Abstract
AIM
To investigate whether M1 or M2 polarization contributes to the therapeutic effects of mesenchymal stem cells (MSCs) in acute hepatic failure (AHF).
METHODS
MSCs were transfused into rats with AHF induced by D-galactosamine (DGalN). The therapeutic effects of MSCs were evaluated based on survival rate and hepatocyte proliferation and apoptosis. Hepatocyte regeneration capacity was evaluated by the expression of the hepatic progenitor surface marker epithelial cell adhesion molecule (EpCAM). Macrophage polarization was analyzed by M1 markers [CD68, tumor necrosis factor alpha (TNF-α), interferon-γ (IFN-γ), inducible nitric oxide synthase (INOS)] and M2 markers [CD163, interleukin (IL)-4, IL-10, arginase-1 (Arg-1)] in the survival and death groups after MSC transplantation.
RESULTS
The survival rate in the MSC-treated group was increased compared with the DPBS-treated control group (37.5% vs 10%). MSC treatment protected rats with AHF by reducing apoptotic hepatocytes and promoting hepatocyte regeneration. Immunohistochemical analysis showed that MSC treatment significantly increased the expression of EpCAM compared with the control groups (P < 0.001). Expression of EpCAM in the survival group was significantly up-regulated compared with the death group after MSC transplantation (P = 0.003). Transplantation of MSCs significantly improved the expression of CD163 and increased the gene expression of IL-10 and Arg-1 in the survival group. IL-4 concentrations were significantly increased compared to the death group after MSC transplantation (88.51 ± 24.51 pg/mL vs 34.61 ± 6.6 pg/mL, P < 0.001). In contrast, macrophages showed strong expression of CD68, TNF-α, and INOS in the death group. The concentration of IFN-γ was significantly increased compared to the survival group after MSC transplantation (542.11 ± 51.59 pg/mL vs 104.07 ± 42.80 pg/mL, P < 0.001).
CONCLUSION
M2 polarization contributes to the therapeutic effects of MSCs in AHF by altering levels of anti-inflammatory and pro-inflammatory factors.
Keywords: Acute hepatic failure, Mesenchymal stem cells, Macrophages, Polarization, Inflammation
Core tip: M1 or M2 polarization governs the therapeutic effect of acute hepatic failure (AHF). Mesenchymal stem cells (MSCs) were transfused into rats with AHF induced by galactosamine. It was found that MSCs alleviated the survival rate and biochemical indicators by promoting hepatocyte regeneration. Immunohistochemistry, flow cytometry, and RT-PCR showed that M2 polarization contributes to the rescue of AHF by MSCs in the survival group after MSC transplantation. In addition, in the death group after MSC transplantation, the number of M1 macrophages increased significantly. Our findings suggest that M2 polarization contributes to the rescue of AHF by MSCs, which result in altered levels of anti-inflammatory and pro-inflammatory factors.
INTRODUCTION
Acute hepatic failure (AHF) is a lethal condition characterized by widespread hepatocyte necrosis, acute deterioration of liver function, and subsequent multiorgan failure. Although many animal and preliminary human studies have shown that stem cell transplantation has substantial potential in treating AHF[1-4], transplantation has rarely produced satisfying therapeutic effects. The exact mechanism through which stem cells assist in organ repair remains elusive. Recent studies have also indicated a substantial role for paracrine effects in delivering overall benefits, although specific cells and signaling molecules have not been identified to mediate these paracrine effects[5]. However, macrophages in the liver play an indispensable role in paracrine mechanisms. There has been a major paradigm shift in the field of macrophage biology with the recognition that macrophages play an important role in homeostasis[6]. Several studies employing selective Kupffer cell depletion in rodents have explored the role of this cell type in hepatocyte proliferation and liver regeneration following partial hepatectomy[7,8]. However, there is little information available regarding the role of macrophages in hepatocyte proliferation. This may be due to the multi-phenotype and multi-functional roles of macrophages in liver regeneration, as they are a major source of both pro-proliferative and anti-proliferative mediators in the liver. Classically activated macrophages (M1 macrophages) mediate host defenses from a variety of bacteria, protozoa, and viruses and have roles in anti-tumor immunity. M2 macrophages have an anti-inflammatory function and regulate wound healing[9,10]. Different phenotypes play various roles in tissue damage and maintenance[11-17]. For example, M1 macrophages are induced by exposure to CD68[18] and are associated with the phagosomes of macrophages, which is consistent with enhanced phagocytosis. These macrophages are characterized by the expression of high levels of inducible nitric oxide synthase (INOS) induced by interferon-γ (IFN-γ) that liberates pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6, which are increased in inflammatory reactions and tissue injury. In contrast, M2 macrophages are characterized by exposure to CD163[19], which is expressed by liver Kupffer cells. These macrophages are activated by IL-4, express high levels of arginase-1 (Arg-1), and release immune-modulatory mediators (such as IL-10) to modulate the inflammatory response and to promote tissue remodeling.
MSC transplantation may be useful in treating AHF conditions. In the present study, we evaluated the contribution of M1 and M2 macrophages in survival and death groups after MSC transplantation to investigate whether macrophage polarization contributes to the rescue of AHF by MSCs.
