Abstract
Background
Efforts are underway to eliminate fetal bovine serum (FBS) from mammalian cell cultures for clinical use. An emerging viable replacement option for FBS is human platelet lysate (PL) either as a plasma (PL-P) or serum (PL-S) based product.
Study Design and Methods
Nine industrial scale PL-S manufacturing runs (i.e. lots) were performed that consisted of an average volume of 24.6±2.2 liters of pooled platelet-rich-plasma (PRP) units that were obtained from apheresis donors. Manufactured lots were compared by evaluating various biochemical and functional test results. Comprehensive cytokine profiles of PL lots and product stability testing was performed. Global gene expression profiles of Mesenchymal Stromal Cells (MSCs) when cultured with PL-S or PL-P were compared with FBS.
Results
Electrolyte and protein levels were relatively consistent among all PL-S lots with only slight variations in glucose and calcium levels. All nine lots were as good as or better than FBS in expanding MSCs. PL-S stored at −80°C remained stable over two years. Quantitative cytokine arrays showed similarities as well as dissimilarities in the proteins present in PL-S. Greater differences in MSC gene expression profiles were attributable to the starting cell source than as to whether PL or FBS were used as culture supplements.
Conclusion
Using a large-scale standardized method, lot-to-lot variations were noted for industrial scale preparations of PL-S. However, all lots were as good as or better than FBS in supporting MSC growth. Together these data indicate that off-the-shelf PL is a feasible substitute for FBS in MSC cultures.
Keywords: Platelet Lysate, Mesenchymal Stromal Cells, Mesenchymal Stem Cells
BACKGROUND
FBS remains the most widely used serum supplement for culturing mammalian cells. With the advent of using cultured derived mammalian cells for therapeutic applications, there is an active pursuit to eliminate xenogeneic supplements in culture medium. Several reasons for this change include avoiding risks associated with transmitting infectious bovine agents (i.e. parvovirus and prions) and exposure to residual xenoproteins that could potentially cause immune reactions in patients. Other motivating factors for replacing FBS include batch-to-batch variations associated with different FBS lots and the rising ethical concerns surrounding the inhumane practices for collecting bovine fetal blood used to manufacture FBS (1).
Strategies under investigation to replace FBS in mammalian cell cultures include the development of chemically defined culture media that are free of xenogeneic components or the use of human serum sources as supplements for basal medium. Progress with developing chemically defined medium is being made (2), but a drawback of the serum-free medium is that they typically show sub-optimal performance (3), and fail to support the attachment of cells to a surface or substrate unless the culture plates are pre-coated with factors that facilitate cellular attachment (i.e. fibronectin) (4). Human protein sources a replacements for FBS include autologous adult serum, pooled AB serum, umbilical cord blood serum and PL (5–7). All of which have been used intermittently in the past to promote mammalian cell cultures (8–12). Of these strategies, PL is emerging as one of the most viable alternatives to FBS.
MSCs when cultured with PL tend to have greater proliferation responses and better cloning efficiencies than MSCs grown with similar concentrations of FBS (13–17). It is also now possible to obtain commercial sources of human PL to replace FBS (18). However, since methods used to produce these off-the-shelf PL products (19) can vary among manufacturers, it is unclear as to how different lots of PL when prepared by different manufacturers compare to one another. It is also unclear as to how different industrial scale lots of PL from the same manufacturer compare to one another.
The aim of this study was to compare the content and functional activity of multiple lots of PL-S that were manufactured on an industrial scale basis when using the same GMP compliant standardized method by the same manufacturer. Using an industrial scale GMP compliant protocol, PL-S was prepared from pooled expired PRP apheresis units that were collected by FDA registered Blood Banks. To manufacture PL-S, PRP apheresis units underwent a freeze/thaw process to obtain PL-P. After conversion of the PL-P to PL-S, the units were pooled, mixed, filtered, and dispensed into defined aliquots.
MATERIALS AND METHODS
Industrial Scale Manufacturing of Platelet Lysate
Single-donor PRP apheresis units ≤3 days post-expiration were obtained from FDA regulated Blood Banks and were placed in storage at −80°C. Donors of platelet apheresis units were screened and tested by Blood Centers for required viral markers (i.e. Hepatitis B Surface Antigen, (hepatitis B core antibody, hepatitis C antibody, Human Immunodeficiency Virus Types 1 and 2, and Syphilis, H
Industrial scale lots of PL-S were manufactured according to our previously published paper (16). Briefly, frozen PRP units from multiple donors (49–109 units) were thawed at 4°C and were transferred into satellite bags. Calcium chloride (20% w/v) was added and each bag was placed at 4°C. The following day bags were centrifuged at 4000×g for 20 min and the PL-S was pooled into a large biocontainer (Pall Corporation, Port Washington, NY). Pooled PL-S was filtered using a proprietary process, the final product was aliquoted, and release testing included sterility, biochemical analyses, and a functional assessment. PL-P was produced essentially the same way as for PL-S, except that CaCl2 was not added and the lot sizes were about half the size as the lots of PL-S.
Sterility, Mycoplasma, and Endotoxin Testing
Sterility testing was performed on pre-processed pooled PL-P, during processing, and on final product. Pre-processing sterility testing involved an inoculation of BACTEC Plus Aerobic/F, Plus Anaerobic/F, and Myco F/Lytic (Becton Dickinson, Sparks, MD) culture bottles and then sending the culture bottles to ARUP Laboratories for testing. Final product sterility testing followed USP <71> guidelines (LABS, Inc., Centennial, CO) and mycoplasma testing followed USP <63> guidelines (Clongen Laboratories, LLC Gaithersburg, MD). Endotoxin was performed using the Endosafe-PTS test system (Charles River, Charleston, SC).
Biochemical Testing
Biochemical tests were sent to ARUP Laboratories (Salt Lake City, UT). Quantitative sandwich enzyme-linked immunosorbent assays (ELISA) for platelet derived growth factor isoform BB (PDGF-BB Quantikine ELISA kit; R & D Systems, Minneapolis, MN), vascular endothelial growth factor (VEGF Quantikine ELISA kit; R & D Systems), epidermal growth factor (EGF Quantikine ELISA kit; R & D Systems) and basic fibroblast growth factor (bFGF Quantikine ELISA kit; R & D Systems) were performed according to the manufacturer’s instructions (R & D, Systems, Minneapolis, MN).
Functional Testing of PL lots
MSCs used for the functional studies were derived from a BM purchased from Lonza Walkersville, Inc. (Walkersville, MD). The MSCs were cultured, harvested at passage 2 (P2), and the single lot of MSCs was aliquoted and stored at <150 °C until use.
