Abstract
Background
Although most African countries offer hepatitis B immunization through a 3-dose vaccine series recommended at 6, 10, and 14 weeks of age, very few provide birth dose vaccination. In support of Cameroon’s national plan to implement the birth dose vaccine in 2017, we investigated predictors of infant hepatitis B virus (HBV) vaccination under the current program.
Methods
Using the 2011 Demographic Health Survey in Cameroon, we identified women with at least one living child (age 12–60 months) and information about the hepatitis B vaccine series. Vaccination rates were calculated and logistic regression modeling was used to identify factors associated with 3-dose series completion. Changes over time were assessed with linear logistic model.
Results
Among 4594 mothers analyzed, 66.7% (95% CI 64.1–69.3) of infants completed the hepatitis B vaccine series; however, an average four week delay in series initiation was noted with median dose timing at 10, 14 and 19 weeks of age. Predictors of series completion included facility delivery (adjusted odds ratio [aOR] 2.1, 95% CI 1.7–2.6), household wealth (aOR 1.9, CI 1.2–3.1 comparing the highest and lowest quintiles), Christian religion (aOR 1.8, CI 1.3–2.5 compared to Muslim religion), and older maternal age (aOR 1.4, CI 1.2–1.7 for 10 year units).
Conclusions
Birth dose vaccination to reduce vertical and early childhood transmission of hepatitis B may overcome some of the obstacles to timely and complete HBV immunization in Cameroon. Increased awareness of HBV is needed among pregnant women and high risk groups about vertical transmission, the importance of facility delivery and the effectiveness of prevention beginning with monovalent HBV vaccination at birth.
Keywords: hepatitis B (HBV) prevention, epidemiology, birth dose vaccine, sub-Saharan Africa, vertical transmission, infants, EPI
Introduction
Viral hepatitis is the 7th leading cause of death worldwide and hepatitis B virus (HBV) is one of the main etiologies with prevalent chronic infection in 250 million people. (1, 2) Hepatitis B is highly endemic in sub-Saharan Africa and an estimated 63 million new HBV cases will occur between 2015 and 2030. (1, 3) In response, the World Health Organization (WHO) has set an ambitious goal of HBV elimination by 2030 and vaccination is a cornerstone of the public health campaign in the absence of a cure. (4) The hepatitis B vaccine has a long track-record of safety and efficacy with more than a billion doses administered since 1981. Vaccination is highly cost-effective and series completion leads to durable protection lasting at least 30 years. (5, 6)
Hepatitis B can be transmitted at the time of delivery (vertically) or later in life (horizontally) through exposure to infected blood or body fluids. Ninety percent of newborns with acute HBV infection will develop chronicity compared to 5–10% of HBV-exposed adults. This strengthens the case for early, targeted prevention efforts. The likelihood of vertical transmission correlates with maternal HBV DNA viral load levels (>200,000 IU/mL) and the presence of e antigen (HBeAg) in the serum.(7) The prevalence of hepatitis B infection among pregnant women in sub-Saharan Africa ranges from 5–13%; approximately 25% are e antigen positive.(8–10) Antenatal screening for hepatitis B is recommended but rarely performed, and most women with infection are asymptomatic and unaware of the risk of vertical transmission. (9, 11, 12) WHO recommends universal administration of the monovalent HBV vaccine within 24 hours of birth to prevent vertical transmission, irrespective of maternal infection status, followed by completion of the series with pentavalent vaccine (HBV/DTP/Hflu) at 6, 10, and 14 weeks of age. Vaccination prevents 75–95% of vertical HBV infections. Other effective interventions to reduce vertical transmission include antiviral therapy and HBV-specific immunoglobulin, but these options are rarely available or affordable in sub-Saharan Africa. (11)
Globally, in 2015, an estimated 87% of children completed the infant hepatitis B vaccine series but only 38% received birth dose vaccine. Birth dose coverage was 10% in sub-Saharan Africa compared to 72% in the United States. (13, 14) An estimated 19 million HBV infections could be averted by 2030 if coverage of the birth dose vaccine increased to 80%. (15) Although 96 countries included the birth dose in their national immunization platforms, the vaccine is not currently available in most of sub-Saharan Africa outside of the private sector. The 2015 WHO Strategic Plan for Immunization in Africa set a goal for 25 countries to adopt the HBV vaccine birth dose by 2020. (16) Although it is not included in the list of vaccines supported by the Global Vaccine Alliance (GAVI), the current feasibility of HBV birth dose vaccination in low-income countries (LIC) has improved since the cost has fallen to 20 cents per dose. Additional challenges to birth dose implementation include home deliveries and cold chain requirements but creative solutions including the use of mobile phones and heat-stable vaccine formulations have been tested and show promise. (17–20)
The National Program for Expanded Immunization in Cameroon was established in 1981 and vaccines on the national platform are widely available with no fees. In Cameroon, the HBV-containing vaccine series was added in 2005.(21) The addition of the monovalent birth dose vaccine to the platform was approved in 2016 with rollout planned for late 2017. This study was designed to highlight gaps and trends in the prevention of pediatric hepatitis B infection in Cameroon. Completion rates and predictors of hepatitis B vaccine series completion were sought to help tailor the new birth dose program.
