Abstract
Standardized evaluation of sperm quality is essential for research, commercial-scale cryopreservation, and induced spawning. However, standardized methods for evaluation of sperm bundles (spermatozeugmata or spermatophores) have not been established. The purpose of the present study was to use Redtail Splitfin (Xenotoca eiseni) as a model for freshwater livebearing fishes to establish initial standardized methods to collect sperm bundles, and quantitatively and qualitatively evaluate quality-related attributes. No sperm or sperm bundles were able to be collected by stripping. Testes were removed, rinsed, weighed, placed in 50 μL of buffer solution on a glass slide, and crushed gently 3 – 5 times with angled spade-tip forceps. Sperm bundles were released into the buffer solution and collected with a pipette into 1.5-mL centrifuge tubes. To quantify size and shape, images of bundles were captured with a CCD camera connected to a microscope, and measured with computer software. There was no significant correlation between body wet weight and major bundle axis length (P = 0.6759), minor axis length (P = 0.5658), average axis length (P = 0.5869), aspect ratio (P = 0.7839), and observed area (P = 0.5727). The concentrations of sperm bundles, estimated with the three methods (Makler® counting chamber, a hemocytometer, and direct counting) were significantly different (P < 0.0001). Hemocytometers were suitable for estimation of bundles from X. eiseni. To evaluate activation of sperm, bundles were viewed with a microscope, and classified into one of five phases by evaluating morphology of the bundles and motion of sperm within the bundles as Phase 0 through Phase 4 that represented early through late activation stages. The frequencies and duration of each activation phase were used to evaluate dissociation of sperm bundles and motility capability of sperm within the bundles. Within 180 min of activation, all five phases were observed. Overall, this study for the first time established standardized methods to collect and evaluate quality-related attributes of sperm bundles. These standardized evaluations provide a basis for further modification, standardization, and generalization, which are useful in research on livebearing fishes involving male gametes, such as studies on cryopreservation, artificial insemination, and in development of germplasm repositories for imperiled species including goodeids.
Keywords: livebearing fish, sperm quality, standardization, sperm bundles, motility, Goodeidae
1. Introduction
Standardized quality evaluation is essential for sperm cryopreservation and induced spawning [1]. In research to develop cryopreservation protocols for aquatic species, sperm quality is used to assess the effects of treatments in key steps such as extender choice, motility activation, cryoprotectant toxicity, cooling and thawing rates, and fertilization assays [2]. For applied and commercial-scale sperm cryopreservation, systematic quality evaluation has been established for humans [3], livestock [4], and has been proposed for aquatic species [5]. In aquatic species the most widely used parameter for the evaluation of sperm quality is percent motility, which can be an important indicator of fertilization success and can be monitored without the time- or sample-consuming observation of fertilization and embryo development [6]. For example, percent motility were correlated with fertilization yield of thawed sperm in Common Carp (Cyprinus carpio) [7]. Percent motility and motility duration can be estimated by direct observation (with naked eye) using a microscope or by computer-assisted sperm analysis (CASA) systems. However, standardization of evaluation approaches is usually overlooked, which can be problematic for the reproducibility of research findings and quality assurance of production [1]. Various procedures of motility evaluation from different sources make it difficult or impossible to directly compare research results, and insufficient checkpoints during commercial-scale cryopreservation make the quality of final products unpredictable. Sperm concentration is another important indicator of initial sperm quality upon collection, and it can significantly affect motility and the level of agglutination of thawed sperm and fertilization rates [8–10], but sperm concentration is often not adjusted or reported in standardized methods in publications regarding sperm cryopreservation for aquatic species.
Species that package sperm in bundles further challenge standardized evaluation of gamete quality. Bundles are formed by packing of numerous sperm cells into unencapsulated (spermatozeugmata) or encapsulated (spermatophores) clusters [11]. The formation of sperm bundles has been identified in invertebrates including nematodes [12], annelids [13], arthropods [14] and molluscs [15], and in vertebrates including amphibians [16], chondrichthyans [17], and teleosts [18]. The occurrence of sperm bundles is sporadic among vertebrates and usually accompanied with internal fertilization and viviparity in fishes [19]. Bundles are believed to facilitate the systematic transfer of sperm from male to female [18], however, they pose difficulty for standardized assessment of male gametes. For example, sperm from most externally fertilized fish do not form bundles (referred as ‘free sperm’). Upon activation by suitable media, the percentage of motile free sperm can be estimated by counting. However, such methods cannot be applied to sperm within bundles, for example, to study the effects of physiochemical factors on activation of sperm within the bundles [20], cryopreservation of sperm bundles, or comparison of fertilization rates between free and bundle-form sperm. In addition to activation, the concentration of free sperm is usually measured with a hemocytometer or specialized counting chamber by identifying the number of sperm cells present in a unit volume, but these devices have not been reliably applied for use with sperm bundles. Sperm morphology is also used to assess sperm quality. For example, morphological examination of fish sperm is an useful tool for monitoring reproductive disruption caused by environmental pollution [21]. Bundle morphology can also be useful to indicate sperm quality, but to date, no standardized approaches have been established to evaluate quality-related attributes, such as activation, concentration, and morphology of sperm bundles from fishes.