Two methods were used to quantitate MSC proliferation responses. The first method used trypan blue staining and a manual cell count to quantitate the proliferative response of MSCs. An aliquot of MSCs was thawed and cells were seeded at 5,000 cells/cm2 in replicates of 3 in 6-well plates. In parallel, the P2 MSCs were also seeded in T75 flasks at 5,000 cells/cm2 for each condition. Cells from the T75 flask were used for expansion and subsequent passaging. All culture were performed with basal medium [(i.e. alpha-minimum essential medium (α-MEM) (ThermoFisher Scientific, Inc.) supplemented with 10% FBS (Life Technologies, Grand Island, NY) or 10% PL. When cells reached 80–90% confluence in the 6-well plates containing 10%-PL as the serum supplement, then cells from both the 10% PL and 10% FBS wells were trypsinized (0.25% trypsin (HyClone, Logan, UT)), harvested, stained with trypan blue and counted (i.e. P3 cell counts used for growth ratio calculation). When cells reached 80–90% confluence in the T75 flasks containing10%-PL then cells from both the 10% PL and 10% FBS flasks were harvested to obtain P3 MSCs and were counted. The P3 MSCs were then re-plated at 5,000 cells/cm2 in replicates of 3 in 6-well plates. When cells reached 80–90% confluence in the 6-well plates containing 10%-PL as the serum supplement, then cells from both the 10% PL and 10% FBS wells were harvested. The P4 cells were stained and counted as before (P4 cell counts used for growth ratio calculation). PLS/FBS growth ratios were calculated by dividing average cell counts of MSC grown in 6-well plates with PL by average cell numbers of MSC grown in 6-well plates with FBS.
The second method we used to quantitate the proliferative response of MSCs employed the Vybrant MTT Cell Proliferation Assay (ThermoFisher Scientific, Inc, Waltham, MA). MSCs at passage 2 (P2) that had been stored at <150 °C were thawed and seeded at 5,000 cells/cm2 in replicates of 5 in 96-well plates containing basal medium supplemented with 10% FBS or 10% PL. After 72 hrs of culture during MSC passage 3 formation, medium was removed from each well. αMEM containing 10% serum plus 12mM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well and to three additional wells to serve as background controls. After 4 hrs of incubation at 37°C, sodium dodecyl sulfate-HCl (100µl) was added and absorbance was read at 570nm. Background absorbance from control wells was subtracted from the test wells of MSCs cultured with PL or FBS. PLS/FBS growth ratios were calculated by dividing the average absorbance of wells containing MSCs cultured with PL by the average absorbance of wells containing MSCs cultured with FBS.
Protein Arrays
PL-S (1mL) from three randomly selected lots were sent to RayBiotec for quantitative screening with Quantibody Human Cytokine Antibody Array 9000 (RayBiotec, In., Norcross, GA). Controls and serial dilutions of cytokine standards were prepared according to the manufacturer’s instructions. Array chips were blocked with Sample Diluent at room temperature. After decanting the diluent from each chip, cytokine standards, controls and test samples were added to chip wells and were incubated at room temperature for 1–2 hr. Each chip was washed three times and then incubated for 1 hr at room temperature in the dark with a Cy3 equivalent dye-streptavidin conjugate. The dye was decanted and chips were washed 5 times with Wash Buffer at room temperature, dried and imaged using a laser scanner equipped with a Cy3 wavelength. Quantitative data analysis was performed using the Quantibody Q-Analyzer software (RayBiotec, Inc.). Hierarchical clustering of individual protein expression levels was performed to generate heat maps and dendrograms.
Expansion of MSCs for Microarrays
MSCs were obtained from the marrow of two different living donors (Lonza, Walkersvile, MD) and from a cadaveric donor. Living donor BM was either directly cultured or was first processed using a ficoll gradient-density sedimentation to obtain a mononuclear fraction (MNC) before plating. Organ donor marrow was obtained as previously described (20) and was subjected to a ficoll gradient-density sedimentation to obtain MNCs that were stored in frozen aliquots that were thawed and then plated.
Whole BM or MNCs were inoculated into T-75 flasks (Corning Costar, Sigma Aldrich, St. Louis, MO) containing basal medium supplemented with 10% FBS, 10% PL-S, or 10% PL-P. In cultures supplemented with PL-P, 2 IU/mL of heparin (APP Pharmaceutical, Schaumburg, IL) was added to preclude clot formation. After 48 hours of incubation, non-adherent cells were removed, fresh medium was added and the medium was changed every 3–4 days until cells reached 80–90% confluence. Cells were detached with 0.25% trypsin (HyClone, Logan, UT), re-seeded and expanded for two more passages (P1 and P2). At each passage, cell counts and viabilities were performed. P2 cells were analyzed for MSC cell surface markers per ISCT guidelines (21) and were frozen in aliquots of 5 × 106 MSC for gene expression analysis.
Microarray gene expression analysis
Total RNA was extracted from MSCs using miRNeasy mini kit (Qiagen, Westburg, Leusden, NL). RNA quality was evaluated by the Agilent Bioanalyzer 2100 (Agilent Technologies, Inc, Santa Clara, CA, USA). Test samples and an Universal Human Reference RNA (Stratagene, Santa Clara, CA, USA) were amplified and labeled by using the Agilent Low Input QuickAmp Labeling Kit, the RNA was amplified and subsequently used on 4 × 44 K Whole Human Genome Microarrays (Agilent technologies). Images of the arrays were acquired using a microarray scanner G2505B (Agilent technologies) and image analysis was performed using feature extraction software version 9.5 (Agilent Technologies). The Agilent GE2-v5_95 protocol was applied using default settings. Resulting data files were analyzed using BRB Array Tools developed by the Biometric Research Branch, National Cancer Institute, Bethesda, MD, USA (http://linus.nci.nih.gov/BRB-ArrayTools.html) and Partek Genomic Suite 6.4 (Partek Inc., St. Louis, MO, USA).
Flow Cytometry Analyses of MSC
Immunophenotyping of harvested cells was performed using fluorescence-conjugated mouse anti-human monoclonal antibodies: CD105-phycoerythrin (CD105-PE), CD45-fluorescein isothiocyanate (CD45-FITC) (both from eBioscience, San Diego, CA, USA) ; CD73-PE, CD90–PE-cyanine dye (CD90-PE-Cy5), (CD34-FITC), CD45 peridinin-chlorophyll proteins (CD45-PerCP), HLA-DR-FITC and CD44-FITC (all from BD Biosciences, San Jose, CA, USA). Appropriate isotype controls were set-up in parallel. A minimum number of 20,000 events was collected on a Partec Cyflow flow cytometer (Partec North America, Inc., Swedesboro, NJ) using FlowMax software for data acquisition and analysis.