Materials and Methods
Study Design and Population
The policy in Cameroon requires HBV-containing pentavalent vaccination at 6, 10 and 14 weeks of age. We conducted a cross-sectional analysis using the National Demographic Health Survey (DHS) carried out nationwide in Cameroon in 2011 as the primary source of data. (22) Our sample was derived from 15,426 women who were interviewed using 2-stage stratified sampling techniques. These recurring, household-level surveys were administered in urban and rural areas and weighting was used to make data nationally representative. Weighting is a mathematical correction to help account for over-sampling, undersampling and differential response rates by region. Standardized DHS survey methods have been previously described.(22) The study population was women with a living child (age 12–60 months) at the time of the survey. Women were included if information was available (yes/no) about the receipt of each dose of the pentavalent series for their youngest child above 12 months of age. The vaccination status of this child became the focus of our study. Survey questions also gathered information about socio-demographics and access to health care including the site of delivery for the most recent pregnancy.
Ethics
We used publicly available, de-identified data provided by DHS and the study was designated exempt by the Institutional Review Board at the University of Alabama at Birmingham, USA. Authorities in Cameroon have approved the distribution of DHS datasets for the purpose of legitimate academic research.
Study Outcomes
The main outcome for this study was completion of the 3-dose infant hepatitis B series based on the infant vaccination card or maternal report if the card was not present. This was chosen as the primary outcome since studies in children have shown that the gold standard (post-vaccination serum antibody levels) is best approximated when vaccination data from the card and maternal report is combined.(23–25) The 12-month age for HBV series completion was used as the standard indicator but 6-month completion rates were also calculated to give a sense of “on time” dosing since the 3rd dose of the series should be given at 14 weeks of age. (26) Vaccine dose timing was calculated for infants with recorded vaccination dates and evidence of receipt of prior doses was required for doses #2, #3.
Additional vaccination outcomes for the group with recorded vaccination dates included the median age at the administration of each HBV vaccine dose, the median number of days between doses and the proportion with delayed initiation of the series (defined as receipt of the first dose after 90 days of life). Vaccination rates were also calculated according to the infant’s year of birth to assess for changes over time. Finally, HBV vaccine completion rates were compared to vaccination rates for other vaccines which are commonly administered during the first year of life: the BCG vaccine (a birth dose vaccine), oral polio vaccine (recommended at 6, 10 and 14 weeks), and measles vaccine (recommended at 9 months of age).
Statistical Analysis
Descriptive statistics were used for survey respondents and infant vaccination outcomes. Univariate and multivariable logistic regression models were created to measure the relationship between individual, household, and access to care parameters in terms of completing the infant HBV vaccination series. Dependent variables in the initial model were: age and education levels for survey respondents and partners (none, primary school, secondary school or higher or “never married” for partner education if women reported no marriage in the past), employment in the past 12 months, marital status (married, living with partner, widowed/divorced/separated or never in union), polygamy (monogamous, polygamous, not known or never in union), urban/rural residence, wealth quintile, transportation ownership (hierarchical categorization: car, motorcycle, bicycle or none), religion (Christian, Muslim, other/none), parity, the number of children at home and access to care. The three access to care parameters were: having visited a health facility in the past 12 months, receipt of prenatal care during the most recent pregnancy and the location of delivery for the most recent birth.