Livebearing has been documented in 54 extant families of fishes, including 40 families of chondrichthyans, one montypic family of coelacanths (Latimeria), and 13 families of teleosts [22]. Among these, species from 5 families inhabit freshwater, including 3 families within Cyprinodontiformes (Poeciliidae, Goodeidae, and Anablepidae), 1 family in Beloniformes (Hemiramphidae), and 1 family in Scorpaeniformes (Comephoridae) [23]. Livebearing fishes employ internal fertilization, and sperm from freshwater livebearing fishes are typically packed into spermatozeugmata [11]. Poeciliidae is the largest freshwater livebearing family, comprising more than 200 species with internal fertilization. Poeciliids are popular ornamental species, important cancer research models, and have been used for mosquito control[24]. Sperm from Poeciliidae has been used in studies addressing reproductive behavior [25], evolution [26], toxicology [27], and establishment of germplasm repositories [28]. Goodeidae, the second largest freshwater livebearing family (about 38 livebearing species), is considered to be one of the most at-risk fish groups in the world [29]. As of 2005, the conservation status of livebearing goodeids included 2 species categorized as extinct in the wild, 17 as critically endangered, 5 endangered, 2 threatened, 11 vulnerable, and only 3 at lower risk rankings [30]. Sperm from poeciliids and goodeids form sperm bundles and the mechanism by which the bundles are dissociated and sperm are activated in the female reproductive tract is not clear. Standardized quantitative or qualitative approaches are necessary to study the activation mechanism of sperm within bundles, improve artificial reproduction, and develop protocols of cryopreservation of sperm bundles for freshwater livebearing species. In the present study, the Redtail Splitfin (Xenotoca eiseni, Goodeidae) was used as a model for freshwater livebearing fishes to establish standardized methods to collect sperm bundles, and quantitatively and qualitatively evaluate quality-related attributes. The specific objectives were to: (1) establish and apply standardized methods to collect sperm bundles, (2) quantitatively evaluate their sizes and concentrations, and (3) classify activation patterns of sperm within bundles.
2. Materials and methods
2.1 Fish husbandry
Protocols for the use of animals in this study were reviewed and approved by the Louisiana State University Institutional Animal Care and Use Committee (Baton Rouge, LA, USA). The X. eiseni used in this study were 2-y old and maintained at the Aquatic Germplasm and Genetic Resources Center (AGGRC) at the Louisiana State University Agricultural Center (Baton Rouge, LA). About 200 fish were cultured indoors at 22 – 26 °C with a 14 h:10 h (light:dark) photoperiod in four individual tanks within an 800-L recirculating system and fed twice daily with tropical flakes (Pentair Aquatic Eco-systems, FL, USA) and twice weekly with brine shrimp (Sally’s Frozen Brine Shrimp™, San Francisco Bay Brand, CA, USA). Males were maintained at a 2:1 ratio with females in each tank until 2 d before experiments. Additional water quality parameters were monitored weekly and held within acceptable ranges including: pH (7.0 – 8.0), ammonia (0 – 1.0 mg/L), and nitrites (0 – 0.8 mg/L).
2.2 Collection of sperm bundles
Fish were anesthetized with 0.01% tricaine methanesulfonate (MS-222, Western Chemical, Inc. WA, USA) diluted with water from the fish tank. To eliminate MS-222 residues, the surface of fish was wiped with a paper towel and rinsed with buffer solution (NaCl solution at 300 mOsmol/kg buffered by 10 mM HEPES-NaOH at pH 7.0). The fish was wiped again and body wet weight was measured. Osmolalities of buffer solutions were measured with a freezing point osmometer (Model 5010 OSMETTE III ™, Precision Systems Inc., MA, USA) and pH was measured with a pH meter (EcoSense® pH100A, YSI Inc., OH, USA). To collect milt by stripping, fish were placed on their back on a sponge and squeezed gently, followed by milt being collected with a 10-μL capillary by mouth suction through a rubber tube. If no milt was collected, testes were removed by dissection. Testes were rinsed, weighed, placed in 50 μL of buffer solution on a glass slide, and crushed gently 3 – 5 times with angled spade-tip forceps. Sperm bundles were released into the buffer solution and collected with a pipette into 1.5-mL centrifuge tubes. Volumes of sperm bundle suspension were adjusted to 100 μL by addition of the buffer solution.