MATERIALS AND METHODS
AHF animal model
Male Wistar rats weighing 190 ± 20 g were obtained from the Huafukang Experimental Animal Center (Beijing, China). The study was reviewed and approved by the Ethics Committee of Shengjing Hospital of China Medical University. The animal study protocol, in compliance with the Guidelines of China for Animal Care, conformed to internationally accepted principles in the care and use of experimental animals. Animals were housed at room temperature (22 ± 2 °C) with light cycles between 08:00 and 22:00 and free access to food and water. A total of 52 rats were randomly divided into four groups: an experimental group (group A, n = 16), a control group (group B, n = 10), an MSC–treated group (group C, n = 16), and a DPBS (Dulbecco phosphate-buffered saline)–treated group (group D, n = 10). Rats in group A were injected intraperitoneally (i.p.) with D-galactosamine (DGalN) (1.2 g/kg; Sigma-Aldrich, St. Louis, MO, United States). Rats in group B were injected i.p. with 2 mL of 0.9% phosphate buffered saline (PBS). At 12 h after DGalN treatment, rats in group C underwent intravenous tail vein transplantation of 5.5 × 105 MSCs dissolved in 1.0 mL of DPBS, and rats in group D were given 1.0 mL of DPBS. All rats were selected for survival analysis at 72 h after treatment. The survival rate of rats remained unchanged at 48 h after treatment. The rats in the survival group were still in good physical condition at 48 h after MSC treatment. The rats in the death group were in poor physical condition or in the state of death before they died at 48 h after MSC treatment. Serum and liver tissues were collected at 48 h after MSCsGFP transplantation for biochemical analyses, inflammatory factor detection, and further evaluation.
MSCsGFP culture and MSCsGFP transplantation
Wistar bone marrow MSCs were obtained from a cell bank (Shanghai, China) and cultured in α-MEM medium with GlutaMAX-I (Gibco, United States), supplemented with 10% fetal bovine serum (Gibco, United States), 100 IU/mL penicillin and 100 μg/mL streptomycin (Thermo, United States). When cells reached 80%-90% confluence, they were trypsinized with 0.05 g/L trypsin-EDTA (Gibco, United States) and replated at a density of 1 × 10 4/cm2 for further expansion. After cells were passaged to the fourth generation, they were infected with an adenovirus encoding the gene encoding green fluorescent protein (GFP), and the multiplicity of infection was determined by fluorescence inverted phase-contrast microscopy.
Biochemical assay and histological evaluation
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), and total bilirubin (TBIL) were monitored with an automatic analyzer (Roche, United States) and liver biochemical indicators were estimated. The liver was fixed in 4% paraformaldehyde for hematoxylin and eosin (HE) or immunohistochemical staining. Paraffin-embedded liver tissue was cut into 3-μm thick sections for histopathological evaluation, deparaffinized in xylene, and rehydrated through a series of decreasing concen–trations of ethanol. Sections were stained with HE and analyzed under a light microscope.
Immunohistochemistry and immunofluorescence staining
Immunohistochemistry was performed with primary rabbit or mouse anti-rat antibodies (Abcam, Cambridge, MA, United States) for EpCAM, CD68, and CD163. Liver sections were deparaffinized in xylene and rehydrated through a series of decreasing concentrations of ethanol. Heat-mediated antigen retrieval was performed using citrate buffer (MVS-0100, MXB Blotechnologies, Fujian, China). Blocking solution and secondary antibodies (KIT-9710, MXB Blotechnologies, Fujian, China) were applied according to standard protocols. Sections were incubated overnight with a primary antibody at 4 °C and visualized with DAB (ZLI-9017, ZSGB-BIO, Beijing, China).
Indirect immunofluorescence was used to detect the phenotype of M1/M2 following overnight incubation at 4 °C with primary antibodies. Secondary antibodies (Abcam, Cambridge, MA, United States) were used at room temperature for 4 h with goat anti-mouse IgG-H&L (Abcam, Cambridge, MA, United States) and goat anti-rabbit IgG-H&L (Abcam, Cambridge, MA, United States). Nuclear staining was performed using DAPI (ZSGB-BIO, Beijing, China). A standard in situ TUNEL (Roche, Indianapolis, IN, United States) method was used for detection of DNA fragmentation in apoptotic cells according to the manufacturer’s instructions. Cell proliferation was determined using anti-Ki67 (Novus, NB500-170).
To determine engraftment of MSCs after GFP transfection, the livers from rats in the survival and death groups were dissected out, fixed in 4% formaldehyde and optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura, Japan), and preserved at -20 °C. GFP expression by transplanted MSCs was detected by fluorescence inverted phase-contrast microscopy.
Measurement of cytokine production
Cytokine production was measured in serum centrifuged at 1500 r/min for 15 minutes. IL-4 and IFN-γ were tested using Multi-Analyte Flow Assay Kit (BioLegend, CA, United States) and analyzed by flow cytometry. Each analysis was performed in duplicate. In this quantitative assay system, specific antibodies directed against each cytokine are conjugated to the surface of fluorescence-coded microbeads, with each fluorescence-coded microbead type being conjugated to one specific capture antibody.