Statistical Analysis
Data are presented as mean ± standard deviation of the mean. A student t-test was used to determine differences or similarities between populations. P-values <0.05 were designated as significant.
RESULTS
Characterization of Large-scale PL-S products
From January 2013 to December 2016, 9 GMP compliant industrial scale PL-S manufacturing runs (i.e. 9 lots) were conducted using a standardized method. The total number of PRP apheresis units per lot production ranged from 48 to 109 units for an average volume of 24.6±2.2 liters (Table 1). The amount of time that the PRP units were stored at −80 °C before being selected for use in a PL-S manufacturing run was ~1 yr for lots 1–7 and ~3 yrs for lots 8 & 9. Post-manufactured average volume recoveries were 18.8± 2.5 liters for an average total volume recovery of 74±6%. In-process sterility testing for all lots were negative and final product sterility testing showed that 8 lots of PL-S tested negative and one lot was positive for Propionibacterium, a gram-positive rod that is associated with normal human skin flora. Confirmatory testing of the alleged contaminated PL-S lot was inconclusive and we suspect that contamination occurred at the time of sterility testing. The 8 lots that tested negative at lot-release were released and the 1 lot that inconclusively tested positive was not released for clinical use.
Table 1.
Production Volumes for Nine Lots of Platelet Lysate
| Lot # | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Mean | S.D. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Apheresis Units per Lot Production | 81 | 49 | 52 | 56 | 109 | 109 | 109 | 107 | 104 | 86 | 27 |
| Total Volume Post-Thaw PRP (L) | 20.1 | 22.2 | 24.2 | 25.8 | 24.9 | 25.4 | 26.6 | 25.6 | 26.6 | 24.6 | 2.2 |
| Estimated PL Final Product Volume (L) | 13.6 | 16.9 | 16.8 | 21.1 | 19.4 | 19.9 | 20.5 | 21.5 | 19.3 | 18.8 | 2.5 |
| % Total Volume Recovery | 68 | 76 | 69 | 82 | 78 | 81 | 65 | 84 | 93 | 74 | 6 |
PRP=Platelet Rich Plasma; PL=Platelet Lysate; S.D.= Standard Deviation; N.D.=Not Done; N. A.=Not Applicable
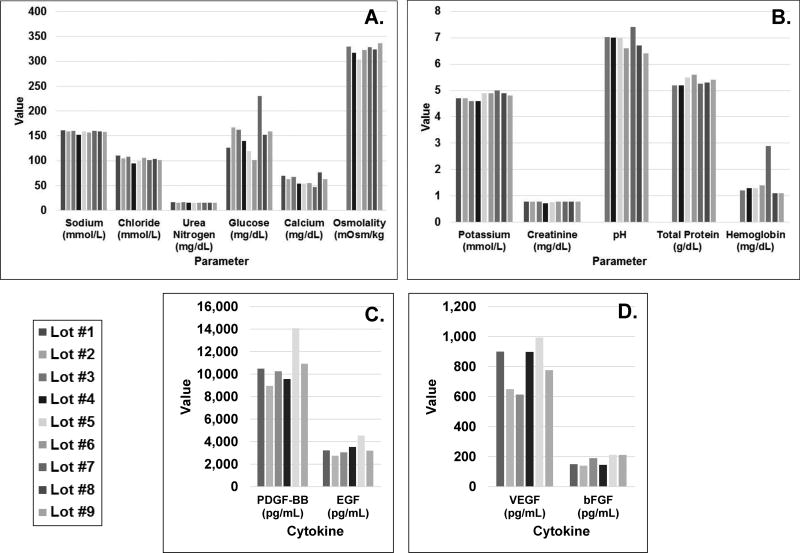
Nine lots of PL-S were tested for biochemical markers. Values for sodium, potassium, chloride, urea nitrogen, and creatinine levels were relatively similar among lots (Fig. 1A & B). Variations in glucose and calcium levels were noted with glucose levels averaging 151 mg/dL (range: 101–230 mg/dL) and calcium levels averaging 70 mg/dL (range: 47–76 mg/dL) (Fig. 1A & B). Average pH, osmolality and total protein levels were 6.9±0.3, 323±10 mOsm/kg, and 5.4±0.2 g/dL, respectively (Fig. 1 A & B). Hemoglobin levels averaged 1.5±0.7 mg/dL (Fig. 1B), and fibrinogen levels were below detectable levels (<30 mg/mL) (data not shown). Mean growth factor levels were 10.7±1.8 ng/ml for PDGF-BB (range: 14.1−9.0 ng/mL), 0.8±0.2 ng/mL for VEGF (range: 1.0−0.6 613 ng/mL), 3.4±0.6 ng/mL for EGF (range: 4.5−2.7 ng/mL) and 0.2±0.03 ng/mL for bFGF (range: 0.2−0.1 ng/mL for bFGF) (Fig. 1C). (Fig. 1C).
Figure 1. Biochemical and growth factor compositions of industrial scale platelet lysate lots.
Aliquots from PL-S lots (average 18.8±2.5 liters) were tested for A) sodium, chloride, urea nitrogen, glucose, calcium, and osmolality; B) potassium, creatinine, pH, total protein and hemoglobin levels. (n=9); C) platelet derived growth factor isoform (PDGF-BB), epidermal growth factor (EGF) D) vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF). The concentration of each growth factor from 6 of 9 lots was determined by an enzyme-linked immunosorbent assay (ELISA).
PL support of MSC cultures
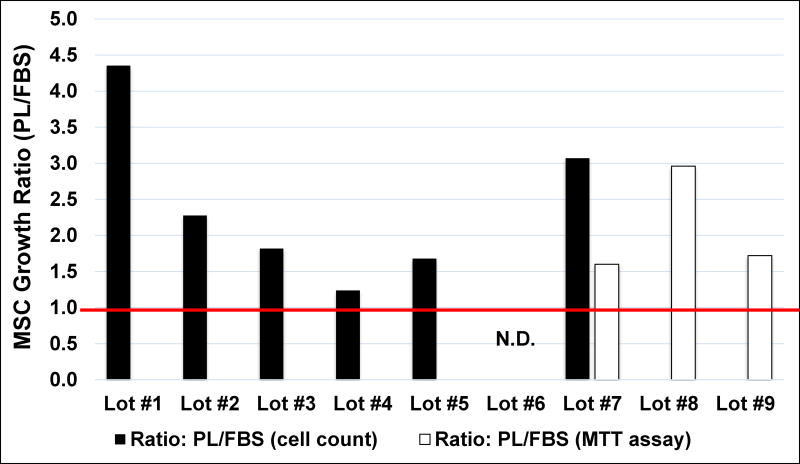
Of 8 lots tested, PL-S was as good as or better than FBS in expanding MSCs. For 6 of 8 PL-S lots, proliferation responses, doubling times and growth ratios for MSCs were determined by manual cell counts at passages 3 and 4. Average MSC fold-expansions at passage 3 were 7.2±2.6 and 3.2±0.7 and MSC doubling times were 39.3±7.2 hr and 71.5±21.0 hr (n=6) when cultured with PL-S versus FBS, respectively. Based on fold-expansion, the growth ratios for MSCs cultured in PL-S for lots 1, 2, 3, 4, 5 & 7 versus FBS (i.e. PL-S/FBS) were determined to be >1.0 for all these lots (Fig. 2; black bars). This represented an overall average MSC (PL-S/FBS) growth ratio of 2.2 for MSCs at passage 3. At passage 4, average fold-expansions with PL-S and FBS were 5.0±1.5 and 3.3±0.1.1 and MSC doubling times were 50.8±9.2 hr and 76.6±24.3 hr (n=6) with PL-S and FBS, respectively. Based on fold-expansion, this represented an overall average MSC (PL-S/FBS) growth ratio for MSCs of 1.25 at passage 4.
