Abstract
Objectives
To determine the ability of a second course of stereotactic radiosurgery (SRS) to control brain metastases as well as to document the incidence of radiation necrosis (RN) after reirradiation with SRS.
Methods and Materials
Between 2001 and 2010, 37 patients with 43 retreated lesions were treated with ≥2courses of SRS to the same brain metastasis. Patient, tumor, and treatment characteristics as well as follow-up data were collected. Magnetic resonance imaging was reviewed to assess tumor response to treatment. Development of RN, as confirmed by pathology or imaging, was recorded. Local control, overall survival, and predictors of RN were analyzed.
Results
The most common histology was melanoma (n=20, 47%) followed by lung (n=9, 21%), and breast (n=8, 19%) cancer. RN was identified in 7/43 (16%) lesions. Using a competing risk model for analysis, with death as the competing risk, the incidence of RN was 11.6% and 16.5% at 6 and 12 months, respectively, and the incidence of local failure was 16.7% and 19.4% at 6 and 12 months, respectively. There was not a statistically significant association between radiation dose, mean tumor size, number of months between SRS courses, use of WBRT, or use of surgery and the development of RN. Median survival after the second course of SRS was 8.3 months, and median survival for those with and without RN was 14.1 and 7.7 months, respectively (p=0.23).
Conclusion
Reirradiation with SRS can lead to tumor response in the majority of patients with a low incidence of RN.
Keywords: brain metastases, stereotactic radiosurgery, reirradiation
1. INTRODUCTION
Radiotherapy for brain metastases (BMs) has evolved from the use of whole brain radiotherapy (WBRT) to increased utilization of focal treatment with stereotactic radiosurgery (SRS). Improvements in oncologic care and lengthening of survival in patients with metastatic disease will necessitate additional treatment options for patients with BMs. At the time of disease progression, salvage options include retreatment with a second course of WBRT [1,2,3], treatment with WBRT if SRS was previously utilized, or salvage SRS for patients previously treated with WBRT [4,5,6,7,8,9]. There is limited data regarding retreatment with SRS for a lesion previously treated with SRS.
The goal of this study was to determine the ability of a second course of SRS to control BMs as well as to document the incidence of radiation necrosis (RN) after SRS reirradiation. All patients with BMs treated with two or more courses of single fraction, linear accelerator based SRS to the same lesion were evaluated.
2. MATERIALS AND METHODS
All patients with BMs treated with SRS between years 2001 and 2010 were identified. In total, 548 patients were treated with SRS for metastatic disease. One-hundred fifteen patients received more than one course of SRS treatment, and of these, 43 patients received two courses of SRS to the same location. Patients were excluded if they did not have follow-up magnetic resonance imaging (MRI) after the second course of SRS (no follow-up brain MRI (n=5). One patient was excluded because the exact location of the retreated lesion could not be identified. A total of 37 patients, with 43 retreated lesions, met inclusion criteria. Patient and tumor characteristics including date of birth, date of death, history of diabetes mellitus, hypertension, and smoking, and tumor histology were collected. Dates of surgical excision were documented. Radiotherapy data, including dates of SRS or WBRT and radiotherapy doses, were recorded.
Radiosurgery was delivered via a linear accelerator based radiosurgical technique utilizing a single isocenter. Until the years 2008/2009, patients were treated with a Brainlab frame-based system, after which institutional planning transitioned to a Brainlab custom mask with ExacTrac image guidance. Lesions were outlined without a margin, and treatments were most frequently delivered using 5 dynamic conformal arcs. In the majority of cases, dose was prescribed per RTOG 9005 [10] guidelines, with lesions 3.01-4.00 cm in diameter treated to 15 Gy and lesions 2.01-3.00 cm treated to 18 Gy. Variation did occur with lesions ≤2 cm, where in the earlier years of the study these lesions were prescribed 20 Gy, and during the last year they were prescribed 24 Gy. Prescription isodose lines were chosen per our institutional standard [11], where the chosen isodose volume covers 95% of the tumor volume, and 95% of the dose covers 99% of the tumor volume.
Spoiled Gradient Echo (SPGR) MRI scans at the time of diagnosis were evaluated and greatest axial dimensions were documented. Axial dimensions were measured on all subsequent SPGR and diagnostic MRI scans. Lesions were categorized as complete response (CR), partial response (PR) (>20% decrease in size), progressive disease (PD) (>20% increase in size), or stable disease (SD) (not a CR, PR, or PD). Development of RN, as confirmed by pathology or imaging, was recorded.
