Abstract
Objectives
Few series analyzing prognostic fac tors predicting for obliteration of arteriovenous malformations (AVMs) following linear accelerator (LINAC)-based stereotactic radiosurgery (SRS) have been reported. We analyzed prognostic variables, outcomes, and toxicities in 88 patients undergoing LINAC-based SRS for AVMs.
Methods
Following IRB approval, patient records were retrospectively analyzed to identify independent predictors of complete response (obliteration) (CR) and time-to-CR. The majority of AVMs were treated using multiple isocenters and non-coplanar arcs. The median AVM volume was 2.67 cm3 (0.05 – 33.51). Median marginal and maximal doses were 17 (12-24) and 26.1 Gy (15-40), with a median prescription isodose surface of 65%.
Results
Spetzler-Martin (SM) grade was determined for 86 patients and was: I–3 pts (3%); II–23 pts (27%); III–45 pts (52%); IV–13 pts (15%); V–2 pts (2%).Of 80 patients with follow-up imaging, 44 (55%) had documented complete responses (CR). Kaplan-Meier estimate probability for CR at 4 years was 62% (95% CI: 0.50, 0.74). Median time to CR was 3 years (95% CI: 2.08, 3.17). Multivariate analysis demonstrated the Spetzler-Martin grade (OR=0.14 for grade III vs. grade I-II; p=0.004 and OR 0.07 for grade IV-V vs. grade I-II; p=0.002) and dichotomized marginal dose > 17 Gy (OR=4.19; p=0.01) to be significantly associated with CR.
Discussion
This report demonstrates that for LINAC-based SRS of AVM, marginal dose and Spetzler-Martin grade are strong predictors of complete AVM obliteration.
Keywords: LINAC radiosurgery, arteriovenous malformations, Spetzler-Martin grade, prognostic factors
INTRODUCTION
Arteriovenous malformations (AVMs) of the brain have a prevalence of approximately 18 per 100,000 in unselected adult populations. AVMs, especially those that show previous hemorrhage at diagnosis, are at a significant risk for more hemorrhages, with accompanying neurological morbidity or mortality. Furthermore the diagnosis of AVM is accompanied by excessive long-term mortality, especially in conservatively managed patients [1]. Therefore, therapeutic interventions are increasingly recommended over observation, unless a particular AVM is at low risk of hemorrhage in a patient with limited life expectancy.
Primary treatment modalities for AVMs include resection and stereotactic radiosurgery (SRS); endovascular embolization is valuable as an adjunctive therapy. Spetzler and Martin [2] published a simple, clinically useful scheme to predict surgical morbidity based on AVM size, location, and venous drainage that assigned 1 to 5 points (Spetzler-Martin grades) to a given nidus. One point is given to lesions < 3 cm, 2 points for lesions between 3.1 and 6.0 cm, and 3 points for malformations greater than 6 cm. Additionally, one point is added for eloquent location, and another for deep venous drainage. In general, grade I and II AVMs can be resected with low morbidity, while surgery on grade IV and V lesions yield worse outcomes with considerably enhanced risk. It is recognized that there are indeed exceptions, but the robustness of this system is illustrated by the fact that it has been prospectively validated [3].
The American Stroke Association issued a recommendation that surgical resection should be strongly considered as primary therapy for Spetzler-Martin grade I and II lesions, while radiosurgery should be considered for small lesions with increased surgical risk based on location or feeding vessel anatomy [4]. No randomized studies have been conducted to guide the choice of primary therapy.
Numerous reports generated by centers utilizing Gama Knife SRS have identified prognostic factors for favorable response after SRS for AVMs [5-11]. Furthermore, Pollock and Flickinger developed a radiosurgery-based AVM score (RBAS) [12] in patients undergoing Gamma Knife SRS that incorporated 3 variables: AVM volume, patient age, and lesion location. Subsequently this group modified the RBAS in 2008 to simplify the scoring of the location into a 2-tiered system [13]. However relatively few series analyzing prognostic factors in linear accelerator (LINAC)-based SRS for AVMs have been reported. Since the dosimetric characteristics, especially in terms of peripheral to central dose-gradient, as well as fall-off for the two methods are different, extrapolation of Gamma Knife SRS conclusions to patients treated with LINAC-based SRS may not be fully valid. We therefore analyzed prognostic variables, outcomes and toxicities in 88 patients undergoing LINAC-based SRS for AVMs at a single institution, to specifically address this knowledge gap.
MATERIALS AND METHODS
Radiosurgery technique
Between 1989 and 2005, 88 AVM patients were treated with LINAC-based SRS at the University of Wisconsin. All patients were treated using a BRW-based head-fixation and localization system, and a stereotactic floor stand. From 1989 to 2000 we employed a floor stand system developed at the University of Wisconsin, which was retired in the first quarter of 2000 and replaced with a LINAC Scalpel (Zmed IsoStand, Ashland, MA). Our current LINAC Scalpel has an isocenter stability of 0.15 +/-0.1 mm for any couch and gantry angle [14]; the prior system also had submillimetric precision [15].