Variables were chosen for the multivariable model based on statistical significance in univariate models (p<0.05), collinearity considerations and relevance based on the literature. A sensitivity analysis was also performed with the primary outcome defined as vaccination completion per card only (excluding maternal report). The test of trend for vaccination rate by year of birth was calculated using linear logistic model. The age and education level of male partners was excluded from the MV model due to missing data. All analyses were performed with SAS 9.4 (Cary, NC) and results were adjusted for weighting, clustering and stratification using the SAS/STAT® “SURVEY” procedures.
Results
Among the 15,426 women surveyed, 4594 (29.8%) met criteria for inclusion in our analysis. We excluded 7157 who did not have a child >12 months of age and 3675 for incomplete HBV vaccination data. The demographic characteristics of survey respondents included in our analysis were stratified by whether or not the vaccine series was completed. The median age of survey respondents was 27.4 years and most women were married (68%), attended at least primary school (74.5%) and employed in the past year (76.6%). The median parity was 2.7 and most women received prenatal care during their most recent pregnancy (86.7%) and delivered at a facility (67.4%). (Table in supplemental digital content).
Table 1 shows the primary outcome of HBV vaccine series completion and timing. In the analysis cohort (n=4594), we observed that 3065 (66.7%; 95% CI 64.1–69.3) completed the HBV vaccine series. Among those who initiated the series (n=3769), the completion rate was 81.3%. There was a 7.5% loss between doses 1–2 and a 12.1% loss between doses 2–3. A majority of the vaccine information on HBV series completion came from vaccination cards (63.1%). When review of the infant vaccination card only was assessed (discounting maternal report), the series completion rate was 42.1% (95% CI 39.9–44.4). Among infants with vaccination dates recorded (n=2189 for dose #1, n=2094 for dose #2, n=1978 for dose #3), the median timing of dose #1 was 10 weeks after birth, dose #2 at 14 weeks and dose #3 at 19 weeks, but the range of timing was broad. Eighty percent of these infants had completed the vaccine series by 180 days, but one in five had not received any vaccine by 90 days of life. The timing between doses was excellent; on average, 32 days separated doses 1–2 and 33 days separated doses 2–3. (Figure 1).
Table 1.
HBV Vaccine Completion Rates * (n=4594)
| Outcome | n (%) or median (Q1, Q3) |
|---|---|
|
| |
| HBV Series Completion | |
| Yes | 3065 (66.7) |
| No | 1529 (33.3) |
|
| |
| Number of HBV Vaccines Received | |
| 0 | 825 (18) |
| 1 | 283 (6.1) |
| 2 | 421 (9.2) |
| 3 | 3065 (66.7) |
|
| |
| Information Source for Series Completion (n=3065) | |
| Per Card | 1935 (63.1) |
| Per Mom | 1130 (36.9) |
|
| |
| Timing of Vaccinationˆ | |
| Age in Weeks | |
| Dose #1 | 9.5 (7.8, 12.2) |
| Dose #2 | 14.3 (12.3, 17.6) |
| Dose #3 | 19.3 (16.9, 23.9) |
Adjusted for weighting, clustering and stratification
Among children with vaccination date recorded on the card (n=2136 for dose #1, n=2046 for dose #2, n=1928 for dose #3)
Figure 1.
