Abstract
AIM
to evaluate gender differences in the aspect of ghrelin, nociception-related genes and psychological aspects and the quality of life (QoL) in Korean functional dyspepsia (FD) patients.
METHODS
Total of 191 persons were prospectively enrolled between March 2013 and May 2016 in Seoul National Bundang Hospital, and classified into control and FD group based on ROME III criteria. Questionnaire included assessment for dyspepsia symptoms, QoL and anxiety or depression. Preproghrelin and nociception genes in the gastric mucosa and plasma acyl/des-acyl ghrelin were measured.
RESULTS
Lower level of plasma acyl ghrelin in FD patients compared to control was significant only in male (15.9 fmol/mL vs 10.4 fmol/mL, P = 0.017). Significantly higher mRNA expressions of nerve growth factor and transient receptor potential vanilloid receptor 1 were observed in male (P = 0.002 and P = 0.014, respectively) than in female. In contrast, female FD patients had a higher anxiety and depression score than male FD (P = 0.029), and anxiety score was correlated with epigastric pain only in female FD patients (female: Spearman rho = 0.420, P = 0.037). The impairment of overall QoL was more prominent in female FD patients than male patients (5.4 ± 0.3 vs 6.5 ± 0.3, P = 0.020).
CONCLUSION
Gender differences of ghrelin and nociception-related genes in male and psychological factors in female underlie FD symptoms. More careful assessment of psychological or emotional status is required particularly for the female FD patients.
Keywords: Functional dyspepsia, Gender differences, Quality of life
Core tip: Gender-specific medicine has become a recently rising medical field in which differences between males and females are recognized and actively utilized in the clinical study, diagnosis and treatment. The lower level of plasma acyl ghrelin and higher expressions of nociception-related genes are associated with pathogenesis of functional dyspepsia (FD) in males, while female FD patients had more serious anxious and depressive mood. Underlying mechanism in FD could be different according to gender, and meticulous attention for psychological predisposition is required particularly in the treatment of female FD patients.
INTRODUCTION
Functional dyspepsia (FD) is a heterogeneous disorder characterized by recurrent upper abdominal discomfort or pain. According to the main symptom, FD is further classified into postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS). FD is not life-threatening but imposes a socio-economic burden due to its high prevalence. Several factors could underlie this disorder including abnormal motor function[1], visceral hypersensitivity[2], genetic predisposition[3] or psychosomatic feature[4]. Interestingly, most functional gastrointestinal (GI) disorder (FGID), including FD, shows female predominance[5].
Gender-specific medicine has become a recently rising medical field in which differences between males and females are recognized and actively utilized in the diagnosis and treatment. Gender is assumed to be a crucial factor in the pathogenesis, disease progression and even prognosis of certain diseases[6-8]. However, there have been only a few reported gender differences in FGIDs, and attention has focused mostly on irritable bowel syndrome (IBS). Moreover, the topic of most studies were restricted to the prevalence of FGID[9], specified GI symptoms[10] or quality of life (QoL)[11].
Ghrelin controls appetite[12] and modulates gastric motility. A reduced acyl ghrelin level has been correlated with impaired gastric emptying[13], leading to postprandial fullness or vomiting[14]. Transient receptor potential vanilloid-1 (TRPV1) is believed to be an important integrator of the transmission and modulation of pain with nerve growth factor (NGF) or glial cell line-derived neurotrophic factor (GDNF). Previously, we demonstrated that the genes encoding these nociception-related proteins are involved in the pathogenesis of FD, particularly in the EPS type[15]. Regarding the PDS type, we found an association of increased plasma acyl ghrelin levels with abatement of dyspepsia following Helicobacter pylori eradication[16]. However, we have not evaluated the differences of expression of ghrelin or nociception-related genes regarding gender specific manner. Female predominance of FGIDs maybe related with extraintestinal conditions such as hysterectomy, which was reported to be 3-fold higher in women with IBS[17]. However, its relationship with female FD has not been evaluated so far.
Against this background, we hypothesized that there might be a difference in the underlying mechanisms of FD which could cause a difference in QoL between males and females. To verify this hypothesis, we analyzed the possible etiological factors including ghrelin, nociception-related genes, psychological aspects and history of abdominal operation as well as basal characteristics, dyspepsia symptoms and QoL between male and female FD patients.
MATERIALS AND METHODS
Subjects
The subjects were enrolled prospectively at the Department of Gastroenterology of Seoul National University Bundang Hospital(SNUBH), between March 2013 and May 2016. All subjects were of Korean and received upper gastrointestinal endoscopies and completed questionnaires about gastrointestinal symptoms including dyspepsia, emotional state and QoL under the supervision of a well-trained interviewer. History of abdominal operations including gynecological surgeries (i.e. hysterectomy, salpingooophorectomy or gynecologic surgery) was evaluated. Subjects were excluded if there was a history of gastrointestinal GI surgery, current duodenal/gastric ulcer and any history of malignancy. Users of non-steroid anti-inflammatory drugs/anticoagulants, patients with systemic diseases requiring chronic medication (except for hypertension and diabetes mellitus) were also excluded.
The subjects were classified into the FD or control group. FD was defined to the according to the Rome III criteria[15,16]. FD patients were categorized into PDS, EPS and mixed subgroups on the basis of the Rome III criteria[18]. Individuals without GI symptoms and any endoscopic lesion were assigned to the controls. The Institutional Review Board of SNUBH approved this study (B-1101/119-010), and written informed consent was obtained from all participants.
Dyspepsia symptom, emotional status and QoL assessment
The severities of epigastric pain/burning, postprandial fullness, early satiation and overall abdominal pain (not restricted to epigastric area) were scored using a five-point scale (0, none; 1, mild; 2, moderate; 3, severe; 4, very severe) using validated Korean version of Talley’s bowel disease questionnaire[19]. Stool consistency based on Bristol Stool Form Scale[20] and the number of bowel habits was evaluated. In order to assess the anxiety and depression of participants, the hospital anxiety and depression scale (HADS) was used[21]. It is subdivided into anxiety and depression subscales, both containing seven items. Each response is ranked on a 4 point (0-3) scale. Higher HADS score indicates that the subject is more depressive or anxious, with a score > 7 of each subscale indicating potential anxiety disorder or depression[22]. History of abdominal operations including hysterectomy and gynecologic surgery were collected. World Health Organization quality of life scale field trial version (WHOQOL-BREF) was used to evaluate the QoL of each subject[23]. It consists of questions about overall QoL and general health with four domains of physical health, psychological health, social relationship and environmental domains. Results are expressed as an overall score (range 0-100) and domain score (range 0-20). Higher scores denote higher QoL. These three questionnaire s have been validated in Korea[19,24,25].
Upper endoscopy and biopsy
During endoscopy, biopsy specimens were obtained from the antrum, body and fundus for histological studies. Specimens taken from the fundus were used to measure the mRNA of preproghrelin, TRPV1, GDNF and NGF[16]. The baseline H. pylori infection status and histology using the updated Sydney scoring system[26] was evaluated (Supplementary document).
Measurement of preproghrelin and nociception-related gene expression
Preproghrelin- and nociception-related gene expression was measured based on previous studies[15,16]. Detailed method was described in Supplementary document.
Measurement of plasma ghrelin level
Acyl/desacyl ghrelin was measured according to previous study (Supplementary document)[13].
Statistical analysis
Categorical variables were analyzed by χ2 test or Fisher’s exact test. Continuous variables presented as mean or median were analyzed by Student t-test or Mann-Whitney test, respectively. Spearman correlation test was used to evaluate potential correlations between HADS score and dyspepsia symptoms. SPSS Statistics version 20.0 (IBM, Armonk, NY, United States) was used. All statistical tests were 2-sided, and P < 0.05 was considered to be statistically significant.
RESULTS
General characteristics
A total of 191 subjects were included in this study. Among them, 87 subjects and 104 patients were classified into the control and FD group, respectively. Demographic characteristics of study population are summarized in Table 1. The control group was older than the FD group. The proportions of males were not significantly different between the control and FD groups (43.7% vs 37.5%. P = 0.386). The mean body mass index (BMI), H. pylori infection positivity, glandular atrophy and intestinal metaplasia were not significantly different between FD and control groups. However, there were more smokers in the FD group than the control group. The proportion of alcohol consumers was not significantly different between the groups.
Table 1.
Characteristics of the subjects n (%)
| Variables |
Control (n = 87) |
FD (n = 104) |
P value |
||||||
| Total (n = 87) | Male (n = 38) | Female (n = 49) | Total (n = 104) | Male (n = 39) | Female (n = 65) | Control vs FD | Control1 | FD1 | |
| Age (mean ± SD, yr) | 54.9 ± 12.1 | 57.4 ± 12.1 | 52.9 ± 11.8 | 50.5 ± 11.3 | 49.6 ± 11.6 | 51.3 ± 11.1 | 0.010 | 0.084 | 0.536 |
| Male | 38 (43.7) | 38 (100) | 0 | 39 ( 37.5) | 39 (100) | 0 | 0.386 | - | - |
| BMI (mean ± SD, kg/m2) | 23.1 ± 2.9 | 22.4 ± 2.9 | 22.9 ± 3.30 | 22.4 ± 3.0 | 23.3 ± 2.7 | 21.9 ± 3.4 | 0.140 | 0.482 | 0.039 |
| H. pylori | 58 (66.7) | 23 (60.5) | 35 (71.4) | 60 (57.7) | 21 (53.8) | 39 (60.0) | 0.204 | 0.360 | 0.547 |
| AG antrum | 33 (37.9) | 15 (39.5) | 18 (36.7) | 28 (26.9) | 9 (23.1) | 19 (29.2) | 0.104 | 0.794 | 0.493 |
| AG body | 13 (14.9) | 5 (13.2) | 8 (16.3) | 11 (10.6) | 4 (10.3) | 7 (10.8) | 0.365 | 0.681 | 0.999 |
| IM antrum/body | 22 (25.3) | 12 (31.6) | 10 (20.4) | 18 (17.3) | 6 (15.4) | 12 (18.5) | 0.177 | 0.234 | 0.688 |
| Smoking | 13 (14.9) | 10 (26.3) | 1 (2.0) | 24 (23.1) | 17 (43.6) | 2 (3.1) | < 0.001 | < 0.001 | < 0.001 |
| Alcohol | 20 (23.0) | 16 (42.1) | 4 (8.2) | 30 (28.8) | 21 (53.8) | 9 (13.8) | 0.342 | < 0.001 | < 0.001 |
| Gynecologic surgery2 | - | - | 13/43 (30.2) | - | - | 25/48 (52.1) | 0.171 | - | - |
| Subtype in FD | - | - | - | - | - | - | - | - | - |
| PDS | - | - | - | - | 9 (23.1) | 15 (23.1) | - | - | 0.408 |
| EPS | - | - | - | - | 6 (15.4) | 17 (26.2) | - | - | - |
| Mixed | - | - | - | - | 24 (61.5) | 33 (50.8) | - | - | - |
Between males and females;
Hysterectomy, salpingooophorectomy or cesarean section were included. Only some patients responded to this question. AG: Atrophic gastritis; BMI: Body mass index; EPS: Epigastric pain syndrome; mixed, postprandial distress syndrome and epigastric pain syndrome; FD: Functional dyspepsia; IM: Intestinal metaplasia; PDS: Postprandial distress syndrome; SD: Standard deviation; -: not available.
Female FD patients had a lower BMI than male FD patients. A higher proportion of men smoked and consumed alcohol than women in the both FD and control groups (all P < 0.001). There were no significant gender differences in H. pylori infection positivity, glandular atrophy, intestinal metaplasia and the proportion of FD subtypes. Regarding history of gynecological surgeries, 87.8% of female subjects in the control and 73.8% of female subjects in the FD group responded. FD patients were more likely to have undergone gynecological surgeries, but this was insignificant (52.1% vs 30.2%, P = 0.171).
Comparison of expression of ghrelin and nociception-related gene
We analyzed whether gender and dyspepsia symptoms were associated with levels of plasma acyl-/desacyl ghrelin and expression of preproghrelin, NGF, GDNF and TRPV1 mRNA (Table 2). While the levels of plasma acyl ghrelin in the control group was higher than in the FD group, those of NGF, GDNF and TRPV1 mRNA expressions in the control group were lower than in the FD group (Table 2) (all P < 0.05). When the comparison was restricted to control subjects, female control subjects tended to show lower levels of plasma acyl/desacyl ghrelin and mRNA expression level of NGF, GDNF and TRPV1 than male subjects, with no statistical significances. Preproghrelin mRNA was higher in female control subjects than male individuals, but was not significantly different. Among the FD group, there were no significant gender differences in expressions of the aforementioned proteins or genes.
Table 2.
Expression of plasma ghrelin, gastric peproghrelin and nociception-related genes in the control and functional dyspepsia groups
| Variables |
Control (n = 87) |
FD (n = 104) |
P value1 |
||||||
| Total | Male | Female | Total | Male | Female | Control vs FD | Control2 | FD2 | |
| (n = 87) | (n = 38) | (n = 49) | (n = 104) | (n = 39) | (n = 65) | ||||
| Plasma acyl ghrelin (fmol/mL, median [IQR]) | 14.1 (9.1-20.8) | 15.9 (9.2-33.7) | 12.2 (9.0-18.6) | 11.2 (6.6-16.8) | 10.4 (6.5-18.4) | 11.4 (6.8-16.5) | 0.018 | 0.204 | 0.388 |
| Plasma des-acyl ghrelin(fmol/mL, median [IQR]) | 67.9 (37.5-162.5) | 108 (31.8-212.9) | 65.4 (38.2-111.0) | 62.1 (32.2-110.6) | 58.5 (28.8-134.3) | 63.6 (32.2-108.0) | 0.297 | 0.389 | 0.913 |
| Ghrelin mRNA (median [IQR]) | 2.6 (0.7-4.9) | 2.1 (0.5-4.2) | 3.2 (1.0-6.1) | 1.7 (0.4-5.4) | 1.9 (0.4-9.0) | 1.5 (0.3-4.7) | 0.435 | 0.076 | 0.308 |
| NGF mRNA (median [IQR]) | 1.1 (0.7-1.7) | 1.2 (0.7-1.8) | 0.9 (0.5-1.6) | 1.6 (0.9-2.3) | 1.8 (1.1-2.7) | 1.4 (0.8-2.2) | 0.006 | 0.056 | 0.129 |
| GDNF mRNA (median [IQR]) | 1.0 (0.7-1.6) | 1.2 (0.8-1.5) | 0.9 (0.7-1.7) | 1.8 (1.0-2.9) | 1.6 (1.1-3.0) | 1.9 (0.9-2.9) | < 0.001 | 0.404 | 0.992 |
| TRPV1 mRNA (median [IQR]) | 1.0 (0.6-1.5) | 1.1 (0.8-1.6) | 0.9 (0.6-1.2) | 1.4 (0.9-2.3) | 1.4 (0.9-2.2) | 1.4 (0.8-2.3) | 0.006 | 0.102 | 0.584 |
Mann-Whitney test was used;
Male vs female. FD: Functional dyspepsia; GDNF: Glial cell-line derived neurotrophic factor; IQR: Interquartile range; NGF: Nerve growth factor; PDS: Postprandial distress syndrome; TRPV1: Transient receptor potential vanilloid receptor 1.
The lower level of plasma acyl ghrelin in FD patients compared to controls subjects was significant only in men (15.9 fmol/mL vs 10.4 fmol/mL, P = 0.017; 12.2 fmol/mL vs 11.4 fmol/mL, P = 0.348). Higher expressions of most nociception-related genes were more prominent in men than in women (Table 3).
Table 3.
Expression of plasma ghrelin, gastric peproghrelin and nociception-related genes in different gender
| Variables |
Male |
Female |
||||
| Control (n = 38) | FD (n = 39) | P value1 | Control (n = 49) | FD (n = 65) | P value1 | |
| Plasma acylghrelin (fmol/mL, median [IQR]) | 15.9 (9.2-33.7) | 10.4 (6.5-18.4) | 0.017 | 12.2 (9.0-18.6) | 11.4 (6.8-16.5) | 0.348 |
| Plasma des-acylghrelin (fmol/mL, median [IQR]) | 108.0 (31.8-212.9) | 58.5 (28.9-134.3) | 0.302 | 65.4 (38.2-111.0) | 63.6 (32.2-108.0) | 0.844 |
| Ghrelin mRNA (median [IQR]) | 2.1 (0.5-4.2) | 1.9 (0.4-9.1) | 0.428 | 3.2 (1.0-6.1) | 1.5 (0.3-4.7) | 0.092 |
| NGF mRNA (median [IQR]) | 1.2 (0.7-1.8) | 1.8 (1.1-2.7) | 0.002 | 0.9 (0.5-1.6) | 1.4 (0.8-2.2) | 0.119 |
| GDNF mRNA (median [IQR]) | 1.2 (0.7-1.5) | 1.6 (1.1-3.0) | 0.003 | 0.9 (0.7-1.7) | 1.9 (0.9-2.9) | 0.018 |
| TRPV1 mRNA (median [IQR]) | 1.1 (0.8-1.6) | 1.4 (0.9-2.2) | 0.014 | 0.9 (0.6-1.2) | 1.4 (0.8-2.3) | 0.089 |
1 Mann-Whitney test was used. FD: Functional dyspepsia; GDNF: Glial cell-line derived neurotrophic factor; IQR: Interquartile range; NGF: Nerve growth factor; PDS: Postprandial distress syndrome; TRPV1: Transient receptor potential vanilloid receptor 1.
Dyspepsia symptoms and bowel habit
Supplementary Table 1 shows the severity of dyspepsia symptom and bowel habit in FD patients according to gender. The score of epigastric burning of female patients was higher compared to male patients (3.5 ± 0.1 vs 2.7 ± 0.2, P = 0.047). On the other hand, overall abdominal pain, early satiation and postprandial fullness were slightly more severe in male patients compared to female patients, but there was no statistical significance (Table 4). In case of nausea, the score was higher in female without statistical significance. In addition, there were no significant differences in stool consistency and number of defecations.
Table 4.
Dyspepsia symptoms, stool consistency and bowel movement between males and females
| Symptoms | Male FD (n = 39) | Female FD (n = 65) | P value1 |
| Overall abdominal pain2 (mean ± SE) | 3.4 ± 0.1 | 3.1 ± 0.2 | 0.195 |
| Early satiation (mean ± SE) | 2.8 ± 0.5 | 2.5 ± 0.3 | 0.434 |
| Postprandial fullness (mean ± SE) | 3.2 ± 0.3 | 3.1 ± 0.2 | 0.221 |
| Epigastric burning/pain (mean ± SE) | 2.7 ± 0.2 | 3.5 ± 0.1 | 0.047 |
| Bloating (mean ± SE) | 2.6 ± 0.3 | 2.2 ± 0.2 | 0.339 |
| Nausea (mean ± SE) | 1.5 ± 0.3 | 1.8 ± 0.2 | 0.327 |
| Vomiting (mean ± SE) | 0.5 ± 0.2 | 0.6 ± 0.1 | 0.203 |
| BSFS (mean ± SE) | 4.8 ± 0.2 | 4.4 ± 0.2 | 0.103 |
| Number (per week) (mean ± SE) | 4.8 ± 0.3 | 4.5 ± 0.2 | 0.532 |
t-test was used;
Pain not restricted to the epigastric area. BSFS: Bristol stool form score (from 1 = very hard to 7= watery); FD: Functional dyspepsia.
Female FD patients were further classified according to the presence of history of gynecologic surgery. There were no answers from 17 patients. Twelve patients had received hysterectomy or salpingooophorectomy, and 13 patients underwent more than one Cesarean section. When patients with/without history of gynecologic surgery were compared, the patients who underwent these operations showed more frequent and severer overall abdominal pain and nausea compared to their counterparts (Supplementary Table 1).
Anxiety, depression and QoL
In order to evaluate the impact of mood and QoL on FD HADS scores and WHOQOL-BREF scores were analyzed between control and FD and between males and females.
When the FD and control groups were compared, FD patients showed higher mean HADS total score and higher mean HADS scores of both anxiety and depression than control subjects (Total, 15.8 ± 1.2 vs 11.0 ± 0.8; anxiety, 7.4 ± 0.7 vs 5.1 ± 0.4 and depression, 8.4 ± 0.6 vs 5.9 ± 0.4, all P < 0.05) (Table 5). In terms of quality of life with WHOQOL-BREF questionnaires, the scores of total, social domain and environmental domain was lower in the FD group than in the control group (Table 5).
Table 5.
HADS and WHOQOL-BREF scores in the control and functional dyspepsia groups according to gender
| Variables |
Control |
FD |
P value1 |
||||||
| Total | Male | Female | Total | Male | Female | Control vs FD | Control2 | FD2 | |
| (n = 87) | (n = 38) | (n = 49) | (n = 104) | (n = 39) | (n = 65) | ||||
| HADS score3 (mean ± SE) | |||||||||
| Total | 11.0 ± 0.8 | 10.8 ± 1.2 | 11.2 ± 1.1 | 15.8 ± 1.2 | 13.2 ± 1.3 | 18.6 ± 2.1 | 0.001 | 0.780 | 0.029 |
| Anxiety | 5.1 ± 0.4 | 4.9 ± 0.6 | 5.3 ± 0.6 | 7.4 ± 0.7 | 6.0 ± 0.7 | 9.0 ± 1.3 | 0.005 | 0.587 | 0.036 |
| Depression | 5.9 ± 0.4 | 5.9 ± 0.7 | 5.9 ± 0.6 | 8.4 ± 0.6 | 7.2 ± 0.8 | 9.7 ± 1.0 | 0.001 | 0.984 | 0.047 |
| WHOQOL-BREF score4 (mean ± SE) | |||||||||
| Total | 59.5 ± 1.6 | 60.7 ± 2.7 | 58.0 ± 1.5 | 54.0 ± 1.9 | 57.5 ± 1.9 | 51.6 ± 2.1 | 0.027 | 0.417 | 0.074 |
| Overall quality of life and general health | 6.2 ± 0.2 | 6.6 ± 0.3 | 5.8 ± 0.3 | 5.9 ± 0.3 | 6.5 ± 0.3 | 5.4 ± 0.3 | 0.278 | 0.080 | 0.020 |
| Physical domain | 13.4 ± 0.4 | 13.6 ± 0.7 | 13.1 ± 0.4 | 12.6 ± 0.4 | 13.6 ± 0.5 | 11.6 ± 0.7 | 0.185 | 0.575 | 0.016 |
| Psychological domain | 12.8 ± 0.4 | 13.2 ± 0.7 | 12.4 ± 0.5 | 11.8 ± 0.4 | 12.4 ± 0.5 | 11.4 ± 0.7 | 0.092 | 0.410 | 0.278 |
| Social domain | 13.7 ± 0.4 | 14.0 ± 0.6 | 13.3 ± 0.4 | 12.1 ± 0.5 | 13.0 ± 0.5 | 11.5 ± 0.6 | 0.012 | 0.370 | 0.064 |
| Environment domain | 13.3 ± 0.5 | 13.3 ± 0.7 | 13.3 ± 0.6 | 11.6 ± 0.4 | 12.1 ± 0.5 | 11.7 ± 0.7 | 0.010 | 0.990 | 0.617 |
t-test was used;
Between males and females;
Higher score denotes more severe symptom;
Higher score denotes better quality of life. FD: Functional dyspepsia; HADS: Hospital anxiety and depression scale; WHOQOL-BREF: World health organization quality of life abbreviated version.
In terms of gender, there were no significant differences in HADS score and every score of WHOQOL-BREF system between male and female in the control group. In contrast, FD group showed very clear gender difference. That is, female patients showed higher mean HADS total, anxiety and depression scores compared to male patients (total, 18.6 ± 2.1 vs 13.2 ± 1.3; anxiety, 9.0 ± 1.3 vs 6.0 ± 0.7; depression, 9.7 ± 1.0 vs 7.2 ± 0.8, all P < 0.05) (Figure 1). Moreover, the severity of epigastric pain correlated with HADS anxiety score only in female FD patients (males: Spearman rho = 0.232, P = 0.128; females: Spearman rho = 0.420, P = 0.037) (Figure 2).
Figure 1.
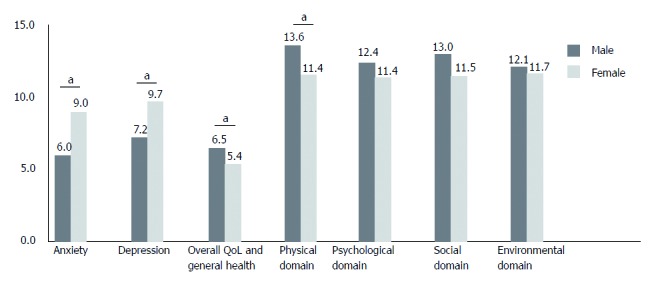
HADS and WHOQOL-BREF scores of patients with functional dyspepsia according to gender. HADS: Hospital anxiety and depression scale; WHOQOL-BREF: World health organization quality of life abbreviated version; QOL, quality of life. “a” denotes statistical significance.
Figure 2.
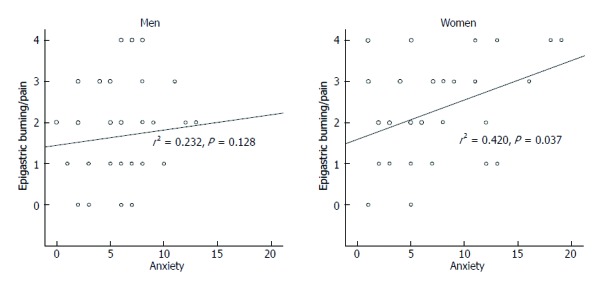
Correlation between epigastric burning/pain score and HADS anxiety score according to gender. Spearman correlation was used.
Similar to HADS score WHOQOL-BREF scoring system showed gender difference only in FD group. That is, female FD patients scored lower in every domain including the scores of total, overall QoL and general health, physical, psychological, social and environmental domains compared to male FD patients (Table 5). In particular, the scores of overall QoL and general health, and physical domain in females were significantly lower than males (P = 0.020 and 0.016, respectively) (Table 5).
DISCUSSION
We demonstrated that differences in plasma acyl ghrelin and the gastric expressions of most nociception-related gene between FD and control groups were significant only in men. In contrast, female FD patients had a more anxious and depressive mood, and showed a more apparent impaired QoL compared to male FD patients. Epigastric burning or pain was correlated with anxiety score only in women. Women who underwent any gynecologic surgery showed more severe overall abdominal symptoms than women who did not. To our knowledge, this is the first study to evaluate the differences between males and females in terms of clinical characteristics of FD and the expression of ghrelin and nociception-related genes.
Women are more likely than men to meet the criteria for most FGIDs[5,9,27,28], although some studies reported no difference in the prevalence of FD between men and women[14,29,30]. One of the most essential factors characterizing an individual biologically male and female is the sex hormone. The representative female hormones estrogen and progesterone can interfere with gastric motility. Gastric emptying in premenopausal females is delayed compared to that in males[31-34] and gastric emptying during luteal phase when the levels of the sex hormones are high is prolonged in comparison with the follicular phase[33]. Generally accepted slower gastric emptying in females than in males[35] may be at least partially attributable to the female sex hormone, which hampers gastric motility by reducing gastric smooth muscle contractility[36].
However, sex hormones may not the single factor contributing to the delayed gastric emptying in females, because this has also been observed during the follicular phase of the menstrual cycle[32]. In terms with gastric motility, a reduced acyl ghrelin level has been reported to be correlated with an impairment of gastric emptying[13]. We previously published the data of decreased plasma acyl ghrelin levels in the PDS type of FD compared to the control group[16]. PDS symptom was thought to be associated with the delayed gastric emptying. Based on the literature, a decreased tendency of plasma acyl ghrelin in the female control group in the present study may reflect the delayed gastric emptying in women. Interestingly, the difference in plasma acyl ghrelin between FD patients and control was statistically significant in male but not in female. Similarly, elevated level of NGF, GDNF and TRPV1 mRNA expression in the FD group was more prominent in men than in women. We also previously reported that FD patients showed an elevated level of NGF, GDNF and TRPV1 mRNA expression[15]. Indeed, visceral hypersensitivity is another important mechanism for the FD and the upregulation of TRPV1 has been proposed to be associated with visceral hypersensitivity of FD. The present results suggest that ghrelin and visceral hypersensitivity are important underlying mechanisms of male FD but female FD needs more other decisive mechanism.
There is evidence of a lack of the association between physiological mechanisms with dyspeptic symptoms[37]. Instead, recent epidemiological studies have provided increasing evidence for the positive association between anxiety or depression and functional gastrointestinal symptoms[4,38]. In the present study, female FD patients showed more anxious and depressive mood than male patients. Although it is unclear whether psychosocial factors mainly determine healthcare seeking or have a direct influence on symptom perception in FGID, anxiety has been recently reported to be negatively correlated with pain threshold[39]. It is interesting to note that the severity of epigastric pain correlated with HADS anxiety score only in female FD patients in the present study. This indicates that dyspepsia symptoms, particularly epigastric burning or pain in females, may be related more with psychological factors. Therefore, more attention is required in terms of psychological evaluation and management when treating female FD patients.
It is also conceivable that the gynecological condition of female may affect the varied clinical presentations in comparison with male, but most studies evaluated the association between GI symptoms and surgeries in IBS patients[17]. In the present study, female FD patients who received gynecologic surgery reported more severe general abdominal pain. However, because this abdominal pain was not restricted to the epigastric area, overlap with gynecologic conditions may contribute to dyspepsia in females. This needs further study.
Although women have been reported to be more likely than men to exhibit dysmotility-like symptoms and men are more likely to experience food regurgitation and heartburn[28,40], there were no significant differences in FD symptoms or FD symptoms subtypes between male and female FD in the present study. Only female FD patients suffered more epigastric pain than male FD patients. Although some studies in Europe and Japan reported that female predominance in the prevalence of FD[41-45], little gender analysis was conducted except for prevalence. The comparison of various aspects including prevalence, symptom subtype, dominant symptoms, natural course and QoL between males and females by different geographical areas or ethnicity could help to better understand the related pathophysiology of FD.
Concerning QoL, FD patients manifested more impaired QoL status than the control group, especially in the social and environmental domains, which indicates the need of more active intervention to ameliorate the FD symptoms. When comparing males and females, even though aspects of QoL did not reach statistical significance, every aspect of QoL was poorer in females than in males. Particularly, overall QoL and general health and physical domain scored significantly lower in females. Thus, modulating physical aspects would be effective for the alleviation of FD symptoms.
There are several limitations in the present study. This study is a single center study with a possible sample bias. Symptom scores were evaluated by questionnaire; this is not free from the risk of a recall bias. The sample size was relatively small. The effect of female sex hormones on the pathogenesis on FD was not evaluated. In spite of these limitations, our study clearly demonstrated gender differences of FD in terms of clinical characteristics of FD and the expression of ghrelin and nociception-related genes.
In conclusion, our study presents that the lower level of plasma acyl ghrelin and higher expressions of nociception-related genes are associated with pathogenesis of FD in males. On the other hand, female FD patients had more serious anxious and depressive mood, and anxiety score was correlated with epigastric pain in female FD patients. This psychological predisposition might underlie the perception of symptom, especially in female FD patients. There was not a large difference in pattern or severity of FD symptoms, except for female predominance in epigastric pain. However, considering that the impairment of overall QoL and general health was more prominent in female FD patients than in male patients, more careful assessment of psychological or emotional status is needed for the better treatment of female FD patients.
ARTICLE HIGHLIGHTS
Research background
Although gender is assumed to be an important factor in the pathogenesis, progression and prognosis of certain diseases, there have been only a few reported gender differences in functional gastrointestinal disorders, and attention has focused mostly on irritable bowel syndrome.
Research motivation
Most functional gastrointestinal disorders, including functional dyspepsia, show female predominance.
Research objectives
We compared the possible etiological factors including ghrelin, nociception-related genes, psychological aspects and history of abdominal operation as well as basal characteristics, dyspepsia symptoms and quality of life between male and female functional dyspepsia patients.
Research methods
Total of 191 persons [87 subjects (male 38, female 49) and 104 patients (male 39, female 65)] were prospectively enrolled between March 2013 and May 2016 in Seoul National Bundang Hospital. They were classified into control and FD group (PDS, EPS and mixed subgroups) on the basis of ROME III criteria. Questionnaire included assessment for dyspepsia symptoms, quality of life by WHOQOL-BREF scores and anxiety or depression by HADS scores were analyzed. Preproghrelin and nociception genes were analyzed by RT-PCR from the gastric mucosa. Plasma acyl/des-acyl ghrelin were measured by ELISA method.
Research results
Differences in plasma acyl ghrelin and the gastric expressions of most nociception-related gene between dyspepsia and control groups were significant only in men. In contrast, female functional dyspepsia patients had a more anxious and depressive mood, and showed a more apparent impaired quality of life compared to male dyspeptic patients. Epigastric burning or pain was correlated with anxiety score only in women. Women who underwent any gynecologic surgery showed more severe overall abdominal symptoms than women who did not.
Research conclusions
Different mechanisms might underlie the perception of dyspeptic symptom by gender and the negative impact of the functional dyspepsia on the quality of life can be more prominent in women than men.
Research perspectives
More careful assessment of psychological or emotional status is required particularly for the female FD patients.
ACKNOWLEDGMENTS
The authors thank the Division of Statistics of the Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Supported by Support Program for Women in Science, Engineering and Technology through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning, no. 2016H1C3A1903202.
Institutional review board statement: The Institutional Review Board of SNUBH approved this study (B-1101/119-010).
Informed consent statement: Written informed consent was obtained from all participants.
Conflict-of-interest statement: All authors declare that they have no conflict of interest.
Peer-review started: August 5, 2017
First decision: September 13, 2017
Article in press: November 8, 2017
P- Reviewer: De la Roca-Chiapas JM S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
Contributor Information
Yoon Jin Choi, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoungnam, Gyeonggi-do 13620, South Korea.
Young Soo Park, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoungnam, Gyeonggi-do 13620, South Korea.
Nayoung Kim, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoungnam, Gyeonggi-do 13620, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, 03080, South Korea. nayoungkim49@empas.com.
Yong Sung Kim, Department of Gastroenterology, Wonkwang Digestive Disease Research Institute, Wonkwang University Sanbon Hospital, Gunpo, Gyeonggi-do 1142, South Korea.
Sun Min Lee, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoungnam, Gyeonggi-do 13620, South Korea.
Dong Ho Lee, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoungnam, Gyeonggi-do 13620, South Korea; Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, 03080, South Korea.
Hyun Chae Jung, Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, 03080, South Korea.
References
- 1.Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, Marengo M, Corinaldesi R. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036–1042. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]
- 2.Tack J, Caenepeel P, Fischler B, Piessevaux H, Janssens J. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526–535. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- 3.Hwang SW, Kim N, Jung HK, Park JH, Choi YJ, Kim H, Kim J, Kim JS, Jung HC. Association of SLC6A4 5-HTTLPR and TRPV1 945G>C with functional dyspepsia in Korea. J Gastroenterol Hepatol. 2014;29:1770–1777. doi: 10.1111/jgh.12596. [DOI] [PubMed] [Google Scholar]
- 4.Wu JC. Psychological Co-morbidity in Functional Gastrointestinal Disorders: Epidemiology, Mechanisms and Management. J Neurogastroenterol Motil. 2012;18:13–18. doi: 10.5056/jnm.2012.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houghton LA, Heitkemper M, Crowell M, Emmanuel A, Halpert A, McRoberts JA, Toner B. Age, Gender and Women’s Health and the Patient. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.017. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Kim N, Kim GH. Sex and Gender Differences in Gastroesophageal Reflux Disease. J Neurogastroenterol Motil. 2016;22:575–588. doi: 10.5056/jnm16138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilminster S, Downes J, Gough B, Murdoch-Eaton D, Roberts T. Women in medicine--is there a problem? A literature review of the changing gender composition, structures and occupational cultures in medicine. Med Educ. 2007;41:39–49. doi: 10.1111/j.1365-2929.2006.02645.x. [DOI] [PubMed] [Google Scholar]
- 8.Annandale E, Hammarström A. Constructing the ‘gender-specific body’: A critical discourse analysis of publications in the field of gender-specific medicine. Health (London) 2011;15:571–587. doi: 10.1177/1363459310364157. [DOI] [PubMed] [Google Scholar]
- 9.Ahlawat SK, Cuddihy MT, Locke GR 3rd. Gender-related differences in dyspepsia: a qualitative systematic review. Gend Med. 2006;3:31–42. doi: 10.1016/s1550-8579(06)80192-0. [DOI] [PubMed] [Google Scholar]
- 10.Flier SN, Rose S. Is functional dyspepsia of particular concern in women? A review of gender differences in epidemiology, pathophysiologic mechanisms, clinical presentation, and management. Am J Gastroenterol. 2006;101:S644–S653. doi: 10.1111/j.1572-0241.2006.01015.x. [DOI] [PubMed] [Google Scholar]
- 11.Welén K, Faresjö A, Faresjö T. Functional dyspepsia affects women more than men in daily life: a case-control study in primary care. Gend Med. 2008;5:62–73. doi: 10.1016/s1550-8579(08)80009-5. [DOI] [PubMed] [Google Scholar]
- 12.van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 13.Shindo T, Futagami S, Hiratsuka T, Horie A, Hamamoto T, Ueki N, Kusunoki M, Miyake K, Gudis K, Tsukui T, et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion. 2009;79:65–72. doi: 10.1159/000205740. [DOI] [PubMed] [Google Scholar]
- 14.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, Whitehead WE, Janssens J, Funch-Jensen P, Corazziari E. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 15.Choi YJ, Kim N, Kim J, Lee DH, Park JH, Jung HC. Upregulation of Vanilloid Receptor-1 in Functional Dyspepsia With or Without Helicobacter pylori Infection. Medicine (Baltimore) 2016;95:e3410. doi: 10.1097/MD.0000000000003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi YJ, Kim N, Yoon H, Shin CM, Park YS, Park JH, Nam RH, Lee DH, Jung HC. Increase in plasma acyl ghrelin levels is associated with abatement of dyspepsia following Helicobacter pylori eradication. J Gastroenterol. 2016;51:548–559. doi: 10.1007/s00535-015-1124-6. [DOI] [PubMed] [Google Scholar]
- 17.Hasler WL, Schoenfeld P. Systematic review: Abdominal and pelvic surgery in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:997–1005. doi: 10.1046/j.1365-2036.2003.01499.x. [DOI] [PubMed] [Google Scholar]
- 18.Vakil N, Halling K, Ohlsson L, Wernersson B. Symptom overlap between postprandial distress and epigastric pain syndromes of the Rome III dyspepsia classification. Am J Gastroenterol. 2013;108:767–774. doi: 10.1038/ajg.2013.89. [DOI] [PubMed] [Google Scholar]
- 19.Song KH, Jung HK, Min BH, Youn YH, Choi KD, Keum BR, Huh KC. Development and Validation of the Korean Rome III Questionnaire for Diagnosis of Functional Gastrointestinal Disorders. J Neurogastroenterol Motil. 2013;19:509–515. doi: 10.5056/jnm.2013.19.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Kim N, Seo PJ, Lee JW, Kim MS, Kim SE, Jo SY, Lee DH, Jung HC. Association of sleep dysfunction and emotional status with gastroesophageal reflux disease in Korea. J Neurogastroenterol Motil. 2013;19:344–354. doi: 10.5056/jnm.2013.19.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 23.Skevington SM, Lotfy M, O’Connell KA; WHOQOL Group. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res. 2004;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 24.Oh SM, Min KJ, Park DB. A study on the standardization of the hospital anxiety and depression scale for Koreans: a comparison of normal, depressed and anxious groups. J Korea Neuropsych Assoc. 1999;38:289–296. [Google Scholar]
- 25.Min SK, Kim KI, Lee CI, Jung YC, Suh SY, Kim DK. Development of the Korean versions of WHO Quality of Life scale and WHOQOL-BREF. Qual Life Res. 2002;11:593–600. doi: 10.1023/a:1016351406336. [DOI] [PubMed] [Google Scholar]
- 26.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Agréus L, Svärdsudd K, Nyrén O, Tibblin G. The epidemiology of abdominal symptoms: prevalence and demographic characteristics in a Swedish adult population. A report from the Abdominal Symptom Study. Scand J Gastroenterol. 1994;29:102–109. doi: 10.3109/00365529409090447. [DOI] [PubMed] [Google Scholar]
- 28.Stanghellini V. Three-month prevalence rates of gastrointestinal symptoms and the influence of demographic factors: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST) Scand J Gastroenterol Suppl. 1999;231:20–28. doi: 10.1080/003655299750025237. [DOI] [PubMed] [Google Scholar]
- 29.Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210–2216. doi: 10.1111/j.1572-0241.2004.40052.x. [DOI] [PubMed] [Google Scholar]
- 30.Bernersen B, Johnsen R, Straume B. Non-ulcer dyspepsia and peptic ulcer: the distribution in a population and their relation to risk factors. Gut. 1996;38:822–825. doi: 10.1136/gut.38.6.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wedmann B, Schmidt G, Wegener M, Coenen C, Ricken D, Althoff J. Effects of age and gender on fat-induced gallbladder contraction and gastric emptying of a caloric liquid meal: a sonographic study. Am J Gastroenterol. 1991;86:1765–1770. [PubMed] [Google Scholar]
- 32.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 33.Gill RC, Murphy PD, Hooper HR, Bowes KL, Kingma YJ. Effect of the menstrual cycle on gastric emptying. Digestion. 1987;36:168–174. doi: 10.1159/000199414. [DOI] [PubMed] [Google Scholar]
- 34.Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med. 1987;28:1204–1207. [PubMed] [Google Scholar]
- 35.Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422–1428. doi: 10.1111/j.1572-0241.2001.03683.x. [DOI] [PubMed] [Google Scholar]
- 36.Bruce LA, Behsudi FM, Danhof IE. Smooth muscle mechanical responses in vitro to bethanechol after progesterone in male rat. Am J Physiol. 1978;235:E422–E428. doi: 10.1152/ajpendo.1978.235.4.E422. [DOI] [PubMed] [Google Scholar]
- 37.Vanheel H, Carbone F, Valvekens L, Simren M, Tornblom H, Vanuytsel T, Van Oudenhove L, Tack J. Pathophysiological Abnormalities in Functional Dyspepsia Subgroups According to the Rome III Criteria. Am J Gastroenterol. 2017;112:132–140. doi: 10.1038/ajg.2016.499. [DOI] [PubMed] [Google Scholar]
- 38.Locke GR 3rd, Weaver AL, Melton LJ 3rd, Talley NJ. Psychosocial factors are linked to functional gastrointestinal disorders: a population based nested case-control study. Am J Gastroenterol. 2004;99:350–357. doi: 10.1111/j.1572-0241.2004.04043.x. [DOI] [PubMed] [Google Scholar]
- 39.Van Oudenhove L, Vandenberghe J, Geeraerts B, Vos R, Persoons P, Demyttenaere K, Fischler B, Tack J. Relationship between anxiety and gastric sensorimotor function in functional dyspepsia. Psychosom Med. 2007;69:455–463. doi: 10.1097/PSY.0b013e3180600a4a. [DOI] [PubMed] [Google Scholar]
- 40.Westbrook JI, Talley NJ, Westbrook MT. Gender differences in the symptoms and physical and mental well-being of dyspeptics: a population based study. Qual Life Res. 2002;11:283–291. doi: 10.1023/a:1015239020403. [DOI] [PubMed] [Google Scholar]
- 41.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–2666. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schlemper RJ, van der Werf SD, Vandenbroucke JP, Biemond I, Lamers CB. Peptic ulcer, non-ulcer dyspepsia and irritable bowel syndrome in The Netherlands and Japan. Scand J Gastroenterol Suppl. 1993;200:33–41. doi: 10.3109/00365529309101573. [DOI] [PubMed] [Google Scholar]
- 43.Corsetti M, Caenepeel P, Fischler B, Janssens J, Tack J. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol. 2004;99:1152–1159. doi: 10.1111/j.1572-0241.2004.30040.x. [DOI] [PubMed] [Google Scholar]
- 44.Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Thjodleifsson B. Natural history of functional dyspepsia: a 10-year population-based study. Digestion. 2010;81:53–61. doi: 10.1159/000243783. [DOI] [PubMed] [Google Scholar]
- 45.Lu CL, Lang HC, Chang FY, Chen CY, Luo JC, Wang SS, Lee SD. Prevalence and health/social impacts of functional dyspepsia in Taiwan: a study based on the Rome criteria questionnaire survey assisted by endoscopic exclusion among a physical check-up population. Scand J Gastroenterol. 2005;40:402–411. doi: 10.1080/00365520510012190. [DOI] [PubMed] [Google Scholar]