The gonadosomatic index (GSI) was calculated as: (testes weight/body wet weight) × 100%. Pearson correlation coefficient (r) with SAS (PROC CORR) (SAS version 9.4, SAS Institute, NC, USA) was used to evaluate the relationship between body wet weight and testis weight. In this study, the results were considered statistically significant at P < 0.05.
2.3 Quantification of size and shape of sperm bundles
Bundle suspension (2 μL) from each fish was pipetted on a glass slide, and images were observed with a microscope (CX41, Olympus Corporation, Tokyo, Japan) at 200-x magnification and captured with the CCD camera of a computer-assisted sperm analysis (CASA) system (HTM-CEROS, version 14 Build 013, Hamilton Thorne Biosciences, MA, US). Buffer solution was added on the slide to assist observation when necessary. The sperm bundles in the images were elliptical in shape with the major and minor axes measured with ImageJ software (National Institutes of Health, MD, USA). ImageJ measurements were calibrated using a Makler® counting chamber (Sefi-Medical Instruments, Haifa, Israel). For each fish, 20 – 30 sperm bundles were measured. Average axis length of a sperm bundle was calculated as (major axis length + minor axis length)/2, aspect ratio of axes was calculated as minor length/major length, and observed area was estimated as π (≈ 3.14) × major axis length × minor axis length. Pearson correlation coefficients were used to evaluate the relationship between body wet weight, and major and minor axis length, average axis length, aspect ratio, and observed area.
2.4 Quantification of concentration of sperm bundles
Three methods were used to estimate concentration of sperm bundles in a pooled sample containing about 500 μL of sperm bundle suspension from 10 fish. The first method was with the Makler® counting chamber, which has been used to measure sperm concentration in humans [31], livestock [32], and fishes [33]. Pooled sperm bundles were resuspended with a vortex mixer, and 5 μL were transferred onto the central area of the base of the counting chamber followed by placing the cover slip on top. With the microscope at 200-x magnification, the number of sperm bundles within 100 squares was counted and recorded (Fig. 1A). In the second method, 10 μL of the suspension were loaded to one of the two separate counting areas of a hemocytometer (Hausser Scientific, Horsham, PA, USA) and covered with a cover slip. The number of sperm bundles within the central 1 × 1 mm square was counted and recorded (Fig. 1B). In the Makler® and hemocytometer methods, sperm bundles located on the top and right boundary lines of the counting area were not recorded, whereas bundles located on top of the bottom and left boundary lines were counted. After each counting, another 5 μL (10 μL for the hemocytometer method) of suspension was measured as a repeated measurement with a total of 10 measurements for each counting method. The average of number of sperm bundles counted in 10 measurements was calculated as N. The concentration of sperm bundles was estimated as N × 102/μL for the counting chamber method and N × 10/μL for the hemocytometer method.
Fig. 1.

Observation of sperm bundles from X. eiseni and evaluation scheme. (A) Observation by use of a Makler® counting chamber and microscopy (dark field, 200-x magnification). The white spots outside spermatozeugmata were debris or free sperm. The observed sizes of spermatozeugmata were larger than their actual size due to compression by the coverslip in this chamber (10 μm chamber height). (B) View of the bundles within a hemocytometer (50-x magnification). Due to magnification and camera limitation, images in (A) and (B) only show a proportion of the total number of squares. (C) Evaluation scheme for the five activation phases of sperm within bundles by categorizing sperm bundles distributed in a viewing area (within the dashed circle) of a microscope. The dashed straight arrows indicate free-swimming sperm released from a sperm bundle and the curved double-arrows indicate sperm vibrating in place but not swimming. In the dashed circle, there are ten sperm bundles including six at P0, one at P1, one at P2, one at P3, and one at P4, thus the FAPs are estimated as 60% FAP0, 10% FAP1, 10% FAP2, 10% FAP3, and 10% FAP4. For demonstration purposes, the sizes of spermatozeugmata, sperm, and viewing area do not reflect actual scale.
A third method was used as a control group, in which 1 μL of suspension was placed onto a glass slide followed by use of a cover glass. The slide was viewed using a stereo microscope (SMZ-U, Nikon, Tokyo, Japan) at 40 – 60-x magnification. Images of all sperm bundles were captured with a digital camera (K-5, Pentax, Tokyo, Japan) and the number of all sperm bundles observed from the image was counted using ImageJ software (ten replicates per sample). The concentration of sperm bundles was estimated as N/μL. The time required to conduct each method (from sample loading to completion of counting) was recorded. Differences in concentration estimations of sperm bundles among the three methods were assessed with one-way ANOVA with Tukey’s multiple comparisons test with SAS. Data were log10-transformed to fulfill all assumptions for one-way ANOVA test prior to statistical analyses.