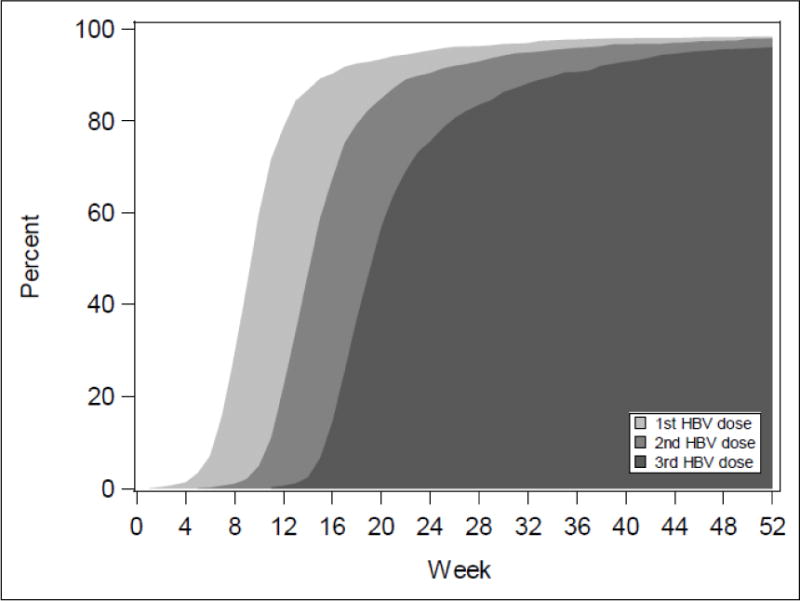
Timing of HBV Vaccination among Children Who Completed the 3-Dose Series (n=1914)*
*Percentages adjusted for weighting, clustering, and stratification.
Table 2 shows results of the models for series completion. The adjusted (MV) model is based on results from 4479 women. The largest effect sizes were seen for improved access to care (aOR 2.1, CI 1.7–2.6 for facility delivery) and household wealth (aOR 1.9, CI 1.2–3.1 comparing highest and the lowest quintiles). Christian religion (aOR 1.8, CI 1.3–2.5 compared to Muslim religion), older age (aOR 1.4, CI 1.2–1.7) and unmarried status (aOR 1.3, CI 1.1–1.7) also predicted series completion. Urban residence, parity and maternal education were not associated with series completion. Region of residence within Cameroon was excluded from the model due to collinearity concerns. In a sensitivity analysis modeled for series completion according to the card alone, similar associations and effect sizes were seen except that maternal age, unmarried status and the association between the two bottom wealth strata were not significant. (data not shown)
Table 2.
Factors associated with Completion of HBV Vaccine Series*
| Factor | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|
|
| ||
| Maternal Age (10 year units) | 1.23 (1.1–1.39) | 1.43 (1.21–1.69) |
|
| ||
| Male Partner Age (10 year units) | 1.04 (0.95–1.13) | N/A |
|
| ||
| Maternal Education | ||
| None | Ref | Ref |
| Primary | 2.76 (2.12–3.59) | 1.11 (0.84–1.48) |
| Secondary or Higher | 4.44 (3.30–5.96) | 1.01 (0.71–1.43) |
|
| ||
| Partner Education | ||
| None | Ref | |
| Primary | 2.67 (1.99–3.58) | N/A |
| Secondary or Higher | 4.02 (2.95–5.48) | |
| Never Married | 6.02 (4.10–8.84) | |
|
| ||
| Religion | ||
| Muslim | Ref | Ref |
| Catholic/Protestant | 2.79 (2.08–3.76) | 1.81 (1.33–2.48) |
| Other/none | 1.21 (0.80–1.81) | 1.43 (0.98–2.07) |
|
| ||
| Location of Residence | ||
| Rural | Ref | Ref |
| Urban | 2.50 (2.02–3.10) | 1.14 (0.85–1.52) |
|
| ||
| Owns Transportation** | ||
| None | Ref | |
| Car/Truck | 2.11 (1.47–3.03) | N/A |
| Motorcycle | 0.97 (0.77–1.21) | |
| Bicycle | 0.47 (0.36–0.62) | |
|
| ||
| Wealth Index | ||
| 1 | Ref | Ref |
| 2 | 2.48 (1.85–3.33) | 1.43 (1.07–1.92) |
| 3 | 3.13 (2.22–4.42) | 1.31 (0.90–1.90) |
| 4 | 4.21 (3.01–5.88) | 1.41 (0.90–2.21) |
| 5 | 6.87 (4.89–9.66) | 1.90 (1.17–3.07) |
|
| ||
| Marital Status | ||
| Married | Ref | |
| Living with Partner | 1.09 (0.85–1.38) | N/A |
| Widowed, divorced, separated | 1.26 (0.91–1.75) | |
| Never in Union | 2.54 (1.87–3.43) | |
|
| ||
| Type of Marital Union | ||
| Monogamous | Ref | Ref |
| Polygamous | 0.67 (0.54–0.83) | 1.14 (0.91–1.43) |
| Not Known | 2.34 (1.50–3.65) | 1.51 (0.95–2.42) |
| Not Married | 1.62 (1.29–2.04) | 1.33 (1.05–1.68) |
|
| ||
| Employment in the Past 12 Months | ||
| No | Ref | N/A |
| Yes | 1.10 (0.89–1.36) | |
|
| ||
| Visited Health Facility in the Past 12 Months | ||
| No | Ref | Ref |
| Yes | 2.10 (1.78–2.48) | 1.37 (1.15–1.62) |
|
| ||
| Prenatal Care During the Last Pregnancy | ||
| No | Ref | Ref |
| Yes | 6.09 (4.49–8.25) | 2.23 (1.63–3.05) |
|
| ||
| Location of Last Delivery | ||
| Home | Ref | Ref |
| Facility | 4.68 (3.74–5.87) | 2.11 (1.69–2.64) |
|
| ||
| Parity | ||
| 1–2 | Ref | Ref |
| 3–4 | 1.05 (0.87–1.28) | 1.13 (0.89–1.42) |
