnicity | |||
| Caucasian | 77.3% | 71.4% | |
| African American | 10.7% | 11.1% | |
| Asian | 4.0% | 4.8% | |
| Hispanic | 8.0% | 12.8% | |
| MMSEa | 28.9 (1.2) | 28.5 (1.3) | |
| HDRS-24b | 19.0 (2.9) | 18.8 (3.2) | |
| AESc | 30.9 (9.7) | 29.9 (9.8) | |
Mean (SD) are presented for continuous variables.
Mini-Mental State Exam
Hamilton Depression Rating Scale
Apathy Evaluation Scale
Table 2.
Cognitive Scores* of Participants
| Females (N=75) |
Males (N=63) |
||
|---|---|---|---|
| Memory | |||
| Domain Z-score | −0.11 (0.81) | 0.00 (0.74) | |
| CVLT LDFRa | −0.19(1.04) | −0.07 (0.92) | |
| Rey Delayed Recallb | 13.01 (6.12) | 13.40(6.47) | |
| Cognitive Control | |||
| Domain Z-score | −0.28 (0.87) | −0.42 (0.82) | |
| Trails Ac | 40.51 (15.66) | 42.79(17.13) | |
| Stroop Colord | 63.29(13.90) | 58.40(12.01) | |
| Trails Bc | 100.67(81.94) | 114.67(71.64) | |
| Stroop Interferencee | −0.30(0.71) | −0.30 (0.75) | |
Mean (SD) are presented for all measures.
California Verbal Learning Test-II long delayed free recall
Rey-Osterrieth Complex Figure Test 30-minute delayed recall
Trail Making Test parts A and B
Stroop Color Trial
Stroop interference task
Mediation Analyses
Increased apathy was significantly associated with more severe depression (a-path: slope = −1.36, t(133) = −5.5, p < .0001) and worse cognitive control (b-path: slope = 0.02, t(133) = 2.3, p < .02) but not memory (slope = −0.001, t(133) = −0.3, p = .8). Higher HDRS-24 scores correlated with lower cognitive control (c-path: slope = −0.06, t(133) = −2.5, p < .01), and this association became non-significant after controlling for apathy (c’-path: slope = −0.04, t(132) = −1.8, p < .09). In contrast, greater depressive scores were not associated with lower memory scores (slope = −0.02, t(133) = −1.0, p = .3).
There was a significant indirect effect on cognitive control by depression through increased apathy scores (a*b = −0.02 (0.01), bootstrap p = .05, 95% CI [-0.03, −0.001]). The mediator accounted for about 21% of the total effect (Table 3; See also Figure 1). Stratified analyses by sex revealed a significant mediation effect in females, (a*b = −0.03 (0.02), bootstrap p = .04, 95% CI [-0.07, −0.002]) with apathy accounting for 47% of the total effect (Table 3). However, there was no mediation by apathy on the relationship between depression and cognitive control in males (a*b = −0.0, bootstrap p = 0.4, CI [-0.02, 0.03]). A moderated mediation did not support a differential effect in females and males, indicating that the difference between females and males was not statistically significant (index of moderated mediation = −.003(.0025), bootstrap p = .2, CI [-0.02, .003]).
Table 3.
Results of Mediation Analysis, for all subjects, and in females and males separately.
| Path Coefficients* | ||||
|---|---|---|---|---|
| Path a | Path b | Path c | Path c’ | |
| All subjects (N=138) | slope = −1.36, t(133) = −5.5, p < 0.0001 | slope = 0.02, t(133) = 2.3, p < .02 | slope = −0.06, t(133) = −2.5, p < .01 | slope = −0.04, t(132) = −1.8, p < .09 |
| Females (N=75) | slope = −1.52, t(71) = −4.4, p < 0.0001 | slope = 0.03, t(71) = 2.6, p < .01 | slope = −0.07, t(71) = −2.0, p < .05 | slope = −0.04, t(70) = −1.0, p < .3 |
| Males (N=63) | slope = −1.15, t(59) = −3.3, p < 0.002 | slope = 0.00, t(59) = 0.0, p < .9 | slope = −0.04, t(59) = −1.4, p < .2 | slope = −0.05, t(58) = −1.5, p < .15 |
The a-path represents the association between depression (HDRS-24) scores and apathy. The b-path denotes the relationship between apathy and cognitive control scores, while also controlling for depression. The c-path and the c’-path represent the associations between HDRS-24 and cognitive control scores without and with apathy included as a mediator, respectively. Age, sex, educational level used as covariates in all analyses. See also Figure 1.