All patients were positioned, scanned (treatment planning CT on the day of SRS), and treated using a modified BRW headframe on the day of the procedure. Prior to SRS, all patients had conventional angiography, MRI with MR angiography, and/or CT angiography, and one or more (usually multiple) image sets were fused with the planning CT for nidus identification and delineation, which was always performed using a team of radiation oncologist, neurosurgeon, and neuroradiologist, as necessary. 6 MV photons were used to treat all lesions. The majority of AVMs were treated using multiple isocenters (range 1-13, median 2) and non-coplanar arcs (range 5-67, median 9) having an arc length ranging from 70 to 100 degrees (median 90). Circular, pre-manufactured collimators ranging from 4-30 mm (in 2 mm increments) were used to provide conformal dose distributions. AVM volume was determined at the time of SRS, using 3-D contouring. Median marginal dose was 17 Gy (12-24 Gy) while median maximal dose was 26.1 Gy (15-40 Gy). The median prescription isodose surface was 65%. The homogeneity index is reported as the ratio of the maximal point dose to the prescription dose (i.e. the inverse of the prescription isodose surface).
Data collection and statistical analysis
Patient records were entered in an IRB-approved, HIPAA-compliant database, and retrospectively collated and analyzed. Achievement of complete obliterative response (CR) was the primary endpoint. Characteristics of AVMs, including venous drainage patterns, location, diameter, and eloquent location were determined by dedicated neuroradiologists using conventional, CT, and MR angiography in a complementary fashion (at least 2/3 of these modalities were available for every patient) [15]. These parameters were used to define the Spetzler-Martin grade. The radiosurgery-based AVM score (RBAS) is defined as: (0.1) (AVM volume in cm3) + (0.02) (patient age in years) + (0.3) (location of lesion: frontal or temporal = 0; parietal, occipital, intraventricular, corpus callosum, cerebellar = 1; or basal ganglia, thalamic, or brainstem = 2) [12]. The 2008 modified RBAS is defined as: (0.1)(AVM volume in cm3) + (0.02) (patient age in years) + (0.5)(location of lesion: hemispheric, corpus callosum, and cerebellar = 0; or basal ganglia, thalamic, or brainstem = 1) [13]. As per Pollock et al [12], for lesions occupying more than one RBAS location (e.g. frontal and basal ganglia) the location score of each AVM portion was weighted by 0.5 (if 2 locations) or 0.33 (if 3 locations) to determine the composite RBAS location score for the nidus.
Patients were followed after SRS with yearly MR angiography (MRA) imaging studies, except in patients with contraindications to MR imaging, where CT angiography (CTA) was utilized. Complete obliterative response (CR) as assessed by MRA was defined as the disappearance of any flow voids in the area of the previously seen AVM as well as the absence of the nidus on the MRA. Confirmation of CR with conventional angiography was recommended in all patients; some patients declined conventional angiography after a personal assessment of its risk-benefit ratio.
The purpose of the analysis was to identify independent predictors of complete response (CR) and estimate time-to-CR using the Kaplan-Meier survival function. The AVM volume and the two RBAS scores were transformed to the natural log, abbreviated in (volume), in (2002 RBAS), and in (2008 RBAS), since the transformed values were approximately normally distributed and generally better behaved in the statistical analysis than the skewed distribution of the original value. Univariate logistic regression was used to determine the effect of each variable on CR. Multivariate logistic regression using backward selection (p<0.05) was conducted in order to identify a set of independently significant predictors of CR. Results were quantified in terms of odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). Time-to-CR was calculated from the date of the treatment to CR or the last recorded date of response. The Kaplan-Meier (product-limit) method was used to determine estimates of time-to-CR. Event rates were defined as the ratio of the number of events to the person-years at risk for the event. For the incidence of hemorrhage after SRS, person-years were measured from the time of SRS to the date of hemorrhage, death, or censoring of data (whichever came first). For the post-SRS mortality rate, person-years were measured from the time of SRS to death or censoring of data. All statistical analyses were performed using SAS 9.1 software (SAS Institute Inc. Cary, NC) and Kaplan-Meier plots were created using R 2.5.0 software for Windows. Statistical significance was defined as a two-tailed p-value < 0.05.
RESULTS
Patient and AVM characteristics
Patient and lesion baseline characteristics are summarized in Table 1.Thirty-one patients had been previously treated; 14 with embolization, 8 with surgery and embolization, 7 with surgical resection alone,one with proton SRS, and one with fractionated stereotactic radiotherapy. Spetzler-Martin (SM) grade was determined for 86 patients. SM grade was not able to be determined in 2 patients due to missing data. Maximum diameter was documented or ascertainable in 80 patients, and the median was 2.4 cm (range 0.5-5.5).