| 5+ | 0.73 (0.60–0.87) | 0.75 (0.56–1.004) |
|
| ||
| Number of Children at Home | ||
| 0 | Ref | |
| 1–2 | 0.77 (0.56–1.06) | N/A |
| 3+ | 0.70 (0.51–0.96) | |
Adjusted for weighting, clustering and stratification
Hierarchical ownership: only one category allowed (in order as: car, motorcycle, bicycle or none)
Completion rates for other recommended childhood vaccines assessed in the DHS survey were as follows. The highest rate of coverage was for the birth dose BCG vaccine (89.1%) and infants who did not receive BCG were less likely to complete the HBV series (OR 75.8, 95% CI 39.5–145.6). The HBV series had a lower completion rate (67%) than the polio series (71.5%) which is also recommended at 6, 10, and 14 weeks, and the measles vaccine which is administered at 9 months (76.5%). Infants born in later years were significantly less likely to have completed the HBV series (71.7% for 2006 compared to 63.4% for 2010; p (trend)=0.0005). This time trend was consistent for other vaccines in the survey but this was driven by maternal report. Among infants with a vaccination card available, vaccination rates improved over the period of study. There were 124 women who were HIV-positive at the time of the survey and 2063 HIV-uninfected (HIV seroprevalence 5.7%). In a univariate model, children born to women with HIV were more likely to have completed the HBV vaccine series compared to HIV-uninfected women (OR 1.9, 95% CI 1.1–3.1) although the timing of HIV infection and diagnosis relative to the most recent pregnancy is unknown.
Discussion
In Cameroon, 67% of infants had completed the HBV vaccine series at the time of the most recent DHS survey and 42% when the vaccination card alone was assessed for documentation. We were able to identify several maternal factors associated with series completion with relevance to the implementation of HBV birth dose vaccine in Cameroon. The four strongest maternal predictors of series completion were: access to health care (including facility delivery), higher household income, older age and Christian religion.
In terms of series completion rates, these findings are consistent with other childhood vaccine studies in sub-Saharan Africa.(26–28) In a 2014 study by Canavan et al looking at childhood vaccination rates and correlates in East Africa, coverage ranged from 13% in Ethiopia to 66% in Rwanda. (26) In another study that pooled childhood vaccination data from DHS surveys conducted in low and middle income countries (including 38 countries in sub-Saharan Africa), fewer than 50% were fully vaccinated in 2009.(29) National DHS survey data have been proven to be a reliable source of information on infant vaccination rates.(13, 25) Serologic surveys provide the gold standard in documenting protection against vaccine preventable diseases, but they are infrequently performed in sub-Saharan Africa. One recent serologic study of 1800 hospitalized children in three African countries demonstrated that 75% of children in Cameroon who had been vaccinated per maternal report were found to have protective antibody levels in the serum (>10mIU/mL) but only 23% had vaccination cards available for review. (21) This provides additional support for other studies showing the benefit of using a combined measure of vaccination per card or maternal report.
