Abstract
Introduction
Gating technique can improve the accuracy of the treatment of lung and liver lesions with SBRT, by monitoring organ tumor motion and irradiating within a selected area of the respiratory cycle.
Methods
We have treated 75 patients (34 lung and 41 liver) with Novalis LINAC SBRT Adaptive Gating Technique. A total of 130 lesions, 49 lung lesions (11 primary NSCLC and 38 metastases) and 81 liver lesions (10 primary and 71 metastases). Prior to treatment, a fiducial marker is implanted and CT simulation is performed in breatholding with infrared external skin markers. Based on these external markers, internal tumor motion is correlated with the external respiratory signal. The outlined PTV includes (CTV=GTV) + 5 mm margin. The following doses are prescribed: liver (5Gy x 10 or 12-20Gy x 3), peripheral lung lesions (15-20 Gy x 3), and central lung lesions (5Gy x 10 or 10 Gy x5). The dose was delivered with multiple coplanar static beams. During patient setup, infrared markers track the respiratory cycle. Exactrac X-Rays localize the internal marker, quantify the tumor movement, and define the “beam on area” by correlating the external marker motion to the internal marker position. Intrafraction verification of the validity of this model is performed in real time by ExacTrac X-Rays.
Results
130 lesions were evaluated with 90.5% local control at two years [93.8% in lung and 87.3% in liver lesions]. Clinical tolerance was excellent and no lung or liver toxicity grade 3 was observed.
Conclusion
Our clinical experience with Novalis SBRT Adaptive Gating shows that this technique is safe and efficient for the treatment of lung and liver lesions, while reducing the volume of irradiated healthy tissue. Intrafraction verification improves the treatment accuracy by a real time verification of tumor position.
Keywords: BED, Colangiocarcinoma, Gating, Hepatocarcinoma, Hypofractionation, IGRT, Liver metastasis, lung metastasis, non small cell lung cancer, SBRT.
INTRODUCTION
Stereotactic body radiation therapy (SBRT) has emerged over the last two decades, built on the expertise of intra-cranial radiosurgery and supported by technological advances and the commercialization of dedicated treatment units. Image-guided techniques greatly improved target localization accuracies and allowed to move out from the cranial static scenarios to extra-cranial targets an apply radioablative doses to moving tumors.
The definition of SBRT is the delivery of hypofractionated radiotherapy using a stereotactic reference system, generally given in multiple fractions ranging from three to five, with techniques accounting for or limiting tumor motion, and image guidance before and during each treatment.
In 2004 the American Society for therapeutic Radiology and Oncology (ASTRO) and the American College of Radiology published a practice guideline for the performance of SBRT [1] with recommendations about personnel’s qualifications and responsibilities, procedure’s specifications and overall treatment’s quality control. In 2010 the American Association of Physicist in Medicine (AAPM) reported the Task Group 101 report [2] which is provided for establishing a SBRT program, including protocols, equipment, resources, and QA procedures.
SBRT has been demonstrated a curative treatment in lung and liver lesions in the literature. From the first publications of Lax and Bloomgren [3,4] in the nineties there have been a great number of publications focusing not only on the clinical features but also on technical possibilities and fractionation schedules. In 2009 a consortium of centers organized by the University of Colorado evaluated SBRT for primary and metastatic liver tumors as well as lung metastases and published [5,6] their results with a 95 and 92% of local control at one and two years respectively for liver lesions and 100 and 96% at one and two years respectively for lung lesions with a dose escalation up to 60Gy in three fractions without dose-limiting toxicity.
For lung NSCLC, several studies have demonstrated high local control with SBRT [7-9] and now there are studies comparing surgery with SBRT in operable NSCLC patients (Radiation Therapy Oncology Group 0618 , Accuray STARS trial, ACOSOG Z4099 trial and Japan Clinical Oncology Group 0403).
Hepatocellular carcinoma has also been investigated in phase I-II trials with good local control percentages and low toxicities even in Child-Turcotte-Pugh class A-B liver cirrhosis patients [5, 10, 11, 12, 13].
MATERIAL AND METHODS
At our institution patients are treated with SBRT using Novalis (Brainlab™), a 6MV monoenergetic LINAC adapted to intracranial and extracranial radiosurgery. To minimize organ tumor motion we use the Exactrac Adaptive Gating technique, which allows us to treat tumors in a selected position within the range of free respiratory movement.
Adaptive Gating monitors the breathing cycle and correlates each step of that cycle to the position of the moving tumor. To follow the tumor we need an implanted marker inside or near the target, placed by CT image-guided puncture with local anesthesia. We use long single markers (Visicoil©) with 3 cm length that can be used for a 3-D spacial localization of the treated lesion.
Once we have placed the implanted marker we proceed with the CT simulation, fixing some external fiducials on the patient’s skin that are tracked by the infrared cameras of the ExacTrac system. The external fiducials allow us to both move the patient automatically to the treatment position in the treatment room, and track the respiratory movement during the treatment. To localize the target volume in the different positions within the respiratory cycle in the treatment room, we use an Image-Guided Radiotherapy (IGRT) technique based on stereoscopic x-rays. This way we can determine in which moment of the respiratory cycle we treat the target and which is the suitable range of movement based on our PTV delineation (beam-on area). ExacTrac is also used during the treatment course to verify the correct position of the implanted marker on the reference point selected within the beam-on area.
We performed a retrospective analysis; from April 2008 to September 2010 we have treated and evaluated75 patients with Novalis Adaptive Gating technique. There were 34 lung patients were and 41 liver patients eligible, with a total of 49 lung lesions and 81 liver lesions; treated tumors included stage I-II NSCLC, lung metastasis, hepatocellular carcinomas, cholangiocarcinomas and liver metastasis; in table1 we describe the patient and lesion characteristics.
Patient simulation was performed after internal marker implantation using a custom-formed vacuum cushion or wing board; for lung tumors 1,5 mm thick contrast enhanced CT scan were recorded, for liver metastasis PET-CT and for hepatocellular carcinoma arterial phase CT scan were ordered. In all cases (CT or PET-CT) CT acquisition was done in breath-hold near the exhalation phase of the respiratory cycle.
Contouring and pacification works were done in iPan RT image and iPlan RT dose (Brainlab™) 4.1.2. A PTV was created adding 5 mm in all directions to the CT scan GTV or the PET-CT biological tumor volume (BTV).
Dose prescription for lung tumors was three fractions of 15 or 20Gy in a dose escalation scheme for peripheral tumors and five fractions of 10Gy for centrally located tumors. For lung tumor reirradiation we prescribed 50Gy in ten fractions.
For liver tumors, the dose prescription was three fractions of 12, 15 or 20Gy in a dose escalation scheme for metastasis, 15-16Gy in three fractions for hepatocellular carcinoma and 50Gy in ten fractions for cholangiocarcinoma. Dose restrictions for organ’s at risk (OARs) were similar to previous reported studies [5,6].
The majority of treatments included multiple coplanar beams and 3-D highly conformal dosimetry; IMRT was exceptionally performed to avoid OARs if necessary. For lung treatments, the Monte Carlo algorithm was introduced in 2010 for all the treatment plans, that results in the verification of a mean under dosage of 10% within the PTV, even up to 20% in small central lesions in the patients treated with pencil-beam.
Patient´s follow-up include an interview and clinical exam, blood test and CT or PET-CT every 3 months for the first two years, then if the patient is still controlled every six months.
RESULTS
One hundred thirty lesions were treated and evaluated (49 lung lesions and 81 liver lesions).
Due to the implantation of internal markers, pneumotorax was observed in 26% of all lung patients. Kaplan-Meier curves show a 93.8% local control in lung lesions 24 months after treatment, and 87.3% at 24 months in liver lesions (Figure 1).
Figure 1.
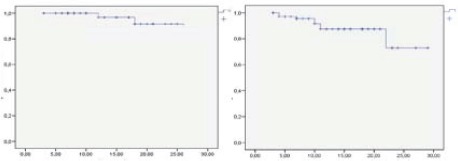
Kaplan-Meier curves for lung and liver lesions local control. Two year estimation 93.8% & 87.3% respectively.
For liver lesions the relationship between low dose and size over 3 cm and local control was analyzed, and as in previous reports [5,6] local control was poorer when dose was beyond 100Gy BED or size was over 3 cm, although no statistic significance was achieved due to the sample size (Figures 2, 3).
Figure 2.
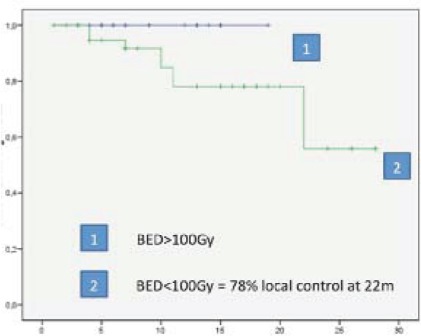
Liver metastasis, 100% local control at two years with BED>100Gy, local control of 78% at two years with BED<100Gy.
Figure 3.
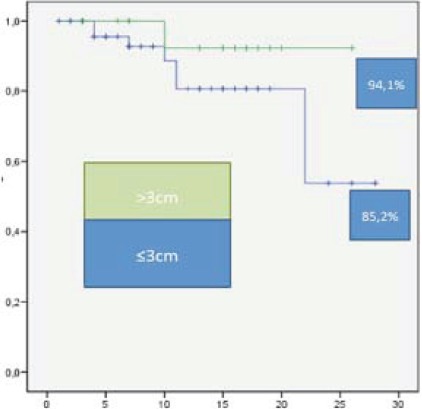
Liver metastasis, 94.1% local control at two years in lesions <3cm, local control of 85.2% at two years in >3cm.
When analyzing the toxicity, we have not found any acute toxicity over G-III (ctcae v4) neither in lung nor in liver lesions. For lung treatments, the most frequent toxicity has been grade I pneumonitis (asymptomatic radiological changes), in liver treatments we observed asymptomatic transaminitis in 14.9% of the patients. Neither skin toxicity has been reported nor rib fractures, but four cases of moderate rib’s pain without x rays demonstrating fracture have been found. No other late toxicity has been recorded at the time of analysis.
Median survival from radiotherapy treatment for lung and liver tumors was 16.8 and 23 months respectively (95%CI, 1,3-14,4 and 20,5-25,5 respectively). Twelve patients died in the lung cohort and thirteen in the liver cohort at the time of analysis.
DISCUSSION
In this study, we report our experience with the treatment of lung and liver lesions with stereotactic body radiation therapy with the adaptive gating technique. We have achieved a 2 years local control of 93.8% for the 49 evaluated lung lesions and 87.3% for the 81 evaluated liver lesions. We have not found any grade III toxicity in our series.
For lung lesions, Rusthoven reported 96% local control at two years in a phase I-II study with doses from 48 to 60 Gy in three fractions [6], previously Milano reported in a Phase II trial local control of 67% for the metastasis and lymph node treated [14]. Hof et al [15] reported local control at two years of 74% with a single dose of 12 to 30 Gy SBRT. Hoyer et al [16] reported a phase II trial of SBRT for colorectal metastasis with 86% local control at two years.
For primary lung cancer, several studies have reported local controls of 85% approximately [7, 8, 18, 19] and phase III studies comparing surgery versus SBRT are ongoing.
Table 1.
Patients &Lesions characteristics
| Lung | Liver | |
|---|---|---|
| Patients | 34 | 41 |
| Age | 66 (44-85) | 66 (30-84) |
| Lesions | 49 | 81 |
| Metastasis | 38 | 71 |
| Primaries | 11 (NSCLC) | 5 (Hepatocarcinoma) & 5 (Cholangiocarcinoma) |
| Tumor cc. | 20.78 (1.20-102.1) | 48.38 (3.6-341) |
| Histology | ||
| Colorectal | 18 (36.7%) | 47 (58%) |
| Breast | 1 (2%) | 10 (12,3%) |
| Primary | 11 (22.4%) | 10 (12.3%) |
| Lung (NSCLC&SCLC) | 7 (14.28%) | |
| Pancreas cancer | 6 (7.4%) | |
| Sarcoma | 3 (6.1%) | 4 (4.9%) |
| Miscellanea | 9 (18.36%) | 4 (4.9%) |
For liver lesions a Phase I-II by Rusthoven (5) reported 95% local control at two years with dose ranging between 36 and 60 Gy in three fractions (only 36Gy in the phase I part of the study). Milano et al (14) reported 67% local control (overall with lung lesions). Herfarth [10] reported in 2001 for single dose SBRT in liver lesions 5 months local control of 78% and in 2005 [20] the same author reported an update with 18 months local control of 66% using a single dose of 22Gy.
The importance of high doses to achieve the ablative expected effect has been described [21], recommending doses of at least 54 Gy (equivalent uniform dose of 65.3 Gy). The local control achieved in our study is comparable to the described series, with almost 94% in lung tumors and liver tumors treated with BED>100 Gy doses.
In our study we did not find any grade III toxicities, even treating lesions up to 6 cm diameter. This is less than observed in other studies. We followed the recommendations of Virginia and Colorado Universities to avoid chest wall toxicity [22]. No major toxicity was found in central lung tumors with the 50 Gy in five fractions use in our series as it is recommended by previous studies [23].
Previous reports have found pneumothorax rates ranging from 13,3 to 53% [24-27]. The highest rates were associated with long markers instead of seeds. In our center we are using a 3 cm long marker (Visicoil©) placed by CT image-guided puncture with local anesthesia; we have found 26% pneumothorax rate, surveillance procedure includes CT images after long marker implantation and chest x rays 4 hours later. A total of nine patients developed this complication, three patients underwent conservative treatment with respiratory rehabilitation and 6 needed chest tube placement (wich represents CTCAE v4.0, 33% Grade I pneumothorax and 66% grade II pneumothorax).
The adaptive gating technique allows us to use a low 0.5 cm margin to create the PTV, due to the creation of a window to treat the tumor within the respiratory cycle and the possibility of precise intra-fraction verification. In comparison to the 1-1.5 cm margin typically used with other motion control systems, adaptive gating represents an advantage for healthy-tissue saving by reducing the safety margin to 5 mm, for example for a 0,69 cm3 GTV the difference between 1-1,5 cm and 0.5 cm margins is up to 19.32 cm3 and up to 36.65 cm3 in a 3.7 cm3 GTV. Althoug there is no scientific evidence in this topic (no comparative studies), in our opinion the more the healthy tissue we save, the less toxicity probability we face. It is known that the toxicity probability in SBRT treatments is low; thus PTV reduction could be more important when treating more than one metastasis or large volume lesions.
In our series, median survival time was 16.8 moths for lung lesions and 23 for liver lesions, comparable with other literature series [5,6] if we take into account the tumor histology that has been demonstrated as a prognostic factor in the Rusthoven studies [5,6], when analyzing good prognostic histology as colorectal, breast, kidney and sarcoma our survival is 21 months compare with 19 months for the rest (p=0.4).
In conclusion, SBRT has been demonstrated as a powerful treatment for oligometastases and localized primary tumors by many studies. Our experience achieves local control and survival percentages comparable to previous data, comparative studies are needed to clarify if there is any advantage on reducing PTV in SBRT treatments.
REFERENCES
- 1. Potters L, Steinberg M, Rose C, Timmerman R, Ryu S, Hevezi JM, Whels J, Mehta M, Larson DA, Janjan NA. American Society of Therapeutic Radiology and Oncology and American College of Radiology practice guideline for the performance of Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol phys 2004; 60: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 2. Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavavagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Soldberg T, Sang DY, Stiever V, Timmerman R, Tomé WA, Verellen D, Wang L, Yin FF. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys 2010; 37: 4078–4101. [DOI] [PubMed] [Google Scholar]
- 3. Lax I, Blomgren H, Näslund I, Svanström R. Stereotactic radiotherapy of extracraneal targets. Med Phys 1994; 4: 112–113. [Google Scholar]
- 4. Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Acta Oncol 1995; 34(6): 861–870. [DOI] [PubMed] [Google Scholar]
- 5. Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Felgenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M, Gaspar LE, Schefter TE. Multi-Institutional phase I/II trial for Stereotactic Body Radiation Therapy for liver metastasis. J Clin Oncol 2009; 27: 1572–1578. [DOI] [PubMed] [Google Scholar]
- 6. Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, Pugh TJ, Kane M, Gaspar LE, Schefter TE. Multi- Institutional phase I/II trial for Stereotactic Body Radiation Therapy for lung metastasis. J Clin Oncol 2009; 27: 1579–1584. [DOI] [PubMed] [Google Scholar]
- 7. Lagerwaard FJ, Haasbeek CJA, Smit EF, Slotman BJ, Senan S. Outcomes of risk-dapted fractionated stereotactic radiotherapy for stage I non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 70: 685–692. [DOI] [PubMed] [Google Scholar]
- 8. Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, Drugge N, Ekberg L, Friesland S, Johansson KA, Lund JA, Morhed E, Nilsson K, Levin N, Paludan M, Sederholm C, Traberg A, Wittgren L, Lewensohn R. Outcome in a prospective phase II trial of medically inoperable stage I non–small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009; 27: 3290–3296. [DOI] [PubMed] [Google Scholar]