2.5 Classification of activation of sperm bundles
To activate motility of sperm within bundles, each suspension was mixed with activation media on the counting chamber and a coverslip was placed on top, followed by viewing at 200-x magnification. The bundles were intact and static before activation, and gradually dissociated accompanied by sperm swimming away if activated (Fig. 2). Towards the end of activation, bundles ceased to dissociate further resulting from the decrease of motility capability of sperm within the bundles (Fig. 2). To evaluate the dissociation and activation, 5 – 15 sperm bundles within a viewing area were classified into one of five phases by evaluating morphology of the bundles and motion of sperm within the bundles as Phase 0 (P0) through Phase 4 (P4) representing early through late activation stages (Fig. 1C). The frequencies of each activation phase (FAP) were referred to as FAP0 through FAP4 and were used to evaluate dissociation of sperm bundles and motility capability of sperm within the bundles (Fig. 1C). A total of 2 or 3 different viewing areas was counted for each measurement. To test the utility of the classification system, sperm bundle suspensions were activated by mixing with activation solution at a ratio of 1:9 (bundle suspension: activation solution). The activation solution was prepared with 40 mM CaCl2 for suitable observation based on our previous studies (adjusted to 300 mOsmol/kg with NaCl and buffered by 10 mM HEPES-NaOH at pH 7.0). The activation of sperm bundles was observed at 1, 5, 10, 20, 30, 60, 120, and 180 min after mixing of activation solution. As a control treatment, 1 μL of sperm bundle suspension was mixed with 9 μL of buffer solution without CaCl2. Five males were used as replicates.
Fig. 2.

Dissociation of sperm bundles and activation of sperm within bundles from X. eiseni partitioned into five phases. As shown in the top figure curve, sperm were quiescent at P0 and P4 with no motility, shaking at P1 and P3 with medium motility capability, and moving freely at P2 with the highest motility capability. The microscopic observation of morphologies of 5 phases are shown below the graph in the dark rectangular areas (the white bars represent 20 μm). Un-dissociated bundles were round or elliptic shape at P0 and P1, and dissociated in dispersed forms at P2 to P4. The bottom histogram shows the frequencies of each activation phase activated by 40 mM CaCl2 at different observation times (0 min indicates the control group). Bars represent means (± SD) of five replicates.
3. Results
The body wet weight of fish (N = 10) used in this study was 1.7 ± 0.6 g (mean ± standard deviation). No sperm or sperm bundles were able to be collected by stripping, but fluid (< 1 μL) without male gametes was collected from three fish. The testis weight of X. eiseni was 14.8 ± 5.5 mg, and was significantly correlated with body wet weight (P = 0.0249, and r = 0.70) (Fig. 3A). The GSI was 0.88 ± 0.19%. With gentle crushing of testes, sperm remained in bundles (Fig. 1A).
Fig. 3.

Relationships between body wet weight and the (A) testis weight, (B) average axis length, and (C) aspect ratio of sperm bundles from X. eiseni.
The major axis length of sperm bundles was 33.1 ± 1.5 μm, the minor axis length was 29.4 ± 1.5 μm, the average axis length was 31.3 ± 1.4 μm, the aspect ratio of the bundles was 88.8 ± 2.5%, and the observed area was 3062.7 ± 278.0 μm2. There was no significant correlation between body wet weight and major axis length (P = 0.6759, and r = 0.15), minor axis length (P = 0.5658, and r = 0.21), average axis length (P = 0.5869, and r = 0.20) (Fig. 3B), aspect ratio (P = 0.7839, and r = 0.10) (Fig. 3C), or observed area (P = 0.5727, and r = 0.20).
The concentrations of sperm bundles estimated with the three methods were significantly different (P < 0.0001): Makler® > hemocytometer = control (Fig. 4). In the 10 measurements, a total of 171 sperm bundles (time required ~ 10 min) were counted with the Makler® chamber, 834 with the hemocytometer (~ 25 min), and 8742 with the control method (~ 70 min).
Fig. 4.
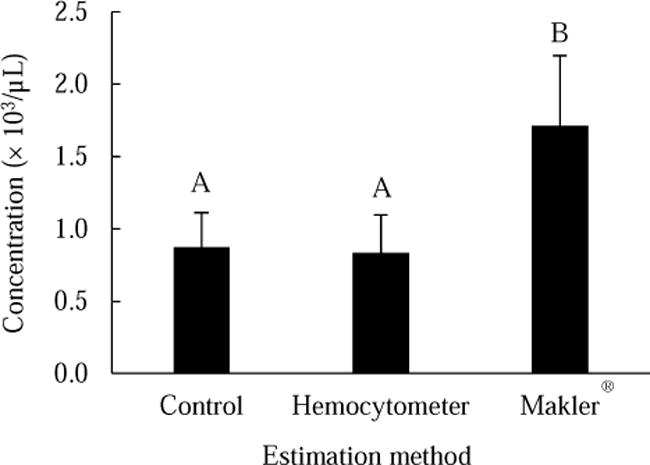
Comparison of sperm bundle concentration estimations with three different methods from pooled suspension of sperm bundles collected from testes of 10 fish (in 0.8 mL buffer solution). Bars represent means (± SD) of 10 repeated measurements. Different capitalized letters above the bars represent significant differences.