In terms of predictors of vaccination, higher household wealth is one of the most consistent predictors of successful vaccination in our study and others. (21, 28) Although the reduction of cost constraints is critical, the provision of free pentavalent vaccine over the past decade in Cameroon has not eliminated barriers posed by transportation costs and facility deliveries. This highlights the need to offer new HBV prevention strategies that can supersede simple cost constraints. Both parental education and smaller family size were predictive of vaccination in prior studies, but these variables did not retain significance in our adjusted model. (21) This may be due to variability by country. Recent studies highlight the fact that there are few common predictors of vaccination across wide geographic areas with the exception of wealth and access to care. (26) The association of vaccine completion with unmarried status was unexpected and it may be explained by additional confounding due to unmeasured characteristics. Furthermore, since it did not persist in our model based on vaccination card alone, the association warrants additional study.
Vaccination programs in sub-Saharan Africa should be tailored based on country-specific data and intimate knowledge of local factors that foster or hinder vaccine uptake. Facility deliveries are predictors of successful vaccination in many settings. (26) Many African countries promote facility deliveries as part of efforts to improve maternal survival, reduce neonatal morbidity and prevent vertical HIV transmission. (30) Children born to the subset of HIV-infected women in our study had improved HBV series completion rates. This may be due to improved access to health care and support for facility deliveries since high vaccine coverage for the birth dose is directly linked to facility delivery rates. In our study, the birth dose BCG vaccine had the highest completion rate at 87% (higher than reported facility delivery rate of 67.4%) and receipt of BCG was strongly associated with completion of the HBV series. The polio series completion rate (71.5%) was similar to the pentavalent series but the measles vaccination rate was higher (76.5%) which may be due the fact that it is a single dose vaccine with administration at 9 months of age.
The HBV birth dose has been shown to be cost-effective in HBV-endemic countries in Asia. (31, 32) In a recent model of global hepatitis B elimination, birth dose vaccination was significantly more effective than pentavalent vaccine, peripartum antiviral therapy, test and treat or cure strategies for HBV. (15) When monovalent hepatitis B vaccine becomes widely available as part of the National Program for Expanded Immunization in Cameroon, it should be administered within 24 hours of birth to the increasing proportion of infants born in health facilities at the same time as the BCG vaccine. (33) Moreover, 81.3% of infants who started the HBV vaccine series in our study were highly likely to complete it by 12 months, so the largest hurdle to vaccination may be overcome by improving access to the first dose. Attention to subsequent vaccine timing is necessary to minimize delays during a period of vulnerability to HBV infection. Some countries have also worked to increase access to vaccination for home deliveries where mobile health strategies have shown an impact. (19, 34) The nationwide rollout of the birth dose vaccine should be accompanied by an awareness campaign targeting pregnant women and communities about the risks of vertical HBV transmission and to help provide the rationale for HBV vaccination and series completion. Special efforts may be needed to reach pregnant women whose infants are less likely to complete the vaccine series: those with barriers to care, higher poverty rates, Muslim religion and younger age.
Study Limitations and Next Steps
Study findings based on survey data from Cameroon may not be generalizable to sub-Saharan Africa, although many similarities exist with respect to HBV epidemiology, barriers to vaccination and prevention strategies. Also, vaccination rates may have changed since the most recent DHS survey in 2011 and we only included women able to provide information about receipt of the 3-dose series. This introduced a selection bias which may have led to an over-estimation of vaccination completion rates. Maternal self-report for vaccination status and socio-demographics is a limitation. Dates of vaccination were only available for a subset, so estimates of vaccine timing may not reflect the timing of vaccination in the larger group. Finally, there may be unique predictors for HBV birth dose vaccination that we could not assess since the DHS survey did not include any queries specific to birth dose vaccine. Future surveys should include these questions. Well-designed epidemiologic studies are also needed to quantify the relative contribution of vertical and horizontal transmission to pediatric HBV disease rates in Africa and to document the cost-effectiveness and public health impact of adding birth dose vaccine to national platforms.
Conclusions
Despite the availability of an effective and safe vaccine, new strategies are needed to prevent hepatitis B infection in sub-Saharan Africa and reach elimination targets by 2030. Facility deliveries should be widely encouraged and a constant supply of univalent HBV birth dose vaccine must be ensured. These goals are aligned with the WHO Regional Strategic Plan for Immunization which aims to mobilize local communities toward demand-driven immunization that is integrated into revitalized and universal primary health care systems. Given the current cost of birth dose vaccine at 20 cents and the global commitment to eliminating hepatitis B, the time to act is now.
Supplementary Material
Acknowledgments
JDO is supported by NIH/NICHD 1K23HD090993. The funding source did not have a role in the development or publication of the findings.
Footnotes
The authors have no conflicts of interest with this work.
Author Contributions: The study was conceptualized and designed by JDO, AW and AT. AW was involved in the statistical analysis. JDO collected the data and drafted the initial manuscript. DN, MJK, and AT helped with data interpretation and along with GH and TW, provided key edits to the manuscript.
References
- 1.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388:1081–8. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clinics in liver disease. 2016;20:607–28. doi: 10.1016/j.cld.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. http://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/. Last accessed 5/31/17.
- 5.Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, et al. Antibody Levels and Protection After Hepatitis B Vaccine: Results of a 30-Year Follow-up Study and Response to a Booster Dose. The Journal of infectious diseases. 2016;214:16–22. doi: 10.1093/infdis/jiv748. [DOI] [PubMed] [Google Scholar]
- 6.Bray F, Jemal A, Torre LA, Forman D, Vineis P. Long-term Realism and Cost-effectiveness: Primary Prevention in Combatting Cancer and Associated Inequalities Worldwide. Journal of the National Cancer Institute. 2015;107 doi: 10.1093/jnci/djv273. djv273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. Journal of hepatology. 2013;59:24–30. doi: 10.1016/j.jhep.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Dionne-Odom J, Mbah R, Rembert NJ, Tancho S, Halle-Ekane GE, Enah C, et al. Hepatitis B, HIV, and Syphilis Seroprevalence in Pregnant Women and Blood Donors in Cameroon. Infectious Diseases in Obstetrics and Gynecology. 2016;2016:8. doi: 10.1155/2016/4359401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ott JJ, Stevens GA, Wiersma ST. The risk of perinatal hepatitis B virus transmission: hepatitis B e antigen (HBeAg) prevalence estimates for all world regions. BMC infectious diseases. 2012;12:131. doi: 10.1186/1471-2334-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasim GI, Murad IA, Adam I. Hepatitis B and C virus infections among pregnant women in Arab and African countries. Journal of infection in developing countries. 2013;7:566–78. doi: 10.3855/jidc.3243. [DOI] [PubMed] [Google Scholar]
- 11.Howell J, Lemoine M, Thursz M. Prevention of materno-foetal transmission of hepatitis B in sub-Saharan Africa: the evidence, current practice and future challenges. Journal of viral hepatitis. 2014;21:381–96. doi: 10.1111/jvh.12263. [DOI] [PubMed] [Google Scholar]
- 12.Keane E, Funk AL, Shimakawa Y. Systematic review with meta-analysis: the risk of mother-to-child transmission of hepatitis B virus infection in sub-Saharan Africa. Alimentary pharmacology & therapeutics. 2016;44:1005–17. doi: 10.1111/apt.13795. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization- UNICEF. Vaccine Coverage Estimates. 2016 http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragedtp3.html Last accessed 5/31/17.
- 14.Hill HA, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M. National, State, and Selected Local Area Vaccination Coverage Among Children Aged 19–35 Months - United States, 2014. MMWR Morbidity and mortality weekly report. 2015;64:889–96. doi: 10.15585/mmwr.mm6433a1. [DOI] [PubMed] [Google Scholar]
- 15.Nayagam S, Thursz M, Sicuri E, Conteh L, Wiktor S, Low-Beer D, et al. Requirements for global elimination of hepatitis B: a modelling study. The Lancet Infectious Diseases. 16:1399–408. doi: 10.1016/S1473-3099(16)30204-3. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Regional Office for Africa. Regional Strategic Plan for Immunization 2014–2020. 2015 https://www.aho.afro.who.int/en/ahm/issue/19/reports/regional-strategic-plan-immunization-2014%E2%80%932020 Last accessed 5/13/17.
- 17.Diamond-Smith N, Sudhinaraset M. Drivers of facility deliveries in Africa and Asia: regional analyses using the demographic and health surveys. Reproductive health. 2015;12:6. doi: 10.1186/1742-4755-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jezek J, Chen D, Watson L, Crawford J, Perkins S, Tyagi A, et al. A heat-stable hepatitis B vaccine formulation. Human vaccines. 2009;5:529–35. doi: 10.4161/hv.5.8.8600. [DOI] [PubMed] [Google Scholar]
- 19.Xeuatvongsa A, Datta SS, Moturi E, Wannemuehler K, Philakong P, Vongxay V, et al. Improving hepatitis B birth dose in rural Lao People’s Democratic Republic through the use of mobile phones to facilitate communication. Vaccine. 2016;34:5777–84. doi: 10.1016/j.vaccine.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonnis WF, Amorij JP, Vreeman MA, Frijlink HW, Kersten GF, Hinrichs WL. Improved storage stability and immunogenicity of hepatitis B vaccine after spray-freeze drying in presence of sugars. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2014;55:36–45. doi: 10.1016/j.ejps.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Bekondi C, Zanchi R, Seck A, Garin B, Giles-Vernick T, Gody JC, et al. HBV immunization and vaccine coverage among hospitalized children in Cameroon, Central African Republic and Senegal: a cross-sectional study. BMC infectious diseases. 2015;15:267. doi: 10.1186/s12879-015-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Statistics, Ministry of Public Health, Yaounde, Cameroon. Cameroon 2011 Demographic Health Survey. Studies in family planning. 2013;44:223–32. [Google Scholar]
- 23.Luman ET, Ryman TK, Sablan M. Estimating vaccination coverage: validity of household-retained vaccination cards and parental recall. Vaccine. 2009;27:2534–9. doi: 10.1016/j.vaccine.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Miles M, Ryman TK, Dietz V, Zell E, Luman ET. Validity of vaccination cards and parental recall to estimate vaccination coverage: a systematic review of the literature. Vaccine. 2013;31:1560–8. doi: 10.1016/j.vaccine.2012.10.089. [DOI] [PubMed] [Google Scholar]
- 25.Cutts FT, Claquin P, Danovaro-Holliday MC, Rhoda DA. Monitoring vaccination coverage: Defining the role of surveys. Vaccine. 2016;34:4103–9. doi: 10.1016/j.vaccine.2016.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canavan ME, Sipsma HL, Kassie GM, Bradley EH. Correlates of complete childhood vaccination in East African countries. PloS one. 2014;9:e95709. doi: 10.1371/journal.pone.0095709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. Journal of epidemiology and community health. 2012;66:e14. doi: 10.1136/jech.2010.124651. [DOI] [PubMed] [Google Scholar]
- 28.Lakew Y, Bekele A, Biadgilign S. Factors influencing full immunization coverage among 12–23 months of age children in Ethiopia: evidence from the national demographic and health survey in 2011. BMC public health. 2015;15:728. doi: 10.1186/s12889-015-2078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao W, Petzold M, Forsberg BC. Routine vaccination coverage in low- and middle-income countries: further arguments for accelerating support to child vaccination services. Global health action. 2013;6:20343. doi: 10.3402/gha.v6i0.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellizzi S, Sobel HL, Mathai M, Temmerman M. Does place and attendance at birth improve early neonatal mortality? Secondary analysis of nine Demographic and Health Surveys. BJOG: an international journal of obstetrics and gynaecology. 2016 doi: 10.1111/1471-0528.14422. [DOI] [PubMed] [Google Scholar]
- 31.Lu SQ, McGhee SM, Xie X, Cheng J, Fielding R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine. 2013;31:1864–9. doi: 10.1016/j.vaccine.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TH, Vu MH, Nguyen VC, Nguyen LH, Toda K, Nguyen TN, et al. A reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine. 2014;32:217–22. doi: 10.1016/j.vaccine.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alam N, Hajizadeh M, Dumont A, Fournier P. Inequalities in maternal health care utilization in sub-Saharan African countries: a multiyear and multi-country analysis. PloS one. 2015;10:e0120922. doi: 10.1371/journal.pone.0120922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiesen E, Lagani W, Sui G, Arava J, Reza S, Diorditsa S, et al. Assessment of the hepatitis B birth dose vaccination program, Papua New Guinea, 2014. Vaccine. 2016;34:367–72. doi: 10.1016/j.vaccine.2015.11.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