Figure 2. PL-S/FBS growth ratios for MSCs.
MSCs were cultured with 10% PL-S or 10% FBS. MSC proliferative responses were measured by either a manual cell count (black bars) or by using an MTT assay (white bars). Proliferative responses of MSCs when cultured with PL-S lots 1, 2, 3, 4, 5, & 7 were determined at passage 3 by performing manual cell counts of MSCs after staining the cells with trypan blue. Growth ratios were then calculated for these lots by dividing the total viable MSC counts from cultures supplemented with 10% PL-S by the total viable MSC counts from cultures supplemented with FBS (black bars). Proliferative responses during passage 3 MSCs formation forots 7, 8 &9 were determined by using an MTT assay Growth ratios were calculated by dividing the average absorbance of wells containing MSCs cultured with PL-S by the average absorbance of wells containing MSCs cultured with FBS (white bars). ND=not done
Given the value of these results, we wanted to develop a less time consuming functional release assay to test the activity of PL lots that uses an MTT assay. For PL-S lots 7, 8, and 9, P2 MSCs were thawed and sub-cultured in 96 well-plates. After 72 hrs of culture during MSC passage 3 formation, a colorimetric measurement of cell proliferation was determined. Using the absorbance measurements, the PL-S/FBS growth ratios were >1.0 (Fig. 2; white bars). This represented an overall average MSC (PL-S/FBS) growth ratio of 2.1 for MSCs at passage 3. Together, these results support an implementation of a functional release test that measures MSC (PL-S/FBS) growth ratios with the MTT assay.
PL-S is stable up to 2 years at −80°C
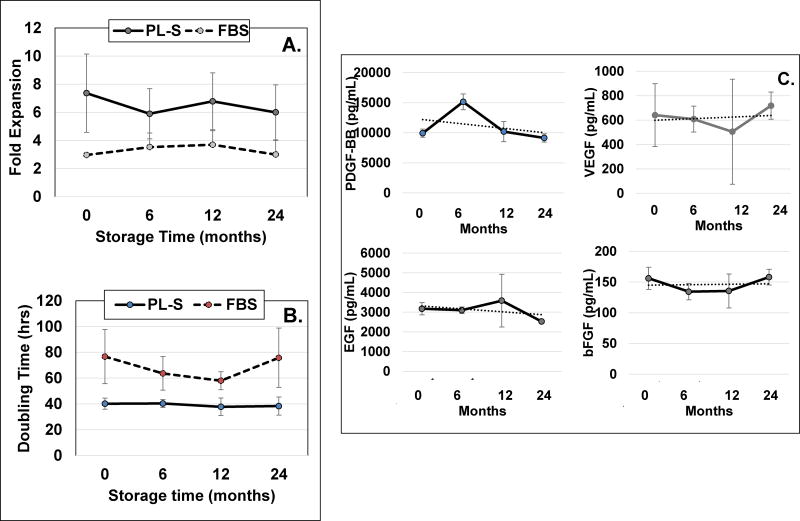
Results showed that MSC fold-expansions were greater (Fig. 3A) and doubling times for MSCs were shorter with PL-S than with FBS when assessed over a 2 year period for each time points tested (Fig. 3B). The stability of growth factors PDGF-BB, VEGF, EGF and bFGF in PL-S when maintained at −80°C showed no apparent decreases in growth factor levels over a 2 year storage period. Growth factor levels at time zero for PDGF-BB, VEGF, EGF and bFGF were 9.9±0.6, 0.6±0.2, 3.2±0.3, and 0.2±0.02 ng/mL, respectively. After two years of storage, growth factor levels were 9.2±0.7, 0.7±0.1, 2.5±0.07, and 0.2±0.01, respectively (Fig. 3C).
Figure 3. PL-S stability testing over a 2-year period.
Three randomly selected industrial scale lots were selected for stability testing. A) fold-expansions, and B) doubling-times were determined from each of the 3 lots of PL-S that were tested immediately after the completion of the PL-S manufacturing run (i.e. time zero) or after 1–10 mL aliquots from each PL-S lot were maintained at −80°C for 6, 12 or 24 months. A single lot of FBS was used and tested over the same time period. Using a single lot of MSC for all time points, MSCs that had been stored frozen were thawed and plated in 6 well plates that contained basal medium supplemented with either PL-S or with FBS. After cells reached confluence, they were harvested to determine fold-expansions and doubling times. C) The concentration of PDGF-BB, VEGF, EGF and bFGF in aliquots of PL-S were assessed upon completion of the PL-S manufacturing run or after being maintained at −80°C for 6, 12 or 24 months. (n=3)
Protein Array Profile
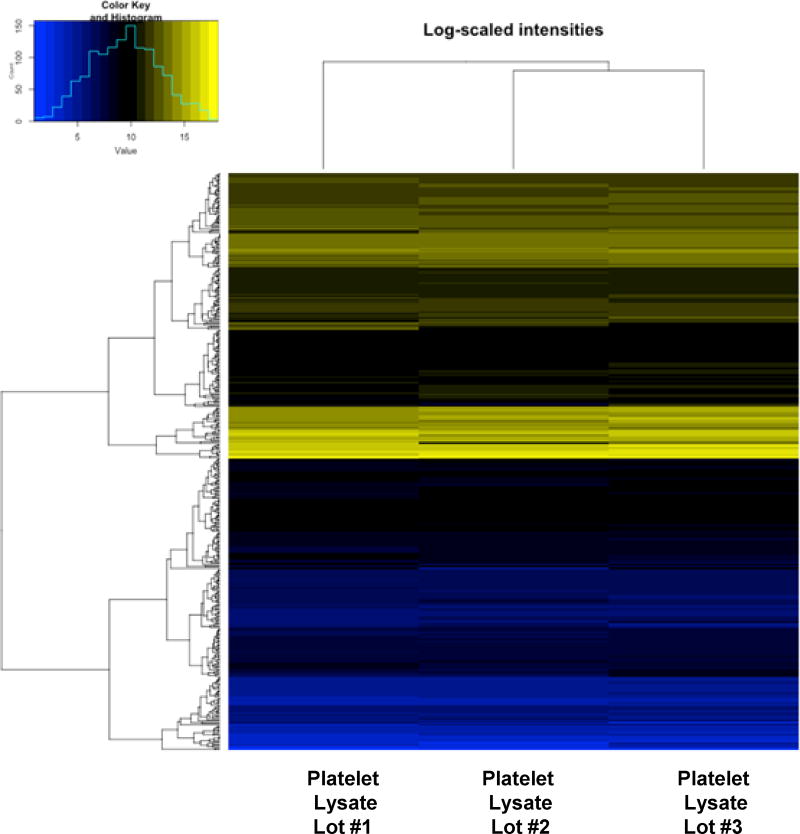
Quantitative measurements of cytokines, growth factors, soluble receptors and other proteins in three randomly selected PL-S lots revealed that there were similarities and as well as dissimilarities among the lots as is evident by hierarchical cluster profiling (Fig. 4). Of 423 cytokines tested, only three cytokines were found to be negative (i.e. 0.0 ng/mL) in all three lots, angiotensin-converting enzyme 2 (ACE-2), tumor necrosis factor [TNF]–related activation-induced cytokine (TRANCE) and single Ig IL-1-related receptor (SIGIRR). Thirty-one proteins were below detection limits in all three lots. Three-hundred-sixty-seven of the 423 proteins (i.e. 87%) tested were present in all three lots with 27 of the 423 cytokines found in one or two of the three PL-S lots (see supplemental data). Of the 367 proteins that were detected in all three lots, 290 of these proteins were present with quantitative level within 50% of one another.
Figure 4. Hierarchical heat map clusteri analysis showing individual protein expression levels in PL-S lots.
Cluster analysis was performed for 3 different lots of PL-S. Low expression protein values are represented as blue, high expression protein values are designated as yellow, and intermediate values are black. Dendrogram shows the similarity of the groups.
MSC Gene Expression in Response to Different Protein Supplements
Two sets of experiments were conducted to determine how the gene expression profiles of MSCs would be affected when they were produced in a culture medium that was supplemented with different protein additives. In the first set of experiments, the MSCs used in the experiments were produced in culture from three different sources, which included whole BM from one living donor, MNCs from a second living donor’s BM and MNCs from the BM of an organ donor. Since at the time of the first gene profile experiment our lab was still considering whether to manufacture large scale lots of PL-S or PL-P, both PL-P and PL-S were evaluated relative to FBS. MSC proliferation responses after two passages showed that similar numbers of MSCs were generated in cultures containing FBS (i.e. 61±14×106) and PL-S (i.e. 73.9±27×106) (P-value=0.50), but cultures supplemented with PL-P produced significantly more cells (i.e. 145±33×106) than cultures with FBS (p-value<0.02) (Table 2a). Cultures supplemented with PL-P also out-performed cultures with PL-S (p-value=0.05) (Table 2a). Independent of the protein supplement that was used in the culture medium, cells expressed markers associated with an MSC phenotype (Table 2b). Additional comparative data regarding the activities of PL-S, PL-P and FBS can be found in one of our previous publications (16).
Table 2.
| a: Expansion | |||
|---|---|---|---|
| *Medium Supplement | Source | Passage 2 (i.e. 3rd Detachment) (×106) |
Days in Culture |
| Fetal Bovine Serum | Living Donor #1 | 71.2 | 20 |
| Living Donor #2 | 44.7 | 24 | |
| Cadaveric Donor | 67.2 | 27 | |
| Platelet Lysate Plasma | Living Donor #1 | 162.6 | 24 |
| Living Donor #2 | 106.2 | 23 | |
| Cadaveric Donor | 166.6 | 27 | |
| Platelet Lysate Serum | Living Donor #1 | 96.3 | 20 |
| Living Donor #2 | 81.6 | 24 | |
| Cadaveric Donor | 43.8 | 27 | |
| b: Immunophenotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| Serum Supplement |
CD73 | CD90 | CD105 | CD34 | CD14 | CD19 | CD45 | HLA-DR |
| Fetal Bovine Serum | 99.9±0.1% | 99.9±0.1% | 67.2±17.5% | 5.5±5.7% | 0.0±0.0% | 0.7±0.8% | 0.0±0.0% | 0.2±0.2% |
| Platelet Lysate-Plasma | 99.4±0.8% | 99.9±0.1% | 66.2±15.8% | 1.7±2.9% | 0.0±0.0% | 0.5±0.9% | 0.0±0.0% | 0.2±0.2% |
| Platelet Lysate-Serum | 99.2±0.8% | 99.9±0.1% | 68.1±15.6% | 1.7±2.9% | 0.0±0.0% | 0.0±0.0% | 0.0±0.0% | 1.3±2.0% |
Cultures were supplemented with 10% FBS, 10% PL-P or 10% PL-S
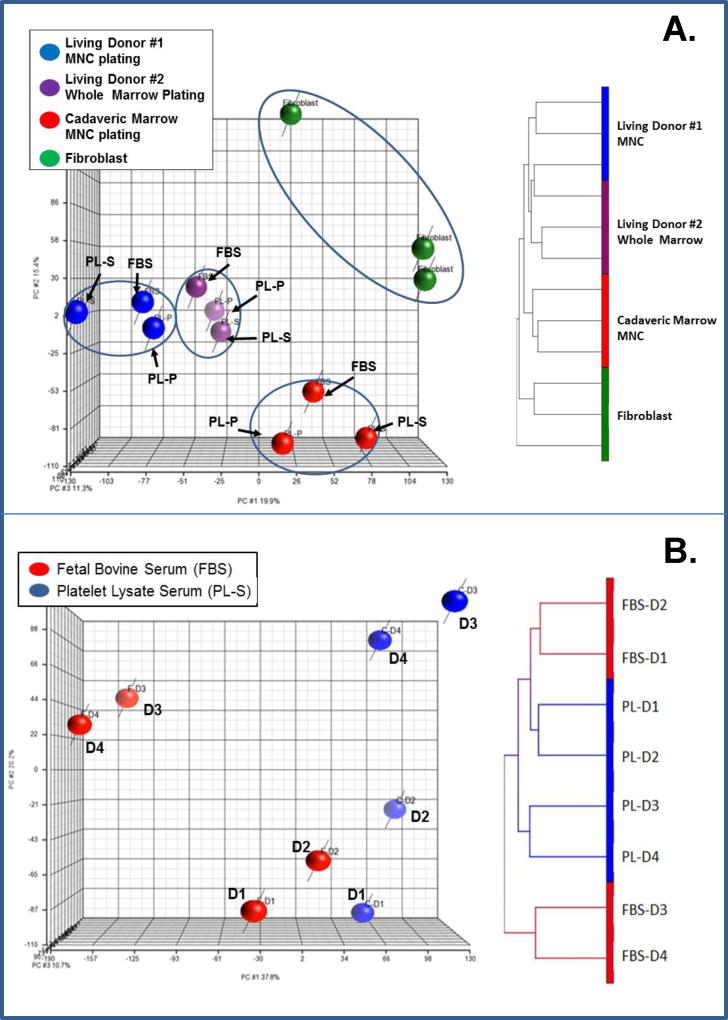