Table 1.
Baseline patient and lesion characteristics:
| Categorical Variables | N | % | Continuous Variables | N | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Sex | Age | ||||||
| Female | 45 | 51.1 | 88 | 35.2 | 8 | 64 | |
| Male | 43 | 48.9 | |||||
| Prior hemorrhage | Hemorrhage to SRS interval | ||||||
| No | 48 | 54.6 | 40 | 6 | 1 | 168 | |
| Yes | 40 | 45.4 | |||||
| Number prior bleeds | 2002 RBAS | ||||||
| 1 | 33 | 82.5 | 85 | 1.33 | 0.25 | 4.45 | |
| 2 | 7 | 17.5 | |||||
| Previous therapy | 2008 RBAS | ||||||
| No | 57 | 64.8 | 85 | 1.23 | 0.25 | 4.15 | |
| Yes* | 31 | 35.2 | |||||
| Eloquent Location | Maximum diameter | ||||||
| No | 24 | 27.3 | 80 | 2.4 | 0.5 | 5.5 | |
| Yes | 64 | 72.7 | |||||
| Spetzler Martin Grade | Volume | 84 | 2.67 | 0.05 | 33.51 | ||
| I | 3 | 3.5 | |||||
| II | 23 | 26.7 | |||||
| III | 45 | 52.3 | |||||
| IV | 13 | 15.1 | |||||
| V | 2 | 2.3 | |||||
| Venous drainage | |||||||
| deep | 59 | 69.4 | |||||
| superficial | 26 | 30.6 |
Previous therapies included: Embolization alone (n=14); surgery and embolization (n=8); surgery alone (n=7); proton radiosurgery (n=1); and fractionated radiotherapy (n=1)
Obliteration rates
Eight patients were excluded from the analysis of obliteration rates as no follow-up imaging was available and these patients were lost to follow-up. Of 80 patients with follow-up imaging, 44 (55%) had documented complete responses (CR), while 36 (45%) had documented residual nidus on their latest post-SRS imaging. All CRs were initially documented by MRA or CTA and in more than half (n=23; 52%), conventional angiographic confirmation was obtained. Of the remaining 21 CRs, 19 (44%) were documented by MRA and 2 (5%) by CTA. Of patients who did not achieve CR (n=36), radiographic follow-up ranged from 9-193 months, with a median length of imaging follow-up of 47 months. Nineteen patients (23.7%) underwent further therapy after failing to achieve complete obliteration. Subsequent therapies included surgery (n=7), repeat SRS (n=5), embolization (n=5), surgery and repeat SRS (n=1), and embolization with repeat SRS (n=1). Kaplan-Meier estimate probability for CR at 3 years was 62% (95% CI: 0.50, 0.74) (Figure 1); this does not include the impact of salvage therapies.
Figure 1.
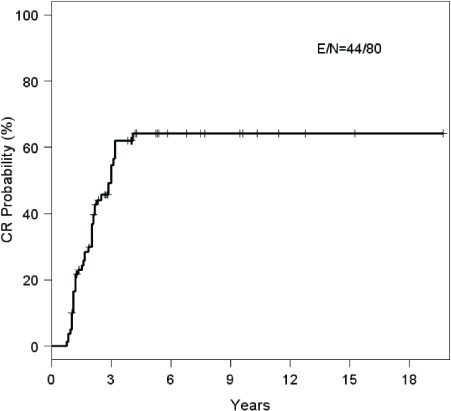
Kaplan-Meier plot for complete response (CR) of arteriovenous malformations after LINAC-based radiosurgery. Kaplan-Meier estimate probability for CR at 3 years was 62% (95% CI: 0.50, 0.74). Median Kaplan-Meier estimate to achievement of CR was 3.0 years (95% CI: 2.08, 3.17). Of patients who did not achieve CR (n= 36), radiographic follow-up ranged from 9-193 months, with a median length of imaging follow-up of 47 months.
Pre-treatment symptoms and symptom response to SRS
Neurologic deficits were documented in 32 patients (36.4%) at presentation (Table 2); four patients had more than two deficits documented. The most common neurologic deficits involved hemiparesis or hemiplegia. One patient with a left hemianopsia was lost to follow up. Of the remaining 31 patients with follow-up, 12 (38.7%) experienced an improvement or resolution of their neurologic symptoms.
Table 2.
Signs and symptoms present in 88 patients at time of radiotherapy evaluation:
| Symptom | N | % of pts |
|---|---|---|
| Neurologic deficit | 32 | 36.4% |
| Hemiparesis/plegia | 14 | 15.9% |
| Memory deficit | 6 | 6.8% |
| Aphasia | 4 | 4.5% |
| Visual deficit | 4 | 4.5% |
| Dizziness/balance | 3 | 3.4% |
| Paresthesias | 2 | 2.3% |
| Cranial nerve deficit | 2 | 2.3% |
| Ataxia | 1 | 1.1% |
| Seizure disorder | 31 | 35.2% |
| Headache | 26 | 29.5% |
Thirty-one patients (35.2%) had a history of seizures at presentation. One of these patients was lost to follow-up; of the remaining 30 patients, 9 (30%) had improvement in their seizure activity, as characterized by a decrease in frequency or ability to terminate antiepileptic medications without resumption of seizure activity.