Quantitative real-time PCR
Total RNA was extracted from liver tissue (~100 mg) using TRIzol reagent (Invitrogen, United States) according to the manufacturer’s instructions, and the amount of isolated RNA was estimated by ribogreen fluorescence. Purity was assessed by the absorbance ratio at 260 and 280 nm. A total of 3 μg was reverse-transcribed using a High Capacity cDNA Reverse Transcription Kit (Promega, United States). Real-time quantitative PCR was performed using SYBR Green I Master and the appropriate primers on a LightCycler 480 instrument. In parallel, mRNA levels of human housekeeping GAPDH were analyzed as an internal normalization control. Primers used are shown in Table 1. Data were calculated using the ΔCt method and were normalized to GAPDH.
Table 1.
Primers used in mRNA expression analysis
| Gene name | Sequence |
| GAPDH | (Forward) 5’-GGCACAGTCAAGGCTGAGAATG-3’ |
| (Reverse) 5’-ATGGTGGTGAAGACGCCAGTA-3’ | |
| CD68 | (Forward) 5’-TCGGGCCATGCTTCTCTT-3’ |
| (Reverse) 5’-AGGGGCTGGTAGGTTGATTGT-3’ | |
| CD163 | (Forward) 5’-CTGGGATGTCCAACTGCCAT-3’ |
| (Reverse) 5’-AATGCTTCCCCCATTCCTGG-3’ | |
| Arg-1 | (Forward) 5’-GCTGTGGTAGCAGAGACCCAGA-3’ |
| (Reverse) 5’-CATCCACCCAAATGACGCATAG-3’ | |
| IL-10 | (Forward) 5’-CAGACCCACATGCTCCGAGA-3’ |
| (Reverse) 5’-CAAGGCTTGGCAACCCAAGTA-3’ | |
| Nos2 | (Forward) 5’-TCCTCAGGCTTGGGTCTTGTTAG-3’ |
| (Reverse) 5’-TTCAGGTCACCTTGGTAGGATTTG-3’ | |
| TNF-α | (Forward) 5’-CCGATTTGCCACTTCATACCA-3’ |
| (Reverse) 5’-TAGGGCAAGGGCTCTTGATG-3’ |
Statistical analysis
Survival statistics were assessed using Log-rank (Mantel-Cox) test. Data are expressed as the mean ± SD. Differences between groups were analyzed by independent sample t-test. Serum concentration and gene expression of cytokine assays were performed in duplicate or triplicate for each specific sample. All data points are the mean of duplicate or triplicate measurements. Differences were considered statistically significant at P < 0.05.
RESULTS
Survival rate is increased and biochemical indicators are altered by MSC transplantation
Implanted MSCs were observed in the liver of treated rats (Figure 1). At 24 h after treatment, 50% (8/16) and 30% (3/10) of the animals had survived in the MSC- and DPBS-treated groups, respectively. At 48 h after transplantation, survival in the MSC- and DPBS-treated groups decreased to 37.5% (6/16) and 10% (1/10), respectively (Figure 1C). Although there was no statistical significance, survival rate was increased by MSC transplantation. At 24 h and 48 h after MSC transfusion, biochemical indicators (ALT/AST/ALB/TBIL) had significantly changed compared with rats in the DPBS-treated group (Figure 1). To investigate the liver histology of rats with AHF after MSC transplantation, HE staining was conducted (Figure 1). At 48 h after MSC transfusion, no obvious histopathological changes were observed in rats infused with MSCs, and most of the tissue showed generalized necrotic areas. Five days after transplantation, most of the tissue had returned to normal with only a few necrotic areas, indicating liver tissue repair after liver function repair.
Figure 1.
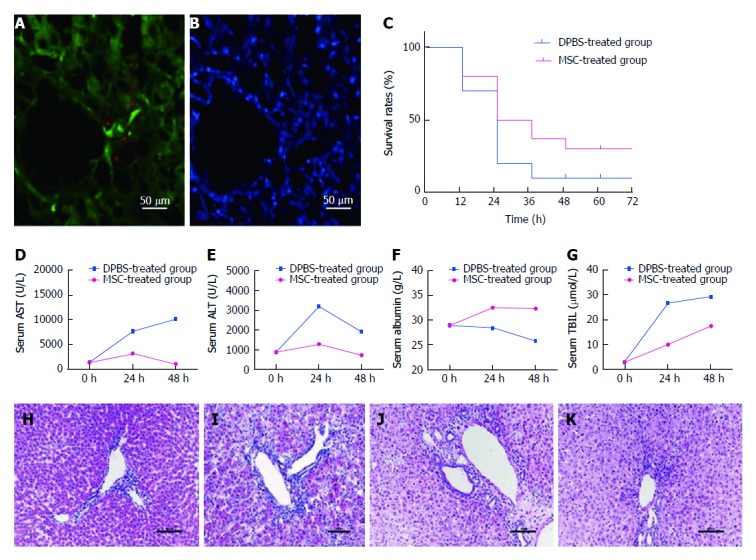
Survival rate and biochemical indicators in rats are improved after mesenchymal stem cell treatment. Colonization of mesenchymal stem cells (MSCs) was observed in the liver (A and B). The red arrow on the left shows engraftment of MSCs and nuclear staining in the same slice. Survival rates were compared between the MSC-treated group and the DPBS-treated group at each time point (C) (P = 0.36). Serum samples collected at various times (0 h, 24 h, 48 h) after MSC treatment were analyzed for levels of ALT, AST, ALB, and TBIL and compared with the DPBS-treated group (D-G). HE staining of liver sections was performed in each group. Compared with the PBS-treated group (H), we observed necrosis of centrilobular hepatocytes, characterized by cell shrinkage and lost nuclei, interstitial hemorrhage, and inflammatory cell infiltration in the DPBS-treated group (I). Liver histomorphology at 48 h after MSC treatment (J) did not change significantly compared with the DPBS-treated group, but the number of hepatocytes with edema, shrinkage, and lost nuclei decreased significantly, with massive inflammatory cell infiltration and increased number of cells observed. The liver histomorphology was gradually repaired after 5 d (K).