The results of our first gene expression profile of MSCs showed that there were fewer differences in gene expression profiles due to different medium supplements (i.e. FBS vs. PL-S vs. PL-P) than to the source of the BM (Fig. 5). This was evident from a Principal Component Analysis that showed that MSCs from living donor #1 formed one cluster, a second cluster was observed with living donor #2, and a third cluster with cadaveric marrow (Fig. 5A). Unsupervised hierarchical culture analysis yielded similar results (Fig. 5B). Two-way ANOVA analysis also revealed significant differences among samples due to source of starting cellular material, but not due to different medium supplements. Analysis showed that the differential expression of 22 genes was attributable to differences in starting cellular material (p<0.05 and FDR<0.05) (Table 3). There were far more differentially expressed genes between living donor marrow #1 and cadaveric marrow culture-derived MSC (i.e. 122 genes) and living donor marrow #2 and cadaveric marrow cultured-derived MSC (i.e. 543 genes) than for MSC obtained from living donor #1 and #2 (i.e. 15 genes; paired t tests, p<0.05 and FDR<0.05) (data not shown). In contrast, a comparison of differentially expressed genes among the MSCs cultured with FBS versus PL-S and PL-P revealed only 2 differentially expressed genes (paired t tests, p<0.05 and FDR<0.05). The 2 genes were protocadherin alpha-C2 (PCDHAC2), a neural cadherin-like cell adhesion protein, and A_32_P206104, a CDC42 binding protein kinase beta.
Figure 5. Transcriptome analysis of MSCs.
A) MSCs were prepared from the marrow of two living donors and one cadaveric donor. The marrow from one living donor was directly plated into culture medium. The Marrow from a second living donor was processed to obtain an MNC fraction, which was then plated. And, the marrow from an organ donor was processed to obtain a MNC fraction that was stored frozen in small aliquots prior to being plated. Each source of marrow was cultured with MEM medium supplemented with 10% FBS, 10% PL-P, or 10% PL-S. Principal component analysis (PCA) of the samples using the entire gene expression data set is shown in on the left and unsupervised hierarchical clustering analysis is shown on the right. B) Transcriptome analysis of MSCs prepared from the marrow of 4 different living donors. Transcriptome analysis of MSCs produced in media supplemented with 10% FBS and PL-S. Marrow aspirates from 4 different healthy subjects were process to obtain a MNC fraction. One-half of the MNC fraction was plated in αMEM medium supplemented 10% FBS and the other half with 10% PL-S. Principal component analysis (PCA) of the samples using the entire gene expression data set is shown on the left and unsupervised hierarchical clustering analysis is shown on the right. MSCs cultured in 10% FBS are shown in red and those cultured in 10% PL-S are shown in blue. D1 denotes donor 1, D2 donor 2, D3 donor 3 and D4 donor 4.
Table 3.
Differential expression of 22 genes in hMSCs that was attributable to different source materials that were used to initiate cultures.
| Gene Symbol | Gene Name | FDR |
|---|---|---|
| ENST00000428332 | 0.0201 | |
| PNMAL1 | PNMA-like 1 | 0.0269 |
| HDDC2 | HD domain containing 2 | 0.0269 |
| CEBPB | CCAAT/ enhancer binding protein (C/EBP) | 0.0269 |
| NTNG1 | Netrin G1 | 0.0342 |
| SNHG5 | Small nucleolar RNA host gene 5 (non-protein coding) | 0.0392 |
| NUDT4 | Nudix (nucleoside diphosphate linked moiety X)-type motif 4 | 0.0392 |
| GSTM3 | Glutathione S-transferase mu 3 (brain) | 0.0392 |
| MRPL19 | Mitochondrial ribosomal protein L19 | 0.0392 |
| LOC644246 | Hypothetical LOC644246 | 0.0434 |
| TMEM57 | Transmembrane protein 57 | 0.0434 |
| FANCD2 | Fanconi anemia, complementation group D2 | 0.0434 |
| RN7SL1 | 0.0434 | |
| C10orf11 | Chromosome 10 open reading frame 11 | 0.0434 |
| C9orf7 | Chromosome 9 open reading frame 7 | 0.0434 |
| A_24_P752208 | 0.0434 | |
| SULT1A2 | Sulfotransferase family, cytosolic, 1A, phenol-preferring, member 2 | 0.0434 |
| HCRTR1 | Hypocretin (orexin) receptor 1 | 0.0434 |
| UBE2T | Ubiquitin-conjugating enzyme E2T (putative) | 0.0452 |
| PPP1R3C | Protein phosphatase 1, regulatory (inhibitor) subunit 3C | 0.0452 |
| PEX6 | Peroxisomal biogenesis factor 6 | 0.0452 |
| HLA-J | Major histocompatibility complex, class I, J (pseudogene) | 0.0477 |
FDR = False Discovery Rate
The differentially expressed genes were identified with 2-way ANOVA analysis using p<0.05 and FDR<0.05.
In a second experiment, a more direct comparison was performed by culturing MNCs from only living donor marrows. Although significance was not reached, cultures supplemented with PL-S produced more MSCs (i.e. 12.6±4.2×106) than with FBS (i.e. 8.0±3.0 ×106) and faster doubling times were noted with PL-S (i.e. 34.9±3.8 hr) than with FBS (i.e. 41.6±5.3 hr). Cultures were maintained for an average of 17.5 days in medium supplemented with FBS and 16.5 days in medium supplemented with PL-S. Principal Component Analysis and hierarchical clustering analysis of the global transcriptome data suggested that there were some differences in MSCs due to both culture media and inter-subject variability (data not shown). However, 2-way ANOVA revealed no significant differences in the gene expression of MSC due to culture media supplement or to marrow source.