Before motility activation with CaCl2, most sperm bundles were in P0 (93 ± 10%) and P1 (7 ± 10%) (Fig. 2). Within 180 min during the activation, all the five phases were observed. FAP0 decreased from 47 ± 28% at 1 min to 0 at 120 min. FAP1 increased from 53 ± 28% at 1 min to a peak of 69 ± 22% at 5 min, and gradually decreased to 16 ± 17% at 180 min. P2 presented from 10 min to 60 min with the FAP2 ranging from 9 ± 7% to 25 ± 11%. P3 and P4 presented from 30 min to 180 min. FAP3 increased 14 ± 10 % to a peak of 22 ± 14% at 60 min, and declined to 9 ± 10% at 180 min. FAP4 constantly increased from 12 ± 14% at 30 min to 75 ± 14% at 180 min.
4. Discussion
Although standardized quality evaluation of free sperm has begun to be established in fishes [34], the bundle forms of male gametes (spermatophores and spermatozeugmata) have been neglected. In the present study, standardized methods were established to collect sperm bundles and evaluate the quality-related attributes including size, concentration, and activation for a freshwater livebearing fish X. eiseni. To evaluate utility, the established methods were applied to compare body wet weight, size and shape of bundles, estimation methods for concentration estimation, and motility activation and duration with attributes of sperm bundles. This study developed for the first time methodologies of collection and evaluation of sperm bundles for Goodeidae, providing a foundation for further standardization and generalization of the methods for other livebearing fishes.
Testis weight had a significant correlation with body wet weight. Therefore, prior to experiments, body wet weight could be a useful indicator to predict the amount of collectable gametes. In this study, no milt was stripped from X. eiseni. Milt collection by stripping has been used in poeciliids, such as Guppy (Poecilia reticulata) [35], Xiphophorus species [25], Mollies (Poecilia spp.)[26], and Mosquitofish (Gambusia affinis)[36]. However, in other goodeids such as Goodea atripinnis, and Ameca splendens milt was not able to be collected from stripping (our unpublished data). The fluid collected from stripping in the present study was presumably urine discharge, mucus, or some other body fluid [37]. Thus, it appears that to collect sperm or sperm bundles from goodeids, the male must be killed and testes dissected. It is unclear whether the annual seasonality of reproductive development of testes can affect the outcome of stripping of goodeids. Collection of sperm by stripping avoids the killing of valuable fish, and individual males can be sampled repeatedly; however, to maximize the volume of sperm available, crushing of dissected testis has been used for sperm collection in studies of small-sized biomedical fishes [24]. As such, a correlation between body and testis weight can be used to avoid killing of males that have limited potential to provide useful sperm samples.
The evaluation methods of bundle size and shape were established with image capture and measurement software. To our knowledge, standardized methods for the estimation of sizes and shapes of non-fixed sperm bundles from livebearing fishes have not been reported. Measurements from histological images in existing publications indicate average axis lengths for X. eiseni ranging from 22.0 to 42.9 μm with an aspect ratio of 77.7 to 78.3% [38, 39], whereas the parameters ranged from 72.2 to 134.9 μm with 66.2 to 71.0% for P. reticulata [27, 40, 41]. Thus, it is possible that bundles from goodeids are generally smaller and rounder than those from poeciliids. However, this possibility should be confirmed in further studies that compare of fresh bundles of more species of the two families by standardized methods. The variation of bundle size and shape in the present study and measurements from other publications may also result from different conditions of growth, seasonality or time of sampling, or reproductive development. In this study, we found that size and shape of bundles had no correlation with body wet weight. However, no study has reported on the relationship between reproductive development and attributes of sperm bundles, including the relationship between different developmental stages of the testis (or spermatogenesis) and the size and shape of sperm bundles. For the purpose of cryopreservation of sperm bundles, further study can target whether the size and shape of bundles are related to sperm quality, and whether it would affect refrigerated storage, cryopreservation, or be related to fertilization rate.