MSC transfusion promotes hepatocyte regeneration
To determine whether MSC treatment promotes hepatocyte regeneration compared to rats in the DPBS-treated group, Ki67-positive hepatocytes were detected. Ki67-positive hepatocytes significantly increased at 48 h after transplantation (P < 0.001) (Figure 2). In DPBS-treated rats with AHF, many TUNEL-positive hepatocytes were observed, yet only a few hepatocytes were observed after MSC treatment (P < 0.001).
Figure 2.
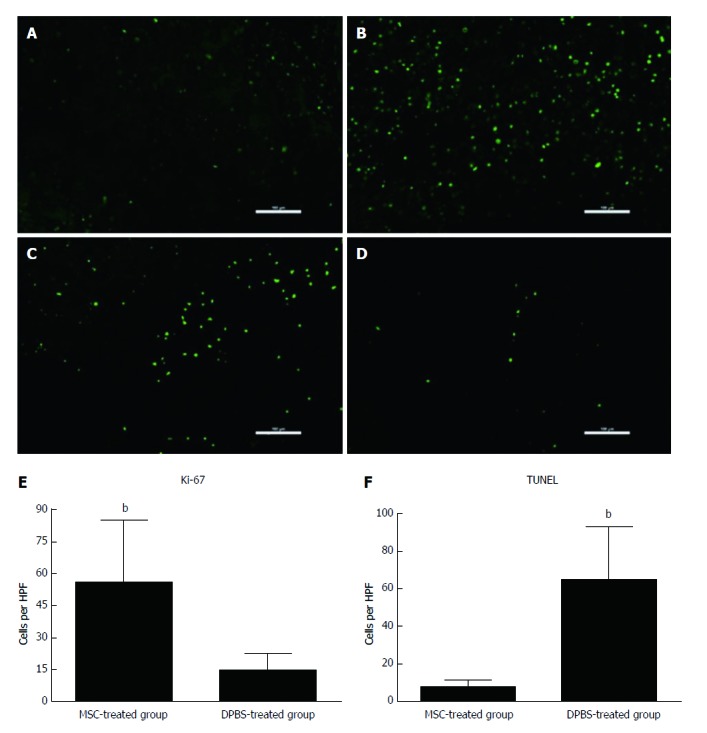
Assessment of hepatocyte apoptosis and proliferation after mesenchymal stem cell transplantation. Immunofluorescence for Ki-67 (A and B) and terminal deoxyribonucleotide transferase (TdT)-mediated deoxyuridine triphosphate nick end labeling (TUNEL) (C and D) staining in MSC-treated and DPBS-treated livers. A and C: MSC-treated group; B and D: DPBS-treated group. The numbers of Ki-67-positive and TUNEL-positive hepatocytes were observed in the DPBS- and MSC-treated groups (E and F). Bar represents the mean ± SD. (n = 5, bP < 0.001). MSC: Mesenchymal stem cell.
EpCAM has been shown to be expressed in a population of rat oval cells, which are composed of liver progenitors[16,17]. In the present study, significant up-regulation of EpCAM was observed in the MSC-treated group compared with DPBS-treated group (P < 0.001) (Figure 3). Compared with the death group, EpCAM expression was increased in the survival group after MSC transplantation (P = 0.003), suggesting the vital roles of progenitor cells in the regeneration process after MSC transplantation.
Figure 3.

Immunohistochemical staining for epithelial cell adhesion molecule expression in each group. A: Survival group after mesenchymal stem cell (MSC) treatment; B: Death group after MSC treatment; C: Survival group after DPBS treatment; D: Death group after DPBS treatment; E: Integrated optical density of immunohistochemical staining for EPCAM+ hepatocytes. Bar represents the mean ± SD (n = 5, aP < 0.05, bP < 0.001).