CONCLUSIONS
This study demonstrates that apathy mediates the association between depression severity and cognitive control in depressed older adults. These findings are supported by recent imaging studies that have suggested differential structural correlates in depressed patients with or without apathy. Specifically, Hollocks and colleagues recently observed that apathy was associated with reduction in widespread white matter integrity in patients with small vessel disease who had depression and apathy, with the strongest reductions observed in limbic association tracts16. The relationship between executive functions, such as cognitive control, and white matter integrity in the limbic association tracts has been related to late-life depression in the literature31. However, Hollocks and colleagues did not observe this relationship in patients with small vessel disease who had depression without apathy16. In addition, Yuen and colleagues have also observed a relationship between apathy and frontolimbic white and grey matter abnormalities, and this association was persistent despite SSRI treatment for depression10. These findings, as well as the result of this study, suggest that while apathy is a common symptom of late-life depression, it appears to be a distinct entity with discrete impacts on structural integrity of the brain, as well as on cognition for older adults with depression.
Interestingly, when we examined whether the mediation effect of apathy was consistent across sex, we found that apathy mediated the effect of depression on cognitive control for women, and not for men, even though the difference between females and males failed to reach statistical significance in this sample. Studies in other clinical populations have found that while women have higher rates of depression overall, men are more likely to have depression with apathy than women,17,18 and older age has also been observed to have higher correlation with apathy for men than women32. In this study, while severity of apathy present among men and women did not differ, apathy appeared to mediate cognitive control for women. Some neuroimaging studies have found differences in white matter microstructural changes between depressed patients with and without apathy16. These vascular changes may also account for sex differences in neural connectivity that have been documented in the literature33, particularly in thalamus-driven brain networks34 involved in complex global integration of cognitive activity. In a recent study, Lei and colleagues observed sex differences within the striatal-lateral prefrontal circuitry that they believe may relate to sex-specific neuroanatomical correlates involved in reward-related cognitive control34. Apathy itself has been theorized as a lack of motivation or interest for future rewards, and has also been linked to inefficient prefrontal activation35, further suggesting the possibility of unexplored sex differences in connectivity and functional activation in these regions. Future research may examine whether apathy itself impacts cognition differently for women with late-life depression than it does for men.
In addition, while we found that higher depression severity was associated with lower cognitive control, we did not observe a similar association with memory. In other words, our results suggest that depression impacts the ability to process information and respond to environmental demands, such as may be needed to complete complex speeded tasks, or hold information in working memory, but we did not observe this association with the recall of words or figures as measured by our memory recall score. While clinicians observe a strong relationship between depression and subjective memory complaints,3 objective memory deficits during cognitive assessment have been inconsistently reported in the literature. A recent meta-analysis indicates that verbal and visual learning and memory performance may not be significantly worse in depressed subjects compared to controls whereas psychomotor speed, attention and executive functioning may be more significantly impacted36. It is possible that subjective memory complaints in late-life depression may be better accounted for by deficits in these cognitive processes, or may be better characterized by alternative methods of assessment not fully captured in this study.
Overall, our study suggests that apathy may be a key mediator of dysfunction in cognitive control in late-life depression, particularly for women. While depression screening has increasingly become more standard during medical visits, apathy has not been a specific target for clinical screening. In light of research demonstrating that apathy is a distinct clinical syndrome with its unique neural correlates14, that may not respond to traditional antidepressants as favorably as other symptoms of depression9,10, this finding has strong implications for treatment of depression in older adults. This research suggests that further apathy screening may be beneficial for depressed patients presenting with cognitive complaints in late life as treatment specifically targeting apathy may be necessary to improve the daily functioning of patients with late-life depression and cognitive impairment. In addition, as traditional antidepressants may not be ideally suited to treating apathy, some research has proposed that memantine, a noncompetitive NMDA antagonist traditionally used to treat cognitive impairment in dementia, may show beneficial therapeutic effects on apathy37. However, that research has not always been replicated, and in a review of current research involved in treating apathy in patients, Harrison and colleagues concluded that substantial pharmacological strategies for treating apathy are yet to be developed38. Indeed, they emphasized that while some pharmacotherapies, including antipsychotics, anticholinergic medications, and psychostimulants have shown some potential benefit, most of the improvements in apathy were found only in combination with nonpharmacological approaches such as exercise, social interaction, or occupational therapy, although these type of studies are sparse. A few formal treatment protocols targeting apathy in Parkinson’s disease and Schizophrenia are beginning to be researched, including interventions involving values clarification, behavioral activation39 as well as meditation and cognitive restructuring40. While promising, these studies require further investigation. Our study also raises the question of whether woman are more heavily impacted by apathy, and further research could lead to more specific interventions for women.