Knowledge of sperm concentration is essential for quality evaluation and protocol standardization for artificial insemination [42] and sperm cryopreservation [9]. Among popular methods to rapidly estimate sperm concentration, the Makler® counting chamber and hemocytometers are relatively low-cost and portable. The principle of each method is to use the counting of cells or objects in a fixed sample volume to estimate concentration of the overall population. In the present study, the estimations made with the control methods had no significant difference with the hemocytometer, but were significantly higher than the Makler® counting chamber. Thus, the hemocytometer instead of the Makler® counting chamber is a viable method to estimate sperm bundles from X. eiseni. The difference in concentrations observed among different methods may be related to the bundle size. The height between cover slip and bottom glass of the counting volume is 10 μm for the Makler® counting chamber and 100 μm for the hemocytometer. However, the diameter of sperm bundles was ~ 31 μm (before placing of cover slip) in the present study. Thus, when the cover glasses were placed, sperm bundles were compressed to the maximum depth of the space between cover glass and base of counting devices, resulting in underestimation of fluid volume containing these sperm bundles. Therefore, the underestimation of the sample volume might cause the overestimation of the population concentration. In addition to counting chambers and hemocytometers, a variety of methods have been used to estimate concentration of free sperm, such as flow cytometry [5], spectrophotometer [42, 43], and CASA [44]. Further study is needed as to whether these latter two methods can be used to estimate bundle concentration.
To assess sperm quality, motility is the most commonly used parameter in aquatic species. However, all existing assays for estimation of sperm motility are based on characterizing movement patterns of free sperm rather than bundles. In the present study, we established a standardized assay to qualitatively evaluate activation of sperm within bundles by classifying behavior patterns into five phases and calculating the frequency of each phase at specific time intervals. During the activation of X. eiseni, the five phases presented in a chronological order of P0 → P1 → P2 → P3 → P4, representing the general sequencing of sperm motility: quiescence → beginning of motility → highest motility → lower motility → end of motility. By applying this standardized approach to other applications, the percentage of any of the five phases can be used to evaluate quality of male gametes, for example, in assessing the frequency of P2 or P1+2 as an indicator of gamete quality. The same concept was also used in the quality evaluation of free-swimming sperm, in which motility capability can be categorized based on average path velocity (VAP), for example, as percent rapid (VAP > 25 μm/s), medium (VAP = 10 – 24 μm/s), slow speed (VAP = 1 – 9 μm/s), percent static (VAP = 0), and percent overall motility (rapid + medium + slow speed) [45]. Among these categories, the percentage of overall motility is the most frequently reported as an indicator of sperm quality. In addition to percent motility, time is also important for the evaluation of sperm quality, such as duration of motility and motility at specific times after activation [44]. The assay established in the present study could be used to assess the duration of specific phases and frequency of certain phases at specific times. A limitation of the method is the accuracy, due to the small number of sperm bundles sampled (15 – 45) for each measurement (compared to usually 200 – 300 free sperm for motility estimation), because categorizing, counting, and recording of sperm bundles was more time-consuming (about 40 s for every 10 bundles) than free sperm estimation (5 s for a measurement). To address this problem, activation can be video-recorded at lower magnification or higher concentration, or classification of fewer phases in each observation could be used to represent overall gamete condition.
5. Conclusions
Using the livebearing fish X. eiseni, the present study established standardized methods to collect, quantitatively evaluate morphology and concentration, and classify dissociation and activation of sperm bundles. These standardized evaluations provide a basis for further modification, standardization, and generalization of these methods, which are useful in research on livebearing fishes involving male gametes, such as studies on cryopreservation, artificial insemination, behavior, taxonomy, toxicology, and evolution. These methods would contribute to conservation of imperiled goodeids by standardization of research and protocol development for artificial reproduction and establishment of germplasm repositories.
Highlights.
Bundled sperm of fishes behave differently than free sperm complicating analysis.
This is the first standardized methodology for evaluation of sperm bundles.
Activation can be evaluated by classification of sperm bundles into 5 phases.
This assists sperm cryopreservation for conservation of imperiled livebearing fishes.