- 9. Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, Timmerman R. Stereotactic body radiation therapy for early-stage non–small-cell lung carcinoma: Fouryear results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009; 75: 677–682. [DOI] [PubMed] [Google Scholar]
- 10. Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, Höss A, Schiegel W, Wannenmacher MF. Stereotactic singledose radiation therapy of liver tumors: Results of a phase I/II trial. J Clin Oncol 2001; 19: 164–170. [DOI] [PubMed] [Google Scholar]
- 11. Tse RV, Hawkins M, Lockwood G, Kimm JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualizad stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008; 26: 657–664. [DOI] [PubMed] [Google Scholar]
- 12. Cardenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J, Johnstone PA. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010; 12: 218–225. [DOI] [PubMed] [Google Scholar]
- 13. Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HP. Stereotactic Body Radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011; in press. [DOI] [PubMed] [Google Scholar]
- 14. Milano MT, Katz AW, Mush AG, Philip A, Buchholz DJ, Schell MC, Okunieff P. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008; 112: 650–658. [DOI] [PubMed] [Google Scholar]
- 15. Hof H, Hoess A, Oetzel D, Debus J, Herfarth K. Stereotactic single-dose radiotherapy of lung metastases. Strahlenther Onkol 2007; 183: 673–678. [DOI] [PubMed] [Google Scholar]
- 16. Hoyer M, Roed H, Treberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, Kill Berthelsen A, Grau C, Aage Engelhom S, Von der Maase H. Phase II study on stereotactic body radiotherapy for colorectal metastases. Acta Oncol 2006; 45: 823–839. [DOI] [PubMed] [Google Scholar]
- 17. Inoue T, Shimizu S, Onimaru R, Takeda A, Onishi H, Nagata Y, Kimura T, Karasawa K, Arimoto T, Hareyama M, Kikuchi E, Shirato H. Clinical outcomes of stereotactic body radiotherapy for small lung lesions clinically diagnosed as primary lung cancer on radiologic examination. Int J Radiat Oncol Biol Phys 2009; 75: 683–687. [DOI] [PubMed] [Google Scholar]
- 18. Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Niibe Y, Karasawa K, Hayakawa K, Takay Y, Kimura T, Takeda A, Ouchi A, Hareyama M, Kokubo M, Hara R, Itami J, Yamada K, Araki T. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007; 2: 94–100. [DOI] [PubMed] [Google Scholar]
- 19. Stephans KL, Djemil T, Reddy CA, Gadjos SM, Kolar M, Mason D, Murthy S, Rice TW, Mazzone P, Machuzak M, Mekhail T, Videtic GM. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: the Cleveland Clinic experience. J Thorac Oncol 2009; 4: 976–82. [DOI] [PubMed] [Google Scholar]
- 20. Herfarth KK, Debus J: Stereotactic radiation therapy for liver metastases. Chirurg 2005; 76: 563–569. [DOI] [PubMed] [Google Scholar]
- 21. McCammon R, Schefter TE, Gaspar LE, Zaemisch R, Gravdahl D, Kavanagh B. Observation of a Dose-Control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2009; 73: 112–118. [DOI] [PubMed] [Google Scholar]
- 22. Dunlap NE, Cai J, Biedermann GB, Yang W, Benedict SH, Sheng K, Schefter TE, Kavanagh BD, Larner JM. Chest Wall volume reveiving more than 30 Gy predicts risk of severe pain and/or rib fracture after lung SBRT. Int J Radiat Oncol Biol Phys 2008; 72 (suppl 36). [DOI] [PubMed] [Google Scholar]
- 23. Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, De Luca J, Ewing M, Abdulrahman R, DesRosiers C, Williams M, Fletcher J. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006; 24: 4833–4839. [DOI] [PubMed] [Google Scholar]
- 24. Le QT, Loo BW, Ho A, Cotrutz C, Koong AC, Wakelee H, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Onco. 2006;1: 802–1809. [PubMed] [Google Scholar]
- 25. Whyte RI, Crownover R, Murphy MJ, Martin DP, Rice TW, DeCamp MM, et al. Stereotactic radiosurgery for lung tumors: preliminary report of a phase I trial. Ann Thorac Surg 2003; 75: 1097–1001. [DOI] [PubMed] [Google Scholar]
- 26. Muacevic A, Drexler C, Wowra B, Schweikard A, Schlaefer A, Hoffmann RT, et al. Technical description phantom accuracy and clinical feasibility for single-session lung radiosurgery using robotic image-guided real-time respiratory tumor tracking. Technol Cancer Res Treat 2007; 6: 321–328. [DOI] [PubMed] [Google Scholar]
- 27. Patrick A Kupelian, Alan Forbes, Twyla R. Willoughby, Karen Wallace, Rafael R Manon, Sanford L. Meeks, Luis Herrera, Alan Johnston, Juan J. Herran. Implantation and stability of metallic fiducials wihin pulmonary lesions. Int. J. Radiation Oncology Biol. Phys., Vol. 69, No. 3, pp. 777–785, 2007. [DOI] [PubMed] [Google Scholar]