Although these results have important treatment implications, several study limitations deserve comment. An important limitation is the use of a cross-sectional study design to examine the mediational effect of apathy. A future study using longitudinal data may shed further light on the proposed mediation model. We also utilized a convenience sample of outpatients with major depression of moderate severity, and this sample was taken from patients willing to undergo a medical trial for depression, which limits its generalizability to other groups. The study also excluded individuals with anxiety disorders, an often-observed comorbidity in late-life depression, which further limits its generalizability. Finally, our study utilized a self-report measure of symptoms associated with apathy, and further research may also consider incorporating an observer reported measure to better assess severity of apathy. Despite such limitations, our study findings provide further impetus for research into possible interventions that target apathy, as targeted treatment may reduce the functional burden of cognitive impairment in late-life depression.
Highlights.
Cognitive impairment in late-life depression can persist after improvements in mood.Apathy, a common symptom of depression, has poorer response to antidepressants. We examined whether apathy mediates cognitive difficulties in a cohort of 138 older adults with depression. Subjects underwent an assessment of depression, apathy and cognition. Results suggest that increased apathy mediates the relationship between cognition and depression, especially in females. These findings may inform treatment strategies focused on improving cognition in late-life depression.
Acknowledgments
This work was supported by the NIH grants MH086481; MH077650; AT009198 to Dr. Lavretsky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Part of this work was presented at the Annual Meeting of the American College of Neuropsychopharmacology, Dec 4–8, 2016, Hollywood, Florida, USA
References
- 1.Sheline YI, Barch DM, Garcia K, et al. Cognitive Function in Late Life Depression: Relationships to Depression Severity, Cerebrovascular Risk Factors and Processing Speed. Biol Psychiatry. 2006;60(1):58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS. Diagnosis and treatment of depression and cognitive impairment in late life. Ann N Y Acad Sci. 2015;1345(1):36–46. doi: 10.1111/nyas.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehrner J, Moser D, Klug S, et al. Subjective memory complaints, depressive symptoms and cognition in Parkinson’s disease patients. Eur J Neurol. 2014;21(10):1276–1e77. doi: 10.1111/ene.12470. [DOI] [PubMed] [Google Scholar]
- 4.Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annu Rev Clin Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill TM, Richardson ED, Tinetti ME. Evaluating the risk of dependence in activities of daily living among community-living older adults with mild to moderate cognitive impairment. [Accessed February 21, 2017];J Gerontol A Biol Sci Med Sci. 1995 50(5):M235-41. doi: 10.1093/gerona/50a.5.m235. http://www.ncbi.nlm.nih.gov/pubmed/7671024. [DOI] [PubMed] [Google Scholar]
- 6.Verhaak PFM, Dekker JH, De Waal MWM, Van Marwijk HWJ, Comijs HC. Depression, disability and somatic diseases among elderly. J Affect Disord. 2014;167:187–191. doi: 10.1016/j.jad.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 7.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: A review of the literature. Maturitas. 2013;75(1):51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Richard E, Reitz C, Honig LH, et al. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70(3):374–382. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavretsky H, Reinlieb M, Cyr NS, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: A randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015;172(6):561–569. doi: 10.1176/appi.ajp.2014.14070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuen G, Gunning F, Woods E. Neuroanatomical correlates of apathy in late-life depression and antidepressant treatment response. J Affect Disord. 2014;166:179–186. doi: 10.1016/j.jad.2014.05.008. http://www.sciencedirect.com/science/article/pii/S0165032714002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen GS, Bhutani S, Lucas BJ, et al. Apathy in late-life depression: Common, persistent, and disabling. Am J Geriatr Psychiatry. 2015;23(5):488–494. doi: 10.1016/j.jagp.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta M, Whyte E, Lenze E, et al. Depressive symptoms in late life: associations with apathy, resilience and disability vary between young-old and old-old. Int J Geriatr Psychiatry. 2008;23(3):238–243. doi: 10.1002/gps.1868. [DOI] [PubMed] [Google Scholar]
- 13.Lim N, Massimo L, Fitts W, Dahodwala N, Grossman M. Differential apathy profiles in Alzheimer’s disease and Parkinson’s disease (P6.198) Neurology. 2015;84(14 Supplement):P6.198. [Google Scholar]