Acknowledgments
This work was supported in part by funding from the National Institutes of Health, Office of Research Infrastructure Programs (R24-OD010441 and R24-OD011120), with additional support provided by the National Institute of Food and Agriculture, United States Department of Agriculture (Hatch project LAB94231). We thank H. Grier for suggestions and for providing fish, and J. Schaff for maintaining fish and assisting with experiments. This manuscript was approved for publication by Louisiana State University Agricultural Center as number 2017-241-31417.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torres L, Hu E, Tiersch TR. Cryopreservation in fish: current status and pathways to quality assurance and quality control in repository development. Reprod Fertil Dev. 2016;28:1105–15. doi: 10.1071/RD15388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiersch T. Process pathways for cryopreservation research, application and commercialization. In: Tiersch TR, Green CC, editors. Cryopreservation in aquatic species. 2nd. Baton Rouge, Louisiana: World Aquaculture Society; 2011. pp. 646–71. [Google Scholar]
- 3.WHO. WHO laboratory manual for the examination and processing of human semen. Fifth. Switzerland: WHO Press; 2010. [Google Scholar]
- 4.Petrunkina A, Waberski D, Günzel-Apel A, Töpfer-Petersen E. Determinants of sperm quality and fertility in domestic species. Reproduction. 2007;134:3–17. doi: 10.1530/REP-07-0046. [DOI] [PubMed] [Google Scholar]
- 5.Torres L, Tiersch TR. Amine reactive dyes: An alternative to estimate membrane integrity in fish sperm cells. Aquaculture. 2016;463:71–8. doi: 10.1016/j.aquaculture.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosson J, Groison AL, Suquet M, Fauvel C, Dreanno C, Billard R. Studying sperm motility in marine fish: an overview on the state of the art. J Appl Ichthyol. 2008;24:460–86. [Google Scholar]
- 7.Linhart O, Rodina M, Cosson J. Cryopreservation of sperm in common carp Cyprinus carpio: sperm motility and hatching success of embryos. Cryobiology. 2000;41:241–50. doi: 10.1006/cryo.2000.2284. [DOI] [PubMed] [Google Scholar]
- 8.Bart A, Dunham R. Effects of sperm concentration and egg number on fertilization efficiency with channel catfish (Ictalurus punctatus) eggs and blue catfish (I. furcatus) spermatozoa. Theriogenology. 1996;45:673–82. doi: 10.1016/0093-691x(95)00413-3. [DOI] [PubMed] [Google Scholar]
- 9.Dong Q, Huang C, Tiersch TR. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: evidence from sperm agglutination in oysters. Cryobiology. 2007;54:87–98. doi: 10.1016/j.cryobiol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Nynca J, Judycka S, Liszewska E, Dobosz S, Ciereszko A. Standardization of spermatozoa concentration for cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture. 2017;477:23–7. [Google Scholar]
- 11.Grier HJ. Cellular organization of the testis and spermatogenesis in fishes. Am Zool. 1981;21:345–57. [Google Scholar]
- 12.Yushin VV, Yoshida M, Spiridonov SE. Riders on the sperm: sperm dimorphism and spermatozeugmata in nematodes from the genus Steinernema (Rhabditida: Steinernematidae) Nematology. 2007;9:61–75. [Google Scholar]
- 13.Braidotti P, Ferraguti M. Two sperm types in the spermatozeugmata of Tubifex tubifex (Annelida, Oligochaeta) J Morphol. 1982;171:123–36. doi: 10.1002/jmor.1051710202. [DOI] [PubMed] [Google Scholar]
- 14.Sahara K, Kawamura N. Roles of actin networks in peristaltic squeezing of sperm bundles in Bombyx mori. J Morphol. 2004;259:1–6. doi: 10.1002/jmor.10168. [DOI] [PubMed] [Google Scholar]
- 15.Lynn JW. The ultrastructure of the sperm and motile spermatozeugmata released from the freshwater mussel Anodonta grandis (Mollusca, Bivalvia, Unionidae) Can J Zool. 1994;72:1452–61. [Google Scholar]
- 16.Piprek RP, Pecio A, Szymura JM. Modifications of the testis in response to sperm bundle formation in the Mediterranean painted frog Discoglossus pictus Otth, 1837 (Amphibia: Anura: Discoglossidae) J Herpetol. 2013;47:331–6. [Google Scholar]
- 17.Jones CJ, Hamlett WC. Glycosylation of the male genital ducts and spermatozeugmata formation in the clearnose skate Raja eglanteria. Histochem J. 2003;34:601–15. doi: 10.1023/a:1026093902502. [DOI] [PubMed] [Google Scholar]
- 18.Grier HJ, Burns JR, Flores JA. Testis structure in three species of teleosts with tubular gonopodia. Copeia. 1981:797–801. [Google Scholar]
- 19.Mann T. Sporadic Occurrence of Spermatophores and Spermatozeugmata in Vertebrata. In: Mann T, editor. Spermatophores: development, structure, biochemical attributes and role in the transfer of spermatozoa. Berlin: Springer; 1984. pp. 165–75. [Google Scholar]
- 20.Tanaka H, Oka Y. Chaotropic ions and multivalent ions activate sperm in the viviparous fish guppy Poecilia reticulata. Biochim Biophys Acta. 2005;1724:173–80. doi: 10.1016/j.bbagen.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Van Look K, Kime D. Automated sperm morphology analysis in fishes: the effect of mercury on goldfish sperm. J Fish Biol. 2003;63:1020–33. [Google Scholar]
- 22.Wourms JP. Viviparity: the maternal-fetal relationship in fishes. Am Zool. 1981;21:473–515. [Google Scholar]
- 23.Goodwin NB, Dulvy NK, Reynolds JD. Macroecology of live-bearing in fishes: latitudinal and depth range comparisons with egg-laying relatives. Oikos. 2005;110:209–18. [Google Scholar]
- 24.Yang H, Tiersch TR. Current status of sperm cryopreservation in biomedical research fish models: zebrafish, medaka, and Xiphophorus. Comp Biochem Physiol, C: Toxicol Pharmacol. 2009;149:224–32. doi: 10.1016/j.cbpc.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith CC. Opposing effects of sperm viability and velocity on the outcome of sperm competition. Behav Ecol. 2012:ars036. [Google Scholar]
- 26.Aspbury AS, Gabor CR. Discriminating males alter sperm production between species. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15970–3. doi: 10.1073/pnas.0405653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinnberg K, Korsgaard B, Bjerregaard P. Effects of octylphenol and 17β-estradiol on the gonads of guppies (Poecilia reticulata) exposed as adults via the water or as embryos via the mother. Comp Biochem Physiol, C: Toxicol Pharmacol. 2003;134:45–55. doi: 10.1016/s1532-0456(02)00206-5. [DOI] [PubMed] [Google Scholar]
- 28.Yang H, Cuevas-Uribe R, Savage MG, Walter RB, Tiersch TR. Sperm cryopreservation in live-bearing Xiphophorus fishes: offspring production from Xiphophorus variatus and strategies for establishment of sperm repositories. Zebrafish. 2012;9:126–34. doi: 10.1089/zeb.2012.0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan JR, Lockwood JL. Extinction in a field of bullets: a search for causes in the decline of the world’s freshwater fishes. Biol Conserv. 2001;102:97–105. [Google Scholar]
- 30.Domínguez-Domínguez O, Mercado-Silva N, Lyons J. Conservation status of Mexican goodeids: Problems, perspectives, and solutions. In: Uribe MC, Grier HJ, editors. Viviparous Fishes. Florida: New Life Publications; 2005. pp. 515–24. [Google Scholar]
- 31.Bonde JPE, Ernst E, Jensen TK, Hjollund NHI, Kolstad H, Scheike T, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 32.Christensen P, Stryhn H, Hansen C. Discrepancies in the determination of sperm concentration using Bürker-Türk, Thoma and Makler counting chambers. Theriogenology. 2005;63:992–1003. doi: 10.1016/j.theriogenology.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 33.He S, Woods LC., Iii Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to striped bass (Morone saxatilis) sperm. Cryobiology. 2004;48:254–62. doi: 10.1016/j.cryobiol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Fauvel C, Suquet M, Cosson J. Evaluation of fish sperm quality. J Appl Ichthyol. 2010;26:636–43. [Google Scholar]
- 35.Zajitschek S, Lindholm A, Evans J, Brooks R. Experimental evidence that high levels of inbreeding depress sperm competitiveness. J Evol Biol. 2009;22:1338–45. doi: 10.1111/j.1420-9101.2009.01738.x. [DOI] [PubMed] [Google Scholar]
- 36.Raut SA, Angus RA. Triclosan has endocrine-disrupting effects in male western mosquitofish, Gambusia affinis. Environ Toxicol Chem. 2010;29:1287–91. doi: 10.1002/etc.150. [DOI] [PubMed] [Google Scholar]
- 37.Alavi SH, Rodina M, Viveiros AT, Cosson J, Gela D, Boryshpolets S, et al. Effects of osmolality on sperm morphology, motility and flagellar wave parameters in Northern pike (Esox lucius L.) Theriogenology. 2009;72:32–43. doi: 10.1016/j.theriogenology.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Uribe MC, Grier HJ, Mejía-Roa V. Comparative testicular structure and spermatogenesis in bony fishes. Spermatogenesis. 2014;4:e983400. doi: 10.4161/21565562.2014.983400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uribe M, Grier H, De la Rosa Cruz G, García-Alarcón A. Modifications in ovarian and testicular morphology associated with viviparity in teleosts. In: Jamieson B, editor. Reproductive Biology and Phylogeny of Fish (Agnatha and Osteichthyes) Enfield (New Hampshire): Science Publisher; 2009. pp. 85–117. [Google Scholar]
- 40.Nielsen L, Baatrup E. Quantitative studies on the effects of environmental estrogens on the testis of the guppy, Poecilia reticulata. Aquat Toxicol. 2006;80:140–8. doi: 10.1016/j.aquatox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Kinnberg K, Toft G. Effects of estrogenic and antiandrogenic compounds on the testis structure of the adult guppy (Poecilia reticulata) Ecotoxicol Environ Saf. 2003;54:16–24. doi: 10.1016/s0147-6513(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 42.Butts IA, Sørensen SR, Politis SN, Pitcher TE, Tomkiewicz J. Standardization of fertilization protocols for the European eel, Anguilla anguilla. Aquaculture. 2014;426:9–13. [Google Scholar]
- 43.Tan E, Yang H, Tiersch TR. Determination of sperm concentration for small-bodied biomedical model fishes by use of microspectrophotometry. Zebrafish. 2010;7:233–40. doi: 10.1089/zeb.2010.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billard R, Cosson MP. Some problems related to the assessment of sperm motility in freshwater fish. J Exp Zool A Ecol Integr Physiol. 1992;261:122–31. [Google Scholar]
- 45.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken R. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33–44. [PubMed] [Google Scholar]