Headaches were a symptomatic concern in 26 patients (29.5%) at presentation. Three of these patients were either lost to follow-up or did not have relevant information on headache resolution or persistence noted in follow-up visits. Eight of the 23 patients (34.8%) with relevant information had improvement or resolution in their headache symptoms after SRS.
Bleeding risk after radiosurgery
Hemorrhagic events were detected before radiosurgery in 40 patients (45.5%) (Table 1). Thirteen of the 80 patients (16.3%) with follow up experienced a hemorrhage after radiosurgery; 4 of these 13 had experienced bleeds before SRS. The median time of these hemorrhages was 24 months (range 3-232). The thirteen hemorrhages in a total of 431 patient follow-up years represent a 3.0% annual hemorrhage risk after radiosurgery. This risk was not higher in patients who had previous hemorrhages (2.3% annual risk) compared to patients who had not previously bled (3.5% annual risk). Two of these hemorrhages occurred in the latent period after SRS before eventual documentation of AVM obliteration. In the 44 patients with documented CR there were 81.6 years of radiographic follow-up before documentation of AVM obliteration; therefore an annual hemorrhage risk during the latent period of 2.5% was observed.
SRS morbidity
Of the 80 patients with follow-up, 17 (21.2%) had either radiographic or clinical sequelae following SRS, attributable to radiation. Fifteen patients (18.8%) had radiographic evidence of post-SRS edema on follow-up imaging. Ten of these were radiographic findings only, with no clinical symptoms and no steroids utilized. Additionally, four patients (5%) had evidence of cyst formation noted on follow-up imaging. Three of these instances were clinically insignificant with no symptoms or therapeutic interventions.
Five patients (6.3%) experienced radiation-induced clinically significant edema. Two of these were associated with parenchymal radiation necrosis. A third patient with documented cerebral edema had associated weakness that resolved after steroid utilization. A fourth patient developed radiation edema and cyst formation resulting in thalamic pain syndrome requiring steroid treatment. Finally, a fifth patient with a 4 cm right frontal lesion had documented post-SRS edema and experienced rapid motor deterioration which resolved with steroid therapy. One additional patient suffered from a non-radiation side effect; they developed local skin infection requiring antibiotics at the site of the surgically-placed headframe.
Mortality after SRS
Of the 80 patients with at least one follow-up imaging study, clinical information following SRS was available for a median of 42.5 months (range 10-236). In these 431 patient-years nine patients died, for a post-SRS annual rate of all-cause death of 2.1%. These nine deaths occurred at a median of 33 months after SRS (range 9-129). Three deaths were clearly attributable to other reasons, including a suicide (in a patient that had prior multiple suicide attempts, and had experienced a complication-free CR), acute myelogenous leukemia, and tuberculosis. Two patient undergoing salvage surgery for persistent AVM died from postoperative complications. One patient with persistent nidus after therapy died 24 months after SRS from an intracerebral hemorrhage, while another patient with persistent nidus died from status epilepticus. Two patients ages 35 and 41 without CR after at least 24 months of imaging follow-up died without clear documentation of the reason/s, and it is assumed that these were related to hemorrhages.
Prognostic factors for CR and time to CR
Univariate analysis
Univariate logistic regression analysis demonstrated that the actuarial complete obliteration rate was significantly related to seven prognostic factors: dichotomized marginal dose, Spetzler-Martin, grade 2002 RBAS location score, in (2002 RBAS), in (2008 RBAS), in (volume), and maximum diameter (Tables 3 & 4). The odds ratios for the log-transformed variables (i.e., in (2002 RBAS), in (2008 RBAS), and in (volume)) can be interpreted with their original scale. For example, ORs of 0.3 for in (2002 RBAS), 0.38 for in (2008 RBAS), and 0.64 for in (volume) imply that a doubling of their original scale (i.e., 2002 RBAS, 2008 modified RBAS, and AVM volume) is associated with a decrease in odds of 56%, 49%, and 27% in favor of CR, respectively. Due to indications of nonlinearity of the marginal dose in the regression analysis, the marginal dose was dichotomized based on its median value (17 Gy). Patient age, preceding hemorrhage, number of isocenters, previous therapy, 2008 RBAS location score, eloquent location, maximum dose, homogeneity index, venous drainage pattern, and bleed to SRS interval were not significant factors for CR. 2002 RBAS, 2008 modified RBAS, AVM volume, and marginal dose remained still significant without log-transformation or dichotomization.
Table 3.
Odds Ratios for Categorical Variables as Related to AVM Complete Obliteration after Radiotherapy:
| Categorical Variables | Comparison (vs. reference group) | Odds Ratio | 95% CI for OR | P-value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Preceding Hemorrhage(s) | Yes vs. No | 2.40 | 0.96 | 5.98 | 0.06 |
| Isocenters | 2-13 vs. 1 | 0.48 | 0.19 | 1.22 | 0.12 |
| Previous therapy | Yes vs. No | 0.81 | 0.33 | 2.03 | 0.66 |
| 2002 Location Score | >0 vs. 0 | 0.38 | 0.14 | 1.01 | 0.05 |
| 2008 Location Score | 1 vs. 0 | 0.90 | 0.31 | 2.63 | 0.85 |
| Eloquent Location | Yes vs. No | 0.71 | 0.27 | 1.91 | 0.50 |
| Spetzler Martin grade | 3 vs. 1-2 | 0.19 | 0.06 | 0.66 | 0.01 |
| 4-5 vs. 1-2 | 0.06 | 0.01 | 0.29 | 0.001 | |
| Venous drainage | Deep vs. Superficial | 0.60 | 0.22 | 1.60 | 0.31 |
| Marginal dose | ≥17 vs. <17 | 3.60 | 1.38 | 9.40 | 0.01 |
Table 4.
Odds Ratios for Continuous Variables as Related to AVM Complete Obliteration after Radiotherapy:
| Continuous Variables | Odds Ratio | 95% CI for OR | P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Max dose | 1.05 | 0.98 | 1.13 | 0.14 |
| Homogeneity index | 0.76 | 0.16 | 3.56 | 0.72 |
| Age | 1.01 | 0.97 | 1.04 | 0.63 |
| Hemorrhage- SRS interval | 1.00 | 0.98 | 1.03 | 0.99 |
| Maximum diameter | 0.44 | 0.28 | 0.70 | <0.001 |
| in (volume) | 0.64 | 0.45 | 0.90 | 0.01 |
| in (2002 RBAS) | 0.30 | 0.11 | 0.79 | 0.02 |
| in (2008 RBAS) | 0.38 | 0.16 | 0.91 | 0.03 |
Spetzler-Martin grade was significantly associated with CR rates (p<0.001, Chi-square test). Patients with low SM (grade I-II) achieved CR in 83% of cases; those with mid SM (grade III) reached CR in 46%, while 21% of those with high SM (grade IV-V) achieved a CR (Figure 2). Kaplan-Meier CR probability at 2 years was 55% for grades I-II; 33% for grade III; and 16% for grades IV-V.
Figure 2.
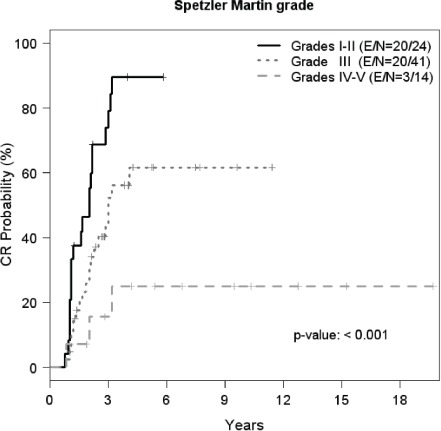
Kaplan-Meier plots of complete response (CR) rate of arteriovenous malformations after SRS. Kaplan-Meier estimates of the three categories of SM grade showed differences in the distributions of time to CR (p<0.001). Kaplan-Meier CR probability at 2 years was 55% for grades I-II; 33% for gradeIII; and 16% for grades IV-V. (E=Number of complete responders, N=Number of subjects).
Multivariate Analysis
Multivariate logistic regression using backward selection (p<0.05) was performed to identify a set of independently significant predictors for the incidence of CR. Correlation analysis (data not shown) indicatedthat the following predictors were moderately or highly correlated with one another (p-values<0.05): Spetzler-Martin grade, maximum diameter, in (volume), in (2002 RBAS), and in (2008 RBAS). In order to identify a set of independently significant predictors for CR, the Spetzler Martin grade was chosen as the primary predictor for assessment in the multivariate model as it is an accessible tool for clinical assessment of AVMs. The multivariate analysis demonstrated the Spetzler-Martin grade (OR=0.14 for grade III vs. grade I-II; p=0.004 and OR = 0.07 for grade IV-V vs. grade I-II; p = 0.002) and marginal dose > 17 Gy (OR=0.19; p = 0.01) to be significantly associated with CR (Table 5).