DISCUSSION
This is the first study that examines the content and function of PL-S lots that are manufactured with a single standardized industrial scale method. The results show that overall the biochemical properties of different manufactured lots of PL-S are essentially similar with some slight variations (Fig. 1). Growth of MSCs cultured with all of the PL-S lots tested are as good as or better than MSCs when grown with FBS. However, variations in MSC growth ratios are present and suggest that some degree of lot-to-lot variability is present (Fig. 2). We also show that PL-S stored at −80 °C is relatively stable over a two year storage period.
Platelets contain proteins that play key regulatory roles in cellular growth, cell development, chemotaxis, angiogenesis and regeneration (22, 23) and when these proteins are released into the supernatant during the manufacturing of PL contribute to the quality/potency of the final product. Of 423 different cytokines/growth factors tested, a large majority (i.e. 87%) are present in all three lots with only 27 proteins present in 1 or 2 of three PL-S lots and only 31 proteins below detection limits in all three lots (see supplemental data). Of the 367 proteins detected in all three lots, a majority of proteins (i.e. 79%) are present with quantitative level within 50% of one another. These data indicate that even though there are differences in protein profiles and levels that a majority of proteins are present at similar quantitative levels (i.e. 79%). Moreover, despite lot-to-lot variations in protein levels, the differences do not preclude the ability of PL-S to surpass FBS in supporting MSC growth. A future challenge will be to identify what are acceptable variations in protein levels for the release of PL-S for specific applications.
Results from this study show that greater differences in MSC gene expression profiles are more attributable to different donor bone marrow sources than to the medium supplement (i.e. PL-S, PL-P & FBS). The greatest differences in MSC gene expression are observed between living donor marrow and cadaveric marrow independent of how marrow is processed (i.e. whole blood vs. MNC). Among differentially expressed genes is HDDC2 (Table 3), a gene that is implicated in maintaining the pluripotency of human induced pluripotent stem cells and embryonic stem cells (24). Also among the 22 genes is CEBPB, a gene that encodes the transcription factor CCATT/enhancer binding protein (C/EBP), an important adipogenic transcription factor (25) and, when expressed by MSC regulates early B cell lymphopoiesis (26). Given differences in MSC gene expression from different BM sources, these data imply that there are distinct populations of MSCs residing within specific BM niches. A notion supported by evidence that MSCs from different tissues of origin are different, such as MSCs from trabecular or from cortical BM (27). Our results are in contrast to another study that shows that significant differences in MSC gene expression are present due to whether FBS, human serum or thrombin-activated platelet releasate plasma are used (28). It is worth noting that a number of factors are different between these two studies.
A major motivating factor for replacing FBS with human PL in cell cultures is to avoid risks associated with transmitting infectious bovine agents (i.e. parvovirus and prions) and to eliminate any exposure to residual xenoproteins that have the potential to cause an immune reaction in recipients. There is no question that exposures to zoonotic diseases and residual xenoproteins are eliminated when using human PL. However, given that PL is derived from human blood products (i.e. PRP), there is still a potential for transmitting human viruses (e.g. hepatitis B, hepatitis C, HIV), for transferring bacterial agents and for exposing someone to allogeneic proteins that may cause an immune reaction. At present, PL has not yet been implicated in any such transmissions or exposures. This may be due in part to the fact that the use of PL as a culture supplement is a relatively new practice. And, it may also be attributable to safety measures that are already in place for blood products that have been put in place by the transfusion community to minimize viral transmissions associated with blood transfusions. For example, the implementation of nucleic acid testing (NAT) for the detection of viral diseases has greatly improved the safety of blood products (29). As well as a more recent adoption by some Blood Centers to pathogen reduce platelet products to eliminate viral and bacterial contamination. A practice that one day may translate into safer PL products (30, 31). Sterile filtration of a PL final product and sterility testing are also strategies to minimize potential bacterial transmissions. Another practice that may reduce the exposure to allogeneic proteins when using PL as a protein supplement in cultures to produce MSC or other cell type that are to be used in a transplant setting is that cell washes are typically incorporated at the end of the culture period that result in a several log reduction of the serum supplement in the wash solution. A step that is performed before cells are diluted with an injectable solution (e.g. Plasmalyte A) to prepare a cell suspension for a clinical injection. Whether washing of the cell suspension before it is infused into a patient successfully eliminates the possibility of any immune reactions attributable to allogenic proteins remains an open question.
Comparative analysis of large scale PL-S lots demonstrate that there are similarities as well as variations in the protein profiles of different PL-S lots. However, despite differences in protein profiles, all PL-S lots out-perform FBS and are effective substitutes for FBS in supporting MSC expansion. We propose that a standardized functional minimum criterion be established to release PL that states that MSC expansion with a specific concentration of PL is equal to or greater than MSC expansion with 10% FBS (i.e. MSC growth ratio ≥ 1.0). Such a specification would allow for some variability in PL composition and at the same time help to define acceptable tolerance limits for cytokines and other active ingredients to achieve a specific response for specific applications (i.e. MSC versus lymphocyte cultures).
Supplementary Material
Acknowledgments
We especially want to thank Mariluz Henshaw, Achut Raj Poudel, Jessica Phibbs, Macon Latimer, Emily Petersen, and Tara Regginello for their technical assistance. We would also like to thank the other staff members at the University of Utah Cell Therapy and Regenerative Medicine Facility for their dedication and support.
Support: This work was supported in part by the University of Utah and the NIH Clinical Center.
ABBREVIATIONS
- AF
Amniotic Fluid
Footnotes
Disclosure of Interest
JAR, DFS, JP, AP, EB and PJin, have no conflicts of interest.