Enhanced M2 polarization in the survival group after MSC transplantation
To investigate the role of macrophage subsets in AHF, liver sections were stained for the recently described M1/M2 specific markers CD68 and CD163, which preferentially detect invading macrophages. The number of CD68+ macrophages was obviously up-regulated in the DGalN-treated group (Figure 4). However, compared to the death group after MSC transplantation, a significantly greater number of CD163+ macrophages was observed in the survival group, while the number of CD68+ macrophages was decreased (Figure 5). Serum protein levels of IL-4 were significantly higher in the survival group than in the death group (88.51 ± 24.51 pg/mL vs 34.61 ± 6.6 pg/mL, P < 0.001) (Figure 6). The mRNA expression of IL-10 and Arg-1 was significantly up-regulated in the survival group (P < 0.001) (Figure 7).
Figure 4.

Immunohistochemistry for polarization of macrophages in liver tissue. A: Distribution of macrophages reacting to CD68 for M1 in PBS-treated group; B: Distribution of macrophages reacting to CD68 for M1 in DGalN-treated group; C: Distribution of macrophages reacting to CD163 for M2 in PBS-treated group; D: Distribution of macrophages reacting to CD163 for M2 in DGalN-treated group (n = 4).
Figure 5.
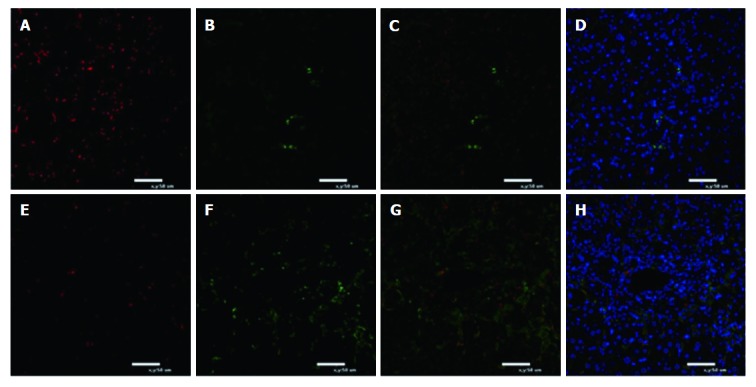
Immunofluorescence for polarization of macrophages in liver tissue. A-D: Death group after mesenchymal stem cell (MSC) treatment; E-H: Survival group after MSC treatment. Green fluorescence indicates CD163+ macrophages. Red fluorescence indicates CD68+ macrophages. Nuclei are stained blue with DAPI. Immunofluorescence for M1 macrophages reacting to CD68 and M2 macrophages reacting to CD163 is shown (n = 5).
Figure 6.

Flow cytometry analysis for serum levels of IFN-y and IL-4 in survival and death groups after mesenchymal stem cell treatment. Bar represents the mean ± SD (n = 6, bP < 0.001).
Figure 7.

mRNA expression of M1- and M2-related factors in survival and death groups after mesenchymal stem cells treatment. The mRNA expression of M1-related factors (TNF-α, INOS, and CD68) and M2-related factors (Arg-1, IL-10, and CD163) was detected in the total liver tissues of death group and survival group after MSC treatment. Bar represents the mean ± SD (n = 6, aP < 0.05, bP < 0.001).
Enhanced M1 polarization in the death group after MSC transplantation
In the death group after MSC transplantation, the number of CD68+ macrophages was significantly increased and the number of CD163+ macrophages was markedly reduced. We investigated serum IFN-γ protein levels and showed that the concentration of IFN-γ was significantly up-regulated in the death group (542.11 ± 51.59 pg/mL vs 104.07 ± 42.80 pg/mL, P < 0.001) (Figure 6). TNF-α and INOS gene expression was dramatically increased (P < 0.001) (Figure 7).
DISCUSSION
As a heterogeneous population of cells, MSCs have the potential for multilineage differentiation. MSCs can differentiate into a variety of liver cells under appropriate culture conditions[20-23]. Many clinical studies have indicated that MSCs are safe and effective in clinical studies and are useful to treat hepatic failure[24-26]. In the present study, MSC infusion was beneficial in improving the survival rate and liver histopathology after altering the concentration of biochemical indicators. To study the reasons for increased survival in the MSC-treated group, we analyzed the expression of EpCAM, which is a marker used to assess liver regeneration[27,28]. The additional study showed that in the death group, implanted MSCs did not fully translate into functional liver cells after treatment and that subsequent liver cell hepatocyte proliferation was unsatisfactory, which cannot completely improve hepatocyte inflammatory necrosis.
There is growing evidence that MSCs increase angiogenesis and improve local cell function through paracrine effects, which are involved in releasing growth factors and signaling molecules[5,29-33]. The pivotal role of paracrine effects in stem cell therapies has been recognized to contribute to many biological processes, such as preventing inflammation, inhibiting apoptosis, improving metabolism, and promoting regeneration. Macrophages are the major cells involved in paracrine effects. We found that M2 macrophages and their associated cytokines can contribute to AHF rescuing by MSCs. The number of CD163+ macrophages and levels of IL-10 and Arg-1 were significantly up-regulated in the survival group. In contrast, CD68+ macrophages and levels of TNF-α and INOS were significantly up-regulated in the death group. During type 2 helper T (TH2)-mediated immune responses, IL-4 can induce macrophages undergoing M2 activation[34], leading to expansion beyond a continuum in multiple activation states. In response to IFN-γ, macrophages undergo M1 activation during type 1 (TH1)-mediated immune responses and represent another extreme in terms of activation states. Our study demonstrates that high IL-4 levels drive M2 polarization, which occurred in the survival group after MSC transplantation. High expression of IFN-γ in the death group stimulated macrophages to undergo M1 activation.
In this study, we investigated the role of macrophage polarization in AHF rescuing by MSCs and found that polarized macrophages from the M2 anti-inflammatory phenotype promote MSC activity. Macrophages to M2 polarization also increase infused MSC activity during myocardial and spinal cord injuries[35,36]. Tremendous research efforts have corroborated the concept that hepatic macrophages are central in the pathogenesis of acute hepatic injury. Elsegood et al[37] showed that the number of macrophages increases in the liver to induce liver progenitor cell proliferation in chronic liver injury models. Our data suggest that the number of macrophages was increased in the pathogenesis of acute hepatic injury. Importantly, the number of M1 macrophages was increased significantly compared to M2 macrophages. Lanthier et al[11] reported that higher liver macrophage expansion could increase proliferative hepatocytes and is associated with a favorable outcome. Here, we determined that TNF-α expression depressed hepatocyte regeneration in AHF. These results differ from those of Lanthier et al[11] and Bihari et al[38], who reported that TNF-α levels contribute to liver cell proliferation in chronic hepatic injury. This disparity could reflect differences in the mechanisms of hepatocyte repair in acute and chronic liver injury. Our results show an increase in IL-10 gene expression in the survival group. Interestingly, these results are in agreement with the suggestion that IL-10 released by MSCs has the potential for therapeutic recovery of liver fibrosis[39,40].
MSCs improve liver function, although the specific mechanism of action is still unknown. Several studies have shown that MSCs have immunomodulatory properties, focusing on their paracrine effect. Studies of macrophage functions in hepatocyte repair have typically not distinguished between M1 and M2 after MSC transplantation. EpCAM+ hepatocytes are able to differentiate into cholangiocytes or hepatocytes and are located in the portal area. CD68+ macrophages and CD163+ macrophages are mainly located in the portal zone. However, a specific signaling pathway between macrophage polarization, associated cytokines, and hepatocyte regeneration has not been examined to date.
In conclusion, MSCs transfused into rats were recruited and increased the survival rate by inhibiting apoptotic hepatocytes and promoting hepatocyte regeneration. This study demonstrates that expression of hepatic progenitor surface marker (EpCAM) is the key to improving the prognosis of AHF. Although this study lacks specific cell numbers of macrophage polarization in the liver, we detected macrophage polarization by cell markers and related cytokines. Importantly, M2 plays a crucial role in the prognosis of AHF, which results in altered levels of anti-inflammatory and pro-inflammatory factors. The mechanism by which M2 macrophages participate in the activation of infused MSCs remains unclear. In such a situation, the observed differential effects of M1 and M2 macrophages suggest that M2 polarization may provide a potential therapeutic application in AHF after MSC transplantation.
ARTICLE HIGHLIGHTS
Research background
Recent studies have demonstrated that macrophages promote stem cell activity via paracrine action. Macrophages can express multi-phenotype and multi-functional roles in the liver and are a major source of both pro-proliferative and anti-proliferative mediators in liver pathology. There is little information available on the role of macrophage polarization in rescuing acute hepatic failure by mesenchymal stem cells.
Research motivation
Different macrophage phenotypes play various roles in tissue damage and maintenance. It is not clear whether M1 or M2 polarization contributes to the therapeutic effects of mesenchymal stem cells (MSCs). Macrophages to M1 or M2 polarization can increase infused MSCs activity during MSC transplantation, and improve the clinical efficacy of MSCs in the treatment of acute hepatic failure.
Research objectives
To investigate whether M1 or M2 polarization contributes to the therapeutic effects of MSCs.
Research methods
The rats were divided into a survival group and a death group at 48 h after MSC treatment. The rats in the survival group were still in good physical condition at 48 h after MSC treatment. The rats in the death group were in poor physical condition or in the state of death before they died at 48 h after MSC treatment. The polarization of M1 and M2 was compared between the two groups. Macrophage polarization was analyzed by M1 markers [CD68, tumor necrosis factor alpha (TNF-a), interferon-γ (IFN-γ), and inducible nitric oxide synthase (INOS)] and M2 markers [CD163, interleukin (IL)-4, IL-10, and arginase-1 (Arg-1)].
Research results
The number of CD163+ macrophages and levels of IL-4, IL-10, and Arg-1 were significantly up-regulated in the survival group. In contrast, CD68+ macrophages and levels of TFN-γ, TNF-α, and INOS were significantly up-regulated in the death group. However, a specific signaling pathway between macrophage polarization, associated cytokines, and hepatocyte regeneration has not been examined to date.
Research conclusions
This study demonstrates that expression of hepatic progenitor surface marker (EpCAM) is the key to improving the prognosis of AHF. We detected macrophage polarization by cell markers and related cytokines. M2 macrophages play a crucial role in the prognosis of AHF, which results in altered levels of anti-inflammatory and pro-inflammatory factors. The mechanism by which M2 macrophages participate in activation of infused MSCs remains unclear. The observed differential effects of M1 and M2 macrophages suggest that M2 polarization may provide a potential therapeutic application in AHF after MSC transplantation.