DISCUSSION
In this report of 88 patients undergoing LINAC-bases SRS for AVMs at a single institution, we analyzed clinical features, obliteration responses, toxicities, bleeding after SRS, improvements in pre-SRS deficits, and prognostic factors related to complete obliteration in the 80 patients with clinical and imaging follow-up. Over half (55%) of our patients had a documented CR after LINAC-based SRS; the actuarial probability for CR at 3 years was 62% (95% CI: 0.50, 0.74). LINAC-based SRS series with similar groups of patients have demonstrated similar obliteration rates [16-19]. In univariate analysis, marginal dose >17 Gy, lower Spetzler-Martin grade, lower 2002 RBAS, lower 2008 RBAS, smaller volume, smaller maximum diameter, and 2002 RBAS location score >0 were identified as prognostic factors for CR. In multivariate analysis, marginal dose > 17 Gy and lower Spetzler-Martin grade were significantly associated with CR.
Given the large number of AVMs treated with the Gamma Knife technology, numerous reports have focused on these patient cohorts to analyze prognostic factors for obliteration after SRS [5-8, 10, 12]. However, high-precision linear accelerator SRS techniques capable of precise intracranial SRS exhibit certain fundamental dose fall-off and gradient differences [20], which potentially limit the applicability of prognostic factors for obliteration and toxicity derived from Gamma Knife series to patients undergoing LINAC-based SRS for AVMs. For example, Gamma Knife SRS has more inherent dose inhomogeneity than LINAC-based SRS, because the median prescription isodose surface is 50%, compared to 65% in this series. This inhomogeneity may result in more internal “hot spots” in this setting, and further a difference in fall-off will also emerge based on the prescription isodose surface.
The Spetzler-Martin grade was initially developed in a retrospective fashion to classify AVMs to predict the probability of significant postoperative neurologic complications [2]. However, numerous large studies of patients undergoing Gamma Knife SRS for AVMs have failed to validate the SM grade as a prognostic indicator for excellent response after SRS [9, 12] while other Gamma Knife series have demonstrated correlation with nidus obliteration [6]. In LINAC-bases SRS series, Spetzler-Martin grade has been identified as an indicator of likely complete obliteration after LINAC-based SRS in some series [16, 19, 21-24]. In this series both lower Spetzler-Martin grade and peripheral dose >17 Gy were strong, independent prognostic factors for CR upon multivariate analysis.
In 2002, Pollock et al developed a radiosurgery-based grading system for arteriovenous malformations (RBAS) that strongly correlated with excellent outcomes following gamma-knife radiosurgery [12]; all patients in their cohort with a RBAS <1 had “excellent” outcomes, while only 39% of patients with an RBAS >1 had excellent outcomes. Of the 19 patients in our study with follow-up imaging and a 2002 RBAS <1, 16 had CRs, with no post-SRS hemorrhages or repeat treatments. Conversely, 18 patients in our study had a RBAS >2, and only 8 had CRs, with 5 repeat treatments and 4 post-SRS hemorrhages noted. Despite the strong prognostic value of the 2002 RBAS, Pollock and Flickinger simplified the grading system in 2008 [13] by modifying the location component of the equation; we chose to examine the robustness of this modification in our LINAC-based SRS cohort. We demonstrated that the more complex 2002 location component score had prognostic significance, while the simplified 2008 location stratification did not in our study population (Table 3). However, when these location components were integrated with the other variables (volume and age) into their respective 2002 and 2008 formulas, both the 2002 and 2008 RBAS score were similarly prognostic (Table 4). Therefore the more straightforward 2008 formula may be useful in future studies of SRS for AVM patients.
It has been demonstrated that large AVMs can take a long time to obliterate, with a significant proportion still showing obliteration between the 3rd and 4th years of follow-up [25].This latent period before obliteration represents a disadvantage when comparing SRS to surgical management. While some studies have demonstrated a decreased risk of hemorrhage during the latent period compared to the expected natural course [26], most series have demonstrated that the risk of rebleeding during the latent period remains similar to the bleeding risk of an untreated patient [27, 28]. Most current series demonstrate that roughly one-half to two-thirds of patients will have experienced an intracranial hemorrhage prior to receiving therapy [6, 18, 19, 23]. Forty (45.5%) patients in our cohort had a hemorrhage prior to SRS, and 13 of the 80 patients (16.3%) with clinical follow-up had a post-SRS hemorrhage, for an overall 3% annual risk of hemorrhage after SRS. Two of the hemorrhages occurred in lesions that eventually achieved CR, and the annual hemorrhage risk during the latent period was 2.5%. These results support the existing evidence that the risk of bleeding during the latent period is in the 2-3% range [23, 29], with cumulative risk for hemorrhage after LINAC-based SRS in the 10% range when including those lesions that persist after SRS [16, 23, 30].
Five patients (6.3%) in the cohort had clinically significant complications; most of these were transient episodes of edema that resolved with steroid therapy. Similar series have reported complication rates in the 5-7% range [16, 17, 30]. Additionally 19 patients (23.7%) required retreatment; both Gamma Knife and LINAC-bases SRS series have demonstrated that roughly 20-25% of patients require a 2nd treatment for significant persistent nidus or other clinical indications [6, 16].
Our results suggest that in multivariate analysis, a marginal dose of at least 17 Gy and low Spetzler-Martin grade are the strongest predictors of AVM complete obliteration after LINAC-based SRS. These data could assist physicians in counseling patients regarding specific obliteration rates, based on dose-selection, and pre-treatment nidus-related characteristics. Both the 2002 and 2008 RBAS scores are also associated with CR, and moderately correlated with the Spetzler-Martin grade; low RBAS scores are associated with high CR rates and very low toxicity, which should help in developing risk predictions for patients being treated with LINAC-based SRS.
ACKNOWLEDGEMENTS
Minesh Mehta serves as a consultant for Tomotherapy, which can be used to treat AVM patients; none of these patients were treated with Tomotherapy.
REFERENCES
- 1. Laakso A, Dashti R, Seppanen J, Juvela S, Vaart K, Niemela M, Sankila R, Hernesniemi JA. Long-term excess mortality in 623 patients with brain arteriovenous malformations. Neurosurgery 2008, 63(2): 244-253; discussion 253–245. [DOI] [PubMed] [Google Scholar]
- 2. Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986, 65(4): 476–483. [DOI] [PubMed] [Google Scholar]
- 3. Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery 1994, 34(1): 2–6; discussion 6–7. [PubMed] [Google Scholar]
- 4. Ogilvy CS, Stieg PE, Awad I, Brown RD, Jr., Kondziolka D, Rosenwasser R, Young WL, Hademenos G. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Circulation 2001, 103(21): 2644–2657. [DOI] [PubMed] [Google Scholar]
- 5. Flickinger JC, Pollock BE, Kondziolka D, Lunsford LD. A doseresponse analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys 1996, 36(4): 873–879. [DOI] [PubMed] [Google Scholar]
- 6. Liscak R, Vladyka V, Simonova G, Urgosik D, Novotny J, Jr., Janouskova L, Vymazal J. Arteriovenous malformations after Leksell gamma knife radiosurgery: rate of obliteration and complications. Neurosurgery 2007, 60(6): 1005–1014; discussion 1015–1006. [DOI] [PubMed] [Google Scholar]
- 7. Maruyama K, Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brainstem arteriovenous malformations: factors affecting outcome. J Neurosurg 2004, 100(3): 407–413. [DOI] [PubMed] [Google Scholar]
- 8. Nataf F, Merienne L, Schlienger M, Lefkopoulos D, Meder JF, Touboul E, Merland JJ, Devaux B, Turak B, Page P, et al. [Cerebral arteriovenous malformations treated by radiosurgery: a series of 705 cases]. Neurochirurgie 2001, 47(2-3 Pt 2): 268–282. [PubMed] [Google Scholar]
- 9. Pollock BE, Flickinger JC, Lunsford LD, Maitz A, Kondziolka D. Factors associated with successful arteriovenous malformation radiosurgery. Neurosurgery 1998, 42(6): 1239–1244; discussion 1244–1237. [DOI] [PubMed] [Google Scholar]
- 10. Pollock BE, Gorman DA, Coffey RJ. Patient outcomes after arteriovenous malformation radiosurgical management: results based on a 5- to 14-year follow-up study. Neurosurgery 2003, 52(6): 1291–1296; discussion 1296–1297. [DOI] [PubMed] [Google Scholar]
- 11. Karlsson B, Lindquist C, Steiner L. Prediction of obliteration after gamma knife surgery for cerebral arteriovenous malformations. Neurosurgery 1997, 40(3): 425-430; discussion 430-421. [DOI] [PubMed] [Google Scholar]
- 12. Pollock BE, Flickinger JC. A proposed radiosurgery-based grading system for arteriovenous malformations. J Neurosurg 2002, 96(1): 79–85. [DOI] [PubMed] [Google Scholar]
- 13. Pollock BE, Flickinger JC. Modification of the radiosurgerybased arteriovenous malformation grading system. Neurosurgery 2008, 63(2): 239–243; discussion 243. [DOI] [PubMed] [Google Scholar]
- 14. Friedman WA, Bova FJ, Spiegelmann R. Linear accelerator radiosurgery at the University of Florida. Neurosurg Clin N Am 1992, 3(1): 141–166. [PubMed] [Google Scholar]
- 15. Petereit D, Mehta M, Turski P, Levin A, Strother C, Mistretta C, Mackie R, Gehring M, Kubsad S, Kinsella T. Treatment of arteriovenous malformations with stereotactic radiosurgery employing both magnetic resonance angiography and standard angiography as a database. Int J Radiat Oncol Biol Phys 1993, 25(2): 309–313. [DOI] [PubMed] [Google Scholar]
- 16. Friedman WA, Bova FJ, Bollampally S, Bradshaw P. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery 2003, 52(2): 296–307; discussion 307-298. [DOI] [PubMed] [Google Scholar]
- 17. Schlienger M, Atlan D, Lefkopoulos D, Merienne L, Touboul E, Missir O, Nataf F, Mammar H, Platoni K, Grandjean P, et al. Linac radiosurgery for cerebral arteriovenous malformations: results in 169 patients. Int J Radiat Oncol Biol Phys 2000, 46(5): 1135–1142. [DOI] [PubMed] [Google Scholar]
- 18. Moreno-Jimenez S, Celis MA, Larraga-Gutierrez JM, de Jesus Suarez-Campos J, Garcia-Garduno A, Hernandez-Bojorquez M. Intracranial arteriovenous malformations treated with linear accelerator-based conformal radiosurgery: clinical outcome and prediction of obliteration. Sur. Neurol 2007, 67(5): 487–491; discussion 491–482. [DOI] [PubMed] [Google Scholar]
- 19. Zabel A, Milker-Zabel S, Huber P, Schulz-Ertner D, Schlegel W, Debus J. Treatment outcome after linac-based radiosurgery in cerebral arteriovenous malformations: retrospective analysis of factors affecting obliteration. Radiother Oncol 2005, 77(1): 105–110. [DOI] [PubMed] [Google Scholar]
- 20. Stieber VW, Bourland JD, Tome WA, Mehta MP. Gentlemen (and ladies), choose your weapons: Gamma knife vs. linear accelerator radiosurgery. Technol Cancer Res Treat 2003, 2(2): 79–86. [DOI] [PubMed] [Google Scholar]
- 21. Touboul E, Al Halabi A, Buffat L, Merienne L, Huart J, Schlienger M, Lefkopoulos D, Mammar H, Missir O, Meder JF, et al. Single-fraction stereotactic radiotherapy: a dose-response analysis of arteriovenous malformation obliteration. Int J Radiat Oncol Biol Phys 1998, 41(4): 855–861. [DOI] [PubMed] [Google Scholar]
- 22. Ellis TL, Friedman WA, Bova FJ, Kubilis PS, Buatti JM. Analysis of treatment failure after radiosurgery for arteriovenous malformations. J Neurosurg 1998, 89(1): 104–110. [DOI] [PubMed] [Google Scholar]
- 23. Zabel-du Bois A, Milker-Zabel S, Huber P, Schlegel W, Debus J. Risk of hemorrhage and obliteration rates of LINAC-based radiosurgery for cerebral arteriovenous malformations treated after prior partial embolization. Int J Radiat Oncol Biol Phys 2007, 68(4): 999–1003. [DOI] [PubMed] [Google Scholar]
- 24. Andrade-Souza YM, Zadeh G, Ramani M, Scora D, Tsao MN, Schwartz ML. Testing the radiosurgery-based arteriovenous malformation score and the modified Spetzler-Martin grading system to predict radiosurgical outcome. J Neurosurg 2005, 103(4): 642–648. [DOI] [PubMed] [Google Scholar]
- 25. Zabel-du Bois A, Milker-Zabel S, Huber P, Schlegel W, Debus J. Linac-based radiosurgery or hypofractionated stereotactic radiotherapy in the treatment of large cerebral arteriovenous malformations. Int J Radiat Oncol Biol Phys 2006, 64(4): 1049–1054. [DOI] [PubMed] [Google Scholar]
- 26. Karlsson B, Lindquist C, Steiner L. Effect of Gamma Knife surgery on the risk of rupture prior to AVM obliteration. Minim Invasive Neurosurg 1996, 39(1): 21–27. [DOI] [PubMed] [Google Scholar]
- 27. Friedman WA, Blatt DL, Bova FJ, Buatti JM, Mendenhall WM, Kubilis PS: The risk of hemorrhage after radiosurgery for arteriovenous malformations. J Neurosurg 1996, 84(6): 912–919. [DOI] [PubMed] [Google Scholar]
- 28. Pollock BE, Flickinger JC, Lunsford LD, Bissonette DJ, Kondziolka D. Hemorrhage risk after stereotactic radiosurgery of cerebral arteriovenous malformations. Neurosurgery 1996, 38(4): 652–659; discussion 659–661. [PubMed] [Google Scholar]
- 29. Engenhart R, Wowra B, Debus J, Kimmig BN, Hover KH, Lorenz W, Wannenmacher M. The role of high-dose, singlefraction irradiation in small and large intracranial arteriovenous malformations. Int J Radiat Oncol Biol Phys 1994, 30(3): 521–529. [DOI] [PubMed] [Google Scholar]
- 30. Bollet MA, Anxionnat R, Buchheit I, Bey P, Cordebar A, Jay N, Desandes E, Marchal C, Lapeyre M, Aletti P, et al. Efficacy and morbidity of arc-therapy radiosurgery for cerebral arteriovenous malformations: a comparison with the natural history. Int J Radiat Oncol Biol Phys 2004, 58(5): 1353–1363. [DOI] [PubMed] [Google Scholar]