References
- 1.Jochems CE, van der Valk JB, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Altern Lab Anim. 2002 Mar-Apr;30(2):219–27. doi: 10.1177/026119290203000208. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Kang H, Liu X, Gao J, Zhao K, Ma Z. Serum and xeno-free, chemically defined, no-plate-coating-based culture system for mesenchymal stromal cells from the umbilical cord. Cell Prolif. 2016 Oct;49(5):579–88. doi: 10.1111/cpr.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Saqi SH, Saliem M, Asikainen S, Quezada HC, Ekblad A, Hovatta O, et al. Defined serum-free media for in vitro expansion of adipose-derived mesenchymal stem cells. Cytotherapy. 2014 Jul;16(7):915–26. doi: 10.1016/j.jcyt.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 4.van der Valk J, Brunner D, De Smet K, Fex Svenningsen A, Honegger P, Knudsen LE, et al. Optimization of chemically defined cell culture media--replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010 Jun;24(4):1053–63. doi: 10.1016/j.tiv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Kocaoemer A, Kern S, Kluter H, Bieback K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem cells. 2007 May;25(5):1270–8. doi: 10.1634/stemcells.2006-0627. [DOI] [PubMed] [Google Scholar]
- 6.Stute N, Holtz K, Bubenheim M, Lange C, Blake F, Zander AR. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Experimental hematology. 2004 Dec;32(12):1212–25. doi: 10.1016/j.exphem.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Shetty P, Bharucha K, Tanavde V. Human umbilical cord blood serum can replace fetal bovine serum in the culture of mesenchymal stem cells. Cell Biol Int. 2007 Mar;31(3):293–8. doi: 10.1016/j.cellbi.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Penttinen K, Saxen E. Growth-Controlling Action of Human Serum in Cell Culture. Nature. 1959;184(4698):1570–1. doi: 10.1038/1841570b0. [DOI] [PubMed] [Google Scholar]
- 9.Treadwell PE, Ross JD. Characterization of Human Cells - Variation in Growth Rate, Volume, Morphology and Growth Efficiency in Media Supplemented with Human Serum or Bovine Fetal Serum. Experimental Cell Research. 1963;29(1–2):356-&. doi: 10.1016/0014-4827(63)90390-2. [DOI] [PubMed] [Google Scholar]
- 10.Choi YC, Morris GM, Sokoloff L. Effect of Platelet Lysate on Growth and Sulfated Glycosaminoglycan Synthesis in Articular Chondrocyte Cultures. Arthritis and Rheumatism. 1980;23(2):220–4. doi: 10.1002/art.1780230213. [DOI] [PubMed] [Google Scholar]
- 11.Cowan DH, Graham J, Paskevich MC, Quinn PG. Influence of Platelet Lysate on Colony Formation of Human-Breast Cancer-Cells. Breast Cancer Res Tr. 1983;3(2):171–8. doi: 10.1007/BF01803560. [DOI] [PubMed] [Google Scholar]
- 12.Eastment CT, Sirbasku DA. Human platelet lysate contains growth factor activities for established cell lines derived from various tissues of several species. In Vitro. 1980 Aug;16(8):694–705. doi: 10.1007/BF02619199. [DOI] [PubMed] [Google Scholar]
- 13.Muller I, Kordowich S, Holzwarth C, Spano C, Isensee G, Staiber A, et al. Animal serum-free culture conditions for isolation and expansion of multipotent mesenchymal stromal cells from human BM. Cytotherapy. 2006;8(5):437–44. doi: 10.1080/14653240600920782. [DOI] [PubMed] [Google Scholar]
- 14.Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, Zander AR. Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. Journal of cellular physiology. 2007 Oct;213(1):18–26. doi: 10.1002/jcp.21081. [DOI] [PubMed] [Google Scholar]
- 15.Bieback K, Hecker A, Kocaomer A, Lannert H, Schallmoser K, Strunk D, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem cells. 2009 Sep;27(9):2331–41. doi: 10.1002/stem.139. [DOI] [PubMed] [Google Scholar]
- 16.Mojica-Henshaw MP, Jacobson P, Morris J, Kelley L, Pierce J, Boyer M, et al. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy. 2013 Dec;15(12):1458–68. doi: 10.1016/j.jcyt.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Doucet C, Ernou I, Zhang Y, Llense JR, Begot L, Holy X, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. Journal of cellular physiology. 2005 Nov;205(2):228–36. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 18.Burnouf T, Strunk D, Koh MB, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2015 Oct 28;76:371–87. doi: 10.1016/j.biomaterials.2015.10.065. [DOI] [PubMed] [Google Scholar]
- 19.Shih DT, Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N Biotechnol. 2015 Jan 25;32(1):199–211. doi: 10.1016/j.nbt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman H, Reems JA, Rigley TH, Bravo D, Strong DM. Donor age and gender are the strongest predictors of marrow recovery from cadaveric vertebral bodies. Cell transplantation. 2003;12(1):83–90. doi: 10.3727/000000003783985133. [DOI] [PubMed] [Google Scholar]
- 21.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 22.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004 Jan;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 23.Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, et al. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012 Oct 11;120(15):e73–82. doi: 10.1182/blood-2012-04-416594. [DOI] [PubMed] [Google Scholar]
- 24.Hasegawa Y, Taylor D, Ovchinnikov DA, Wolvetang EJ, de Torrente L, Mar JC. Variability of Gene Expression Identifies Transcriptional Regulators of Early Human Embryonic Development. PLoS Genet. 2015 Aug;11(8):e1005428. doi: 10.1371/journal.pgen.1005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. The Journal of biological chemistry. 1998 Nov 13;273(46):30057–60. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- 26.Yoshioka S, Miura Y, Yao H, Satake S, Hayashi Y, Tamura A, et al. CCAAT/enhancer-binding protein beta expressed by bone marrow mesenchymal stromal cells regulates early B-cell lymphopoiesis. Stem cells. 2014 Mar;32(3):730–40. doi: 10.1002/stem.1555. [DOI] [PubMed] [Google Scholar]
- 27.Corradetti B, Taraballi F, Powell S, Sung D, Minardi S, Ferrari M, et al. Osteoprogenitor cells from bone marrow and cortical bone: understanding how the environment affects their fate. Stem cells and development. 2015 May 1;24(9):1112–23. doi: 10.1089/scd.2014.0351. [DOI] [PubMed] [Google Scholar]
- 28.Bieback K, Ha VA, Hecker A, Grassl M, Kinzebach S, Solz H, et al. Altered gene expression in human adipose stem cells cultured with fetal bovine serum compared to human supplements. Tissue engineering Part A. 2010 Nov;16(11):3467–84. doi: 10.1089/ten.TEA.2009.0727. [DOI] [PubMed] [Google Scholar]
- 29.Funk MB, Heiden M, Volkers P, Lohmann A, Keller-Stanislawski B. Evaluation of Risk Minimisation Measures for Blood Components - Based on Reporting Rates of Transfusion-Transmitted Reactions (1997–2013) Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2015 Jul;42(4):240–6. doi: 10.1159/000381996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iudicone P, Fioravanti D, Bonanno G, Miceli M, Lavorino C, Totta P, et al. Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. Journal of translational medicine. 2014 Jan 27;12:28. doi: 10.1186/1479-5876-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonsdottir-Buch SM, Sigurgrimsdottir H, Lieder R, Sigurjonsson OE. Expired and Pathogen-Inactivated Platelet Concentrates Support Differentiation and Immunomodulation of Mesenchymal Stromal Cells in Culture. Cell transplantation. 2015;24(8):1545–54. doi: 10.3727/096368914X683043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.