- 14.Eyre HA, Siddarth P, van Dyk K, et al. Neural correlates of apathy in late-life depression: a pilot [ 18 F]FDDNP positron emission tomography study. Psychogeriatrics. 2017 Jan; doi: 10.1111/psyg.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butterfield LC, Cimino CR, Oelke LE, Hauser RA, Sanchez-Ramos J. The independent influence of apathy and depression on cognitive functioning in Parkinson’s disease. Neuropsychology. 2010;24(6):721–730. doi: 10.1037/a0019650. [DOI] [PubMed] [Google Scholar]
- 16.Hollocks MJ, Lawrence AJ, Brookes RL, et al. Differential relationships between apathy and depression with white matter microstructural changes and functional outcomes. Brain. 2015;138(12):3803–3815. doi: 10.1093/brain/awv304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrin AJ, Nosova E, Co K, et al. Gender differences in Parkinson’s disease depression. Park Relat Disord. 2017;26:93–97. doi: 10.1016/j.parkreldis.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Novo AM, Batista S, Tenente J, et al. Apathy in multiple sclerosis: gender matters. J Clin Neurosci. 2016;33:100–104. doi: 10.1016/j.jocn.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Vol. Washington: 2000. [DOI] [Google Scholar]
- 20.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. [Accessed February 21, 2017];Psychiatry Res. 1991 38(2):143–162. doi: 10.1016/0165-1781(91)90040-v. http://www.ncbi.nlm.nih.gov/pubmed/1754629. [DOI] [PubMed] [Google Scholar]
- 23.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test – second edition. Adult version. Manual. Test. 2000 [Google Scholar]
- 24.Meyers JE, Meyers KR. Rey complex figure test under four different administration procedures. Clin Neuropsychol. 1995;9(1):63–67. doi: 10.1080/13854049508402059. [DOI] [Google Scholar]
- 25.MacLeod C. Half a century of research on the Stroop effect: An integrative review. [Accessed March 7, 2017];Psychol Bull. 1991 109(2):163–203. doi: 10.1037/0033-2909.109.2.163. http://melaniestefan.net/MacLeod1991.pdf. [DOI] [PubMed] [Google Scholar]
- 26.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery and REHABIT: A model for integrating evaluation and remediation of cognitive impairment. Cogn Rehabil. 1988;6:10–17. [Google Scholar]
- 27.Golden CJ, Marsella AJ, Golden EE. Cognitive relationships of resistance to interference. J Consult Clin Psychol. 1975;43(3):432–432. doi: 10.1037/h0076772. [DOI] [PubMed] [Google Scholar]
- 28.Selnes OA, Jacobson L, Machado AM, Becker JT. Normative data for a brief neuropsychological screening battery. Percept Mot Skills. 1991;73(2):539–550. doi: 10.2466/pms.1991.73.2.539. [DOI] [PubMed] [Google Scholar]
- 29.Hayes A. Introduction to mediation, moderation, and conditional process analysis. New York, NY Guilford. 2013:3–4. doi:978-1-60918-230-4. [Google Scholar]
- 30.Hesterberg T, Moore DSD, Monaghan S, Clipson A, Epstein R. Bootstrap Methods and Permutation Tests. 2005;5 doi: 10.1002/wics.182. [DOI] [Google Scholar]
- 31.Murphy CF, Gunning-Dixon FM, Hoptman MJ, et al. White-matter integrity predicts Stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61(8):1007–1010. doi: 10.1016/j.biopsych.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer A, Bousleiman H, Chaturvedi M, et al. P136. Relation of EEG frequency and apathy in patients with Parkinson’s disease (PD) Clin Neurophysiol. 2015;126(8) doi: 10.1016/j.clinph.2015.04.259. [DOI] [Google Scholar]
- 33.Ingalhalikar M, Smith A, Parker D, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111(2):823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei X, Han Z, Chen C, Bai L, Xue G, Dong Q. Sex differences in fiber connection between the striatum and subcortical and cortical regions. Front Comput Neurosci. 2016;10:1–9. doi: 10.3389/fncom.2016.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fazio L, Logroscino G, Taurisano P, et al. Prefrontal activity and connectivity with the basal ganglia during performance of complex cognitive tasks Is associated with apathy in healthy subjects. PLoS One. 2016;11(10):1–15. doi: 10.1371/journal.pone.0165301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee RSC, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. 2012;140(2):113–124. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Links KA, Black SE, Graff-Guerrero A, et al. A case of apathy due to frontotemporal dementia responsive to memantine. Neurocase. 2013;19(3):256–261. doi: 10.1080/13554794.2012.667120. [DOI] [PubMed] [Google Scholar]
- 38.Harrison F, Aerts L, Brodaty H. Apathy in dementia: systematic review of recent evidence on pharmacological treatments. Curr Psychiatry Rep. 2016;18(103):1–12. doi: 10.1007/s11920-016-0737-7. [DOI] [PubMed] [Google Scholar]
- 39.Butterfield LC, Cimino CR, Salazar R, Sanchez-Ramos J, Bowers D, Okun MS. The Parkinson’s Active Living (PAL) Program. J Geriatr Psychiatry Neurol. 2017;30(1):11–25. doi: 10.1177/0891988716673467. [DOI] [PubMed] [Google Scholar]
- 40.Acevedo BP, Pospos S, Lavretsky H. The neural mechanisms of meditative practices: novel approaches for healthy aging. Curr Behav Neurosci reports. 2016;3(4):328–339. doi: 10.1007/s40473-016-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]