Fisher’s exact test was utilized to analyze predictors of RN with statistical significance set at p ≤ 0.05. The median tumor size at the time of the second SRS course was 1.5cm and the median number of months between treatment was 9 months, and therefore for comparison, lesion size was categorized as ≤1.5 cm versus >1.5cm, and number of months between SRS courses was categorized as ≤9 months versus >9 months. The “cuminc” function in the “cmprsk” package in “R” statistical computing software was used to analyze the competing risk of radiation necrosis and death, and local failure and death. The software uses the method of Gray (1988) to estimate cumulative incidence. Kaplan-Meier curves were generated for overall survival.
3. RESULTS
A total of 43 lesions in 37 patients were evaluable. There were 13 males and 24 females, and the median age at the time of reirradiation was 51 years (range 27-84) (Table 1). The most common histology was melanoma (n=20, 47%) followed by lung (n=9, 21%), breast (n=8, 19%), sarcoma (n=4, 9%), and renal cell carcinoma (n=2, 5%). At the time of retreatment, 32 lesions were ≤2 cm in greatest dimension, 8 lesions were 2-3cm, and 3 lesions were >3cm. A total of eight (22%) patients had a medical history significant for hypertension requiring medical management, and 12 (32%) patients had a history of smoking >10 pack years. No patients had a history of diabetes mellitus.
Table 1.
Patient, tumor and treatment characteristics for 43 lesions treated with two courses of SRS to a single brain metastases.
| n (%) | |
| Patient Characteristics | |
| Median age at diagnosis (years) | 51 |
| History of diabetes mellitus | 0 (0%) |
| History of hyptertension | 8 (19%) |
| Smoking history > 10 pack years | 12 (28%) |
| Primary tumor type | |
| Melanoma | 20 (47%) |
| Non-small-cell lung cancer | 9 (21%) |
| Breast adenocarcinoma | 8 (19%) |
| Sarcoma | 4 (9%) |
| Renal cell carcinoma | 2 (5%) |
| Treatment characteristics | |
| Median SRS dose, 1stcourse | 18 Gy |
| Median SRS dose, 2ndcourse | 18 Gy |
| Median time (months) between courses | 9 months |
| Median tumor size at 2ndcourse of SRS | 1.5 cm |
| History of WBRT | 17 (40%) |
| History of surgical resection of brain metastasis | 13 (30%) |
RN was identified in 7/43 (16%) lesions. Using a competing risk model for analysis, with death as the competing risk, the incidence of radiation necrosis was 11.6% at 6 months and 16.5% at 12 months. Three cases of RN were confirmed by pathologic evaluation after surgery, and 4 cases were identified by imaging. Table 2 summarizes diagnostic and treatment details for patients with RN. The median number of months from the second course of SRS to the diagnosis of RN was 2.8 months. Table 3 provides a comparison of patients with and without RN. The mean duration between SRS courses was 10.9 months for patients without RN and 8.5 months for patients with RN. The mean tumor size at the second course of SRS was 14.7 mm for patients without RN and 19.3mm for patients with RN (p=0.2). There was not a statistically significant association between mean tumor size (≤1.5 versus >1.5 cm), number of months between SRS (≤9 versus >9 months), use of WBRT, or use of surgery and the development of RN. Of the patients with RN, none had a history of hypertension, and one patient had a >10 pack year history of smoking.
Table 2.
Seven cases of radiation necrosis in patients treated with a second course of stereotactic radiosurgery to a single lesion
| Diagnostic method | Histology | Months from 2nd SRS course to diagnosis of RN | Treatment | Months from 2nd SRS course to death |
| Diagnostic MRI | Lung | 2.1 | Surgery | 9.7 |
| Melanoma | 2.4 | Surgery | alive | |
| Melanoma | 2.6 | Surgery | 15.5 | |
| MR Spectroscopy | Breast | 2.8 | Steroids | 16.4 |
| Melanoma | 3.4 | Steroids | 4.3 | |
| Melanoma | 8.1 | Steroids | 11.5 | |
| Sarcoma | 9.4 | Steroids | 14.1 | |
| Median= 2.8 | Median=14.1 |
Abbreviations: RN= radiation necrosis; SRS= stereotactic radiosurgery
Table 3.
Treatment characteristics for lesions without and with radiation necrosis
| RN absent (n=36) | RN present (n=7) | p-value | |
| Median radiation dose | |||
| First course | 20 Gy | 18 Gy | |
| Second course | 20 Gy | 18 Gy | |
| Treatment prior to or between SRS courses | |||
| Surgical resection of brain metastasis | 10 (28%) | 3 (43%) | 0.35 |
| WBRT | 14 (39%) | 3 (43%) | 0.58 |
| Tumor size at time of 2nd SRS | 0.36 | ||
| ≤1.5 cm | 21 (58%) | 3 (43%) | |
| >1.5 cm | 15 (42%) | 4 (57%) | |
| Number of months between SRS courses | 0.53 | ||
| ≤9 months | 18 (50%) | 3 (43%) | |
| >9 months | 18 (50%) | 4 (57%) |
Abbreviