Abstract
The prevailing opioid crisis has necessitated the need to understand mechanisms leading to addiction and tolerance, the major contributors to overdose and death and to develop strategies for developing drugs for pain treatment that lack abuse liability and side-effects. Opioids are commonly used for treatment of pain and symptoms of inflammatory bowel disease. The significant effect of opioids in the gut, both acute and chronic, include persistent constipation and paradoxically may also worsen pain symptoms. Recent work has suggested a significant role of the gastrointestinal microbiome in behavioral responses to opioids, including the development of tolerance to its pain-relieving effects. In this review, we present current concepts of gut-brain interaction in analgesic tolerance to opioids and suggest that peripheral mechanisms emanating from the gut can profoundly affect central control of opioid function.
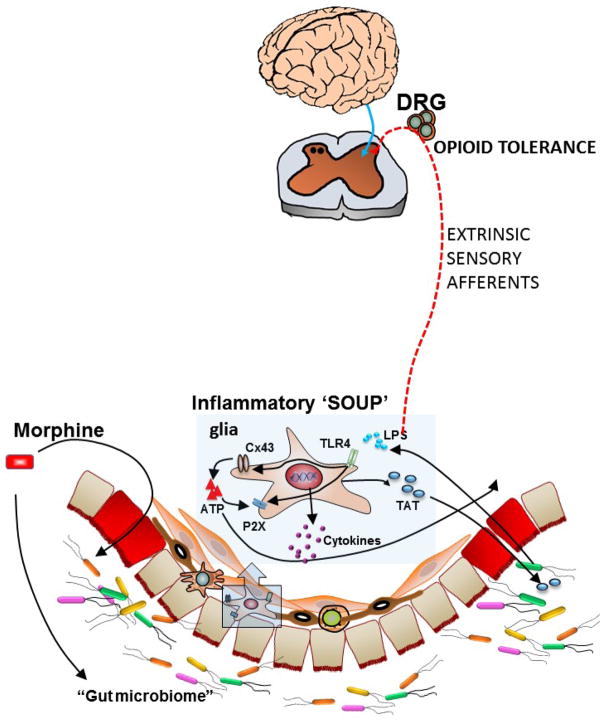
Graphical Abstract
INTRODUCTION
Opioids are the most effective and highly prescribed analgesics. The therapeutic effects of morphine and other opioids are limited by their side effects that include nausea, vomiting, abdominal pain and constipation that are collectively referred to as “opioid induced bowel dysfunction (1, 2). Opioid-induced constipation is the most common of these symptoms and arises largely due to inhibition of peristaltic activity, increased sphincter tone and segmental non-propulsive contractions. These effects of opioids result in dry and hard feces and chronic use paradoxically may also be associated with increased abdominal pain. The over-prescription of opioids in the recent decades, and the current crisis of increasing deaths due to opioid overdose has highlighted the need for better understanding of mechanisms of opioid function. More recently, the role of the gut – brain axis in physiological processes has been identified and novel concepts have emerged regarding mechanisms by which the effects of opioids in the gastrointestinal tract alter what was thought to be an exclusive central effect of analgesic tolerance and hyperalgesia. In this review we summarize some of these current findings and illustrate potential areas for further research to develop better therapeutics for pain treatment.
Of the three major G-protein coupled and naloxone – sensitive opioid receptors, μ, κ, and δ, the major aspects of opioid function on gastrointestinal motility occur as a result of the activation of the μ opioid receptor. The classes of opioid receptors and their functional localization within the gastrointestinal tract have been reviewed recently (3–5). In the GI tract, opioid receptors are mainly expressed on neurons within the myenteric and submucosal plexus throughout the gastrointestinal tract at both pre- and post-synaptic membranes and on the cell bodies within the ganglia. The activation of the μ receptor in neurons within the myenteric ganglia or on nerve terminals innervating smooth muscle cells reduce motility, while activation of submucosal neurons reduces secretion. The inhibition of neuronal activity affects pain transmission in the central neurons whereas in the gastrointestinal tract, both secretory functions and muscle contractility are altered upon opioid receptor activation in enteric neurons. Reduced excitability of neurons is due to opioid modulation of ionic conductances, particularly inwardly rectifying potassium channels and inhibition of sodium and calcium channels. Several μ-opioid agonists increase potassium conductance (6) that results in membrane hyperpolarization thus preventing action potential generation. More recently, voltage clamp studies in isolated myenteric neurons have shown that morphine inhibits tetrodotoxin (TTX)-resistant sodium channels on neuronal cell bodies thus affecting the threshold for action potentials (7, 8). Sodium channels were inactivated in the presence of morphine thus rendering the neurons incapable of firing multiple action potentials resulting in an overall decreased excitability. The receptor mediated biophysical alterations of the sodium channel by opioids underlie the potential cellular basis for reduced neuronal excitability of enteric neurons. Interaction of morphine with activation of inwardly rectifying potassium channels and inhibition of calcium channels involves direct interaction of G-protein subunits in a membrane delimited pathway following receptor activation(9). Whether similar mechanisms are involved with inhibition of TTX-resistant sodium channels in enteric neurons is not known. The reduced excitability of motor and sensory neurons in the myenteric plexus reduces motility and sensory transmission, including the extrinsic pathway whose cell bodies lie within the dorsal root ganglia thus modulating pain transmission to central neurons emanating from the gastrointestinal tract.
In addition to coupling to ion channels, μ opioid receptor mediated analgesia is linked to several signaling pathways including inhibition of adenylate cyclase, β-arrestin recruitment, receptor phosphorylation by protein kinases C and A, and ERK 1/2, particularly in the context of tolerance development (10). The relationship between β-arrestin2 and opioid tolerance development has been investigated recently with regard to drug development for novel pain analgesics with less side-effects, including opioid-induced constipation. Understanding tolerance development to repeated use of opioids is central to drug development for novel analgesics. It is now clear that in both animals and man the rate and extent of tolerance to most opioids develops at different rates and to different extents. For example, tolerance to analgesia and euphoria occurs faster than to respiratory depression and predisposes to the potential for death due to overdose (11). Tolerance does not develop to the constipating effects of opioids, resulting in persistent constipation (4).
It is well documented that recruitment of β-arrestin2 following receptor activation mediates additional signaling pathways and also results in receptor internalization and forms one of the basic pathways for tolerance development (10). Thus, antinociceptive tolerance does not occur in the β-arrestin2 knock out mice. The role of β-arrestin2 in tolerance to morphine mediated inhibition of gastrointestinal motility differs from that to antinociceptive tolerance. Chronic exposure to morphine in the mouse ileum reduces β-arrestin2 expression and induces tolerance (12). In the colon however, β-arrestin2 remains elevated following chronic morphine and is associated with lack of tolerance development. The disparate effects of morphine tolerance between one clinical effect i.e. constipation versus another i.e. analgesia have been exploited to develop biased agonists that would preferentially activate G proteins but do not recruit β-arrestin2 resulting in agents with significant analgesic effects without the adverse effects of analgesic tolerance and constipation (13). The experimental and clinical evidence for reduced gastrointestinal motility remains unclear. Oliceridine (TRV130) is a G-protein biased ligand with low levels of β-arrestin2 recruitment and was reported to produce antinociception with reduced signs of constipation (14). However, recent studies show that with repeated administration in-vivo, TRV130 significantly reduced gastrointestinal motility and prevented antinociceptive tolerance (15). In a phase 2 trial in a bunionectomy model, TRV130 at 1 mg was equi-analgesic to morphine (4 mg) but induced constipation in 15% (6/38) of patients compared to 5% (2/39) for morphine (16). A structurally different μ opioid compound, PZM21, recently reported as a G-protein biased agonist with minimal β-arrestin2 recruitment, also reduced gastrointestinal motility. Although it was reported that PZM21 was less potent in reducing gastrointestinal motility than morphine, an equi-analgesic dose to morphine was not tested (17). These findings highlight the complexity of the different mechanisms between opioid-induced constipation and analgesia.
OPIOID-INDUCED GUT BRAIN INTERACTION
Clinical studies have demonstrated that narcotic use escalates the disease severity of patients with Crohn’s disease (CD) and may be associated with increased recurrence from remission (18). Data from the Crohn’s Therapy, Resource, Evaluation, and Assesment Tool (TREAT) Registry comprised of over 6,000 patients with CD, suggest that narcotic use was associated with a 1.5 fold increased risk of mortality and 3-fold increased risk of infection compared to patients not taking opioid analgesics (19, 20). The increased risk of infection can arise from bacterial translocation in the colon and lead to sepsis (21) and immune dysregulation that occurs not only in the GI tract but also systemically (22). Recent studies show that chronic opioid use is associated with microbial dysbiosis in man (23, 24)} and mice (25–27). The disruption of gut epithelial barrier by chronic morphine in mice enhances “leakiness” allowing for bacterial translocation in ileum (26) and in colon (27). Meng et al. (26) reported disruption of the gut epithelial barrier as a result of morphine mediated activation of the toll-like receptors (TLR) on the epithelial cells, allowing for bacterial products to translocate. Both Gram-positive and Gram-negative bacterial products can activate toll-like receptors (TLRs) on immune cells and enteric glia (28). Enteric glia are key players in mediating GI functions and are importantly involved in immune modulation through their interaction with enteric neurons. Bhave et al.(29) found that activation of the connexin-purinergic pathway in enteric glia by the bacterial product, lipopolysaccharide, was a significant source of cytokine release with chronic morphine treatment. In-vivo treatment with morphine resulted in upregulation of P2X purinergic receptors and enhanced ATP induced currents in the enteric glia from the mouse colon. P2X receptors are cation channels allowing for calcium entry, an essential component in cytokine release. Furthermore, the bacterial product, LPS upregulated connexin43 (Cx43) expression in enteric glia cells. Cx43 are hemichannels through which ATP is released. Thus, the combination of Cx43 and P2X receptor upregulation in enteric glia with chronic morphine provides a significant amplification of an inflammatory response as purines are also a large component of the inflammatory soup (30). Inhibition of Cx43 by carbenoxolone reversed chronic opioid-induced constipation in mice (29).
Opioid use also worsens GI dysmotility in HIV-1 infected individuals. Bacterial infections within the enteric nervous system due to opportunistic infection occur very commonly among HIV-1 infected patients who are often opioid users. Fitting et al. (31) showed that the HIV viral protein, Tat, sensitizes enteric neurons to morphine such that morphine-induced motility is markedly reduced at much lower doses. The increase in sensitivity to morphine was not due to increased expression of the μ opioid receptor, but may underlie inhibition of tetrodotoxin-resistant sodium channels (see (32). The enhanced sensitivity to morphine cautions the use of peripherally restricted opioids in treating chronic diarrhea in HIV-1 infected individuals.
The role of microbial dysbiosis and potential increased infection in the colon was recently addressed with regard to the development of tolerance to chronic morphine. Treatment of mice with oral gavage of an antibiotic cocktail prevented the development of antinociceptive tolerance to chronic morphine in mice (27). Further analysis of individual antibiotics showed that oral vancomycin, which has poor bioavailability (33), was sufficient to prevent tolerance, suggesting that the microbial translocation, specifically Gram – positive bacteria from the gut lumen is intricately involved in this process. Tolerance to morphine was also determined in isolated neurons from the dorsal root ganglia (DRG), which are primary “relay stations” between the periphery and the brain. In nociceptive neurons from the DRG of chronic morphine treated mice, patch clamp studies showed that morphine challenge did not reduce neuronal excitability indicative of the development of tolerance. However in DRG neurons from chronic morphine with antibiotics treated mice, morphine challenge reduced neuronal excitability indicating that tolerance did not develop in these nociceptors. These findings implicate that changes in the gut microbiome affect opioid tolerance in the cell bodies of extrinsic sensory neurons. The concept that peripheral sites may be importantly involved in modulating opioid tolerance in-vivo is also supported by recent study by Corder et. al. (34). These authors observed loss of analgesic tolerance to chronic morphine upon conditional deletion of the μ opioid receptor in DRG nociceptors. While tolerance was blocked, the acute analgesic effects to morphine remained intact suggesting that tolerance to chronic opioids involves peripheral nociceptors, while analgesia is maintained by central mechanisms within the brain. These distinctive effects were also seen when animals were treated with the peripheral opiate antagonist, methylnaltrexone. In models of orthotrauma inflammatory pain and constriction injury, the combination of repeated morphine with methylnaltrexone continued to produce antinociception without the development of tolerance.
The symptoms of Inflammatory Bowel Disease (IBD) such as abdominal pain and diarrhea also often require opioids as potential treatments. However, IBD can be a significant risk factor for heavy opioid use and lead to increased mortality (35). Opioids are also associated with an increased risk of complications of diverticular disease (36) partly as a result of enhanced colonic contractions due to opioid induced inhibition of enteric neurotransmission.
The effects of opioids on gastrointestinal function have been known since the first recorded use of opium. It is now becoming increasing clear that alterations in GI function also affect mechanisms initially thought to be entirely of central origin. An interesting aspect of the information presented in this review is the discovery that the microbiome in the lumen of the gut has such a pronounced role in the development of opioid tolerance in the intestine but most surprisingly also in the brain. A number of recent reports have shown the influence of the microbiome on certain aspects of brain function such as mood and related phenomena (37). The effects of drugs of abuse such as cocaine on the gut environment to affect behavioral responses are now being further recognized (38). However previous studies on the mechanism of opioid induced tolerance development to analgesia and related effects have concentrated on alterations at the neuronal level of the brain, concentrating on alterations of opioid sensitivity at the μ receptor. Now, it is clear that peripheral mechanisms, specifically the microbiome is an important modulator of the effects of chronic opioid administration. The mechanisms by which changes in the gut microbiome alter opioid analgesic tolerance remain to be defined, however, bacterial translocation and ensuing effects on extrinsic sensory afferents are potential pathways (Figure 1). It will be important to determine if similar mechanisms affect tolerance to euphoria and respiratory depression. Opioid related mortality has reached epidemic proportions in the USA resulting in staggering costs to the nation both economically and socially with current estimates of 100 deaths/day. Newer strategies are necessary and our increasing understanding of the gut-brain interaction towards opioid tolerance provides a novel avenue to pursue.
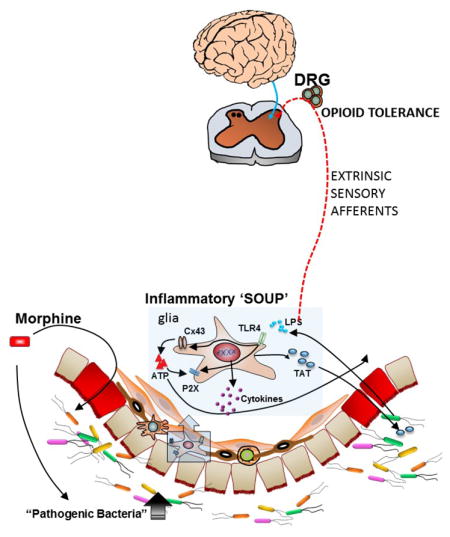
Figure 1.
Schema of gut-brain interaction through the dorsal root ganglia neurons. The gut microbiome is altered in the presence of chronic morphine. Bacterial translocation due to disruption of the epithelial barrier results in activation of TLR’s on enteric glia increasing connexin43 and P2X expression. Cytokines (Inflammatory soup) released from glia affect extrinsic sensory afferents whose cell bodies lie within the dorsal root ganglia (DRG) and induce tolerance to opioids.
HIGHLIGHTS.
Chronic opioids produce tolerance to analgesic but not constipating effects
Chronic opioids can worsen GI diseases
The gut microbial diversity is altered with chronic opioids in man and mice
Changes in the microbiome alters neuronal tolerance in extrinsic sensory afferents
The gastrointestinal microbiome may mediate opioid tolerance to analgesia
Acknowledgments
We would like to thank the past and present members of the Akbarali/Dewey laboratories who have made untiring contributions to our understanding of opioid tolerance.
This work was supported by the National Institute of Health (R01DA024009, R01DA036975.P30DA033934)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. American journal of surgery. 2001;182:11S–18S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 2.Holzer P. Treatment of opioid-induced gut dysfunction. Expert Opin Investig Drugs. 2007;16:181–194. doi: 10.1517/13543784.16.2.181. [DOI] [PubMed] [Google Scholar]
- 3.Galligan JJ, Akbarali HI. Molecular Physiology of Enteric Opioid Receptors. Am J Gastroenterol. 2014;2:17–21. doi: 10.1038/ajgsup.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akbarali HI, Inkisar A, Dewey WL. Site and mechanism of morphine tolerance in the gastrointestinal tract. Neurogastroenterol Motil. 2014;26:1361–1367. doi: 10.1111/nmo.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M, Lembo A, Katzka DA. Opioids in Gastroenterology: Treating Adverse Effects and Creating Therapeutic Benefits. Clin Gastroenterol Hepatol. 2017;15:1338–1349. doi: 10.1016/j.cgh.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.North RA, Tonini M. The mechanism of action of narcotic analgesics in the guinea-pig ileum. Br J Pharmacol. 1977;61:541–549. doi: 10.1111/j.1476-5381.1977.tb07546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TH, Grider JR, Dewey WL, Akbarali HI. Morphine Decreases Enteric Neuron Excitability via Inhibition of Sodium Channels. PloS one. 2012;7:e45251. doi: 10.1371/journal.pone.0045251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TH, Ngwainmbi J, Grider JR, Dewey WL, Akbarali HI. An in-vitro preparation of isolated enteric neurons and glia from the myenteric plexus of the adult mouse. Journal of visualized experiments: JoVE. 2013;(78) doi: 10.3791/50688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, Christie MJ. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White JM, Irvine RJ. Mechanisms of fatal opioid overdose. Addiction. 1999;94:961–972. [PubMed] [Google Scholar]
- 12.Kang M, Maguma HT, Smith TH, Ross GR, Dewey WL, Akbarali HI. The role of beta-arrestin2 in the mechanism of morphine tolerance in the mouse and guinea pig gastrointestinal tract. J Pharmacol Exp Ther. 2012;340:567–576. doi: 10.1124/jpet.111.186320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Violin JD, Crombie AL, Soergel DG, Lark MW. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci. 2014;35:308–316. doi: 10.1016/j.tips.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 14.DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344:708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 15.Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol. 2017 doi: 10.1177/0269881116689257. 269881116689257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viscusi ER, Webster L, Kuss M, Daniels S, Bolognese JA, Zuckerman S, Soergel DG, Subach RA, Cook E, Skobieranda F. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157:264–272. doi: 10.1097/j.pain.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 17.Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang XP, Sassano MF, Giguere PM, Lober S, Da D, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, Shoichet BK. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537:185–190. doi: 10.1038/nature19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones JL, Loftus EV., Jr Avoiding the vicious cycle of prolonged opioid use in Crohn’s disease. Am J Gastroenterol. 2005;100:2230–2232. doi: 10.1111/j.1572-0241.2005.50803.x. [DOI] [PubMed] [Google Scholar]
- 19.Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100:2225–2229. doi: 10.1111/j.1572-0241.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Price S, Langholff W, Londhe A, Sandborn WJ. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–1422. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilburger ME, Adler MW, Truant AL, Meissler JJ, Jr, Satishchandran V, Rogers TJ, Eisenstein TK. Morphine induces sepsis in mice. J Infect Dis. 1997;176:183–188. doi: 10.1086/514021. [DOI] [PubMed] [Google Scholar]
- 22.Roy S, Barke RA, Loh HH. MU-opioid receptor-knockout mice: role of mu-opioid receptor in morphine mediated immune functions. Brain Res Mol Brain Res. 1998;61:190–194. doi: 10.1016/s0169-328x(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 23*.Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, Ganapathy D, Fagan A, Sikaroodi M, Bajaj JS. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2017;45:319–331. doi: 10.1111/apt.13858. This study examines the effect of opioids on readmissions and on gut microbiota composition in a cohort of cirrhotic patients. There is significant dysbiosis with chronic opioids and is predictive of readmission. [DOI] [PubMed] [Google Scholar]
- 24*.Xu Y, Xie Z, Wang H, Shen Z, Guo Y, Gao Y, Chen X, Wu Q, Li X, Wang K. Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Sci Rep. 2017;7:3628. doi: 10.1038/s41598-017-03706-9. This study examines the composition and changes in the gut microbiota of 45 patients with substance abuse disorders in China. Abundance analysis showed that both age-matched and long-term substance abuse results in significant changes, particularly in the abundance of Bacteioides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng J, Sindberg GM, Roy S. Disruption of gut homeostasis by opioids accelerates HIV disease progression. Frontiers in microbiology. 2015;6:643. doi: 10.3389/fmicb.2015.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One. 2013;8:e54040. doi: 10.1371/journal.pone.0054040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Kang M, Mischel RA, Bhave S, Komla E, Cho A, Huang C, Dewey WL, Akbarali HI. The effect of gut microbiome on tolerance to morphine mediated antinociception in mice. Sci Rep. 2017;7:42658. doi: 10.1038/srep42658. Depletion of gut bacteria with antibiotics prevents morphine tolerance development to antinociception. Lack of tolerance also occurs in isolated DRG neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow AK, Gulbransen BD. Potential roles of enteric glia in bridging neuroimmune communication in the gut. Am J Physiol Gastrointest Liver Physiol. 2017;312:G145–G152. doi: 10.1152/ajpgi.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Bhave S, Gade A, Kang M, Hauser KF, Dewey WL, Akbarali HI. Connexin-purinergic signaling in enteric glia mediates the prolonged effect of morphine on constipation. FASEB J. 2017;31:2649–2660. doi: 10.1096/fj.201601068R. This paper shows increased P2X mediated currrents in isolated enteric glia from chronic morphine treated mice. P2X and Connexin 43 mRNA were upregulated with lipopolysaccharide treatment of enteric glia. Inhibition of connexin43 with carbenoxolone reduced inflammation in the colon and reduced opioid-induced constipation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linan-Rico A, Turco F, Ochoa-Cortes F, Harzman A, Needleman BJ, Arsenescu R, Abdel-Rasoul M, Fadda P, Grants I, Whitaker E, Cuomo R, Christofi FL. Molecular Signaling and Dysfunction of the Human Reactive Enteric Glial Cell Phenotype: Implications for GI Infection, IBD, POI, Neurological, Motility, and GI Disorders. Inflamm Bowel Dis. 2016;22:1812–1834. doi: 10.1097/MIB.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitting S, Ngwainmbi J, Kang M, Khan FA, Stevens DL, Dewey WL, Knapp PE, Hauser KF, Akbarali HI. Sensitization of enteric neurons to morphine by HIV-1 Tat protein. Neurogastroenterol Motil. 2015;27:468–480. doi: 10.1111/nmo.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galligan JJ. HIV, opiates, and enteric neuron dysfunction. Neurogastroenterol Motil. 2015;27:449–454. doi: 10.1111/nmo.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao S, Kupfer Y, Pagala M, Chapnick E, Tessler S. Systemic absorption of oral vancomycin in patients with Clostridium difficile infection. Scandinavian journal of infectious diseases. 2011;43:386–388. doi: 10.3109/00365548.2010.544671. [DOI] [PubMed] [Google Scholar]
- 34*.Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, Scherrer G. Loss of mu opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nature medicine. 2017;23:164–173. doi: 10.1038/nm.4262. This study utilized conditional mu-opioid receptor knock out in DRG neurons and shows that loss of signaling in DRG prevents onset of tolerance and opioid-induced hyperalgesia. Similar effects were obtained with the peripheral opioid antagonist, methylnaltrexone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Targownik LE, Nugent Z, Singh H, Bugden S, Bernstein CN. The prevalence and predictors of opioid use in inflammatory bowel disease: a population-based analysis. Am J Gastroenterol. 2014;109:1613–1620. doi: 10.1038/ajg.2014.230. [DOI] [PubMed] [Google Scholar]
- 36.Piekarek K, Israelsson LA. Perforated colonic diverticular disease: the importance of NSAIDs, opioids, corticosteroids, and calcium channel blockers. Int J Colorectal Dis. 2008;23:1193–1197. doi: 10.1007/s00384-008-0555-4. [DOI] [PubMed] [Google Scholar]
- 37.Vuong HE, Yano JM, Fung TC, Hsiao EY. The Microbiome and Host Behavior. Annu Rev Neurosci. 2017;40:21–49. doi: 10.1146/annurev-neuro-072116-031347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiraly DD, Walker DM, Calipari ES, Labonte B, Issler O, Pena CJ, Ribeiro EA, Russo SJ, Nestler EJ. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci Rep. 2016;6:35455. doi: 10.1038/srep35455. [DOI] [PMC free article] [PubMed] [Google Scholar]