Research perspectives
M2 macrophages and their associated cytokines can contribute to AHF rescuing by MSCs. It is unclear whether M2 related cytokines originate from the liver or from the implanted MSCs. Further localization studies and relevant cell experiments are needed to confirm the results.
ACKNOWLEDGMENTS
The authors would like to thank Li-Li Wang for her assistance with experiments.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Supported by Liaoning Provincial Science and Technology Key Project for Translational Medicine, No. 2014225020; Outstanding Scientific Fund of Shengjing Hospital, No. 201102; and Liaoning Provincial Science and Technology Key Project for Translational Medicine, No. 2016509.
Institutional review board statement: The study was reviewed and approved by Shengjing Hospital of China Medical University Institutional Review Board.
Institutional animal care and use committee statement: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Ethics Committee of Shengjing Hospital of China Medical University Institutional. (IACUC protocol number: 2016PS248K).
Conflict-of-interest statement: To the best of our knowledge, no conflict of interest exists.
Data sharing statement: The technical appendix, statistical code and dataset are available from the first author Yan-Wei Li at liyanwei201510296@163.com. All the participants gave informed consent for data sharing.
Peer-review started: September 3, 2017
First decision: September 20, 2017
Article in press: November 1, 2017
P- Reviewer: Hashimoto N, Kawakubo K, Ramsay MA, Tanabe S S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Yan-Wei Li, Department of Infectious Diseases, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China.
Chong Zhang, Department of Infectious Diseases, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China.
Qiu-Ju Sheng, Department of Infectious Diseases, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China.
Han Bai, Department of Infectious Diseases, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China.
Yang Ding, Department of Infectious Diseases, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China.
Xiao-Guang Dou, Department of Infectious Diseases, Shengjing Hospital of China Medical University, Shenyang 110022, Liaoning Province, China. douxg@sj-hospital.org.
References
- 1.Viswanathan P, Gupta S. New directions for cell-based therapies in acute liver failure. J Hepatol. 2012;57:913–915. doi: 10.1016/j.jhep.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding J, Roberts RM, Mirochnitchenko O. Large animal models for stem cell therapy. Stem Cell Res Ther. 2013;4:23. doi: 10.1186/scrt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang DW, Yin YM, Yao YM. Advances in the management of acute liver failure. World J Gastroenterol. 2013;19:7069–7077. doi: 10.3748/wjg.v19.i41.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi D, Zhang J, Zhou Q, Xin J, Jiang J, Jiang L, Wu T, Li J, Ding W, Li J, et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut. 2017;66:955–964. doi: 10.1136/gutjnl-2015-311146. [DOI] [PubMed] [Google Scholar]
- 6.Gundra UM, Girgis NM, Ruckerl D, Jenkins S, Ward LN, Kurtz ZD, Wiens KE, Tang MS, Basu-Roy U, Mansukhani A, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123:e110–e122. doi: 10.1182/blood-2013-08-520619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang S, Dong HH, Liang HF, He SQ, Zhang W, Li CH, Zhang BX, Zhang BH, Jing K, Tomlinson S, et al. Oval cell response is attenuated by depletion of liver resident macrophages in the 2-AAF/partial hepatectomy rat. PLoS One. 2012;7:e35180. doi: 10.1371/journal.pone.0035180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey A, Allen J, Hankey-Giblin PA. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front Immunol. 2015;5:683. doi: 10.3389/fimmu.2014.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanthier N, Rubbia-Brandt L, Lin-Marq N, Clément S, Frossard JL, Goossens N, Hadengue A, Spahr L. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J Hepatol. 2015;63:609–621. doi: 10.1016/j.jhep.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Carty F, Mahon BP, English K. The influence of macrophages on mesenchymal stromal cell therapy: passive or aggressive agents? Clin Exp Immunol. 2017;188:1–11. doi: 10.1111/cei.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. doi: 10.3389/fphys.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, French B, Morgan T, French SW. The liver is populated by a broad spectrum of markers for macrophages. In alcoholic hepatitis the macrophages are M1 and M2. Exp Mol Pathol. 2014;96:118–125. doi: 10.1016/j.yexmp.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Yang F, Lin R, Han C, Liu J, Ding Z. Induction of m2 polarization in primary culture liver macrophages from rats with acute pancreatitis. PLoS One. 2014;9:e108014. doi: 10.1371/journal.pone.0108014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tugal D, Liao X, Jain MK. Transcriptional control of macrophage polarization. Arterioscler Thromb Vasc Biol. 2013;33:1135–1144. doi: 10.1161/ATVBAHA.113.301453. [DOI] [PubMed] [Google Scholar]
- 17.Wijesundera KK, Izawa T, Tennakoon AH, Golbar HM, Tanaka M, Kuwamura M, Yamate J. M1-/M2-macrophage polarization in pseudolobules consisting of adipohilin-rich hepatocytes in thioacetamide (TAA)-induced rat hepatic cirrhosis. Exp Mol Pathol. 2016;101:133–142. doi: 10.1016/j.yexmp.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Damoiseaux JG, Döpp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994;83:140–147. [PMC free article] [PubMed] [Google Scholar]
- 19.Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8:e80908. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pihlajamäki J, Kuulasmaa T, Kaminska D, Simonen M, Kärjä V, Grönlund S, Käkelä P, Pääkkönen M, Kainulainen S, Punnonen K, et al. Serum interleukin 1 receptor antagonist as an independent marker of non-alcoholic steatohepatitis in humans. J Hepatol. 2012;56:663–670. doi: 10.1016/j.jhep.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Yan D, Gao J, Xu C, Yuan Y, Zhu R, Xiang D, Weng S, Han W, Zang G, et al. rhIL-1Ra reduces hepatocellular apoptosis in mice with acetaminophen-induced acute liver failure. Lab Invest. 2010;90:1737–1746. doi: 10.1038/labinvest.2010.127. [DOI] [PubMed] [Google Scholar]
- 22.Brückner S, Tautenhahn HM, Winkler S, Stock P, Jonas S, Dollinger M, Christ B. Isolation and hepatocyte differentiation of mesenchymal stem cells from porcine bone marrow--”surgical waste” as a novel MSC source. Transplant Proc. 2013;45:2056–2058. doi: 10.1016/j.transproceed.2013.01.101. [DOI] [PubMed] [Google Scholar]
- 23.Vandermeulen M, Grégoire C, Briquet A, Lechanteur C, Beguin Y, Detry O. Rationale for the potential use of mesenchymal stromal cells in liver transplantation. World J Gastroenterol. 2014;20:16418–16432. doi: 10.3748/wjg.v20.i44.16418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eshkenazy R, Dreznik Y, Lahat E, Zakai BB, Zendel A, Ariche A. Small for size liver remnant following resection: prevention and management. Hepatobiliary Surg Nutr. 2014;3:303–312. doi: 10.3978/j.issn.2304-3881.2014.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Chen L, Liu T, Zhang B, Xiang D, Wang Z, Wang Y. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A. 2012;18:1352–1364. doi: 10.1089/ten.tea.2011.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas JA, Pope C, Wojtacha D, Robson AJ, Gordon-Walker TT, Hartland S, Ramachandran P, Van Deemter M, Hume DA, Iredale JP, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M, Okabe M, Suzuki K, Kamiya Y, Tsukahara Y, Saito S, Miyajima A. Mouse hepatoblasts at distinct developmental stages are characterized by expression of EpCAM and DLK1: drastic change of EpCAM expression during liver development. Mech Dev. 2009;126:665–676. doi: 10.1016/j.mod.2009.06.939. [DOI] [PubMed] [Google Scholar]
- 28.Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47:636–647. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- 29.Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42:145–158. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 31.Viebahn CS, Benseler V, Holz LE, Elsegood CL, Vo M, Bertolino P, Ganss R, Yeoh GC. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;53:500–507. doi: 10.1016/j.jhep.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Ezquer F, Bruna F, Calligaris S, Conget P, Ezquer M. Multipotent mesenchymal stromal cells: A promising strategy to manage alcoholic liver disease. World J Gastroenterol. 2016;22:24–36. doi: 10.3748/wjg.v22.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berardis S, Dwisthi Sattwika P, Najimi M, Sokal EM. Use of mesenchymal stem cells to treat liver fibrosis: current situation and future prospects. World J Gastroenterol. 2015;21:742–758. doi: 10.3748/wjg.v21.i3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhan Peng, 2016. Wei Gao, Qin Shi. Promotion of neurological recovery in rat spinal cord injury by mesenchymal stem cells loaded on nerve-guided collagen scaffold through increasing alternatively activated macrophage polarization. J Tissue Eng Regen Med; p. Nov 15. [DOI] [PubMed] [Google Scholar]
- 36.Freytes DO, Kang JW, Marcos-Campos I, Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J Cell Biochem. 2013;114:220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 37.Elsegood CL, Chan CW, Degli-Esposti MA, Wikstrom ME, Domenichini A, Lazarus K, van Rooijen N, Ganss R, Olynyk JK, Yeoh GC. Kupffer cell-monocyte communication is essential for initiating murine liver progenitor cell-mediated liver regeneration. Hepatology. 2015;62:1272–1284. doi: 10.1002/hep.27977. [DOI] [PubMed] [Google Scholar]
- 38.Bihari C, Anand L, Rooge S, Kumar D, Saxena P, Shubham S, Sukriti, Trehanpati N, Kumar G, Pamecha V, et al. Bone marrow stem cells and their niche components are adversely affected in advanced cirrhosis of the liver. Hepatology. 2016;64:1273–1288. doi: 10.1002/hep.28754. [DOI] [PubMed] [Google Scholar]
- 39.Chai NL, Zhang XB, Chen SW, Fan KX, Linghu EQ. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J Gastroenterol. 2016;22:6036–6048. doi: 10.3748/wjg.v22.i26.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh YG, Kim JK, Byun JS, Yi HS, Lee YS, Eun HS, Kim SY, Han KH, Lee KS, Duester G, et al. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology. 2012;56:1902–1912. doi: 10.1002/hep.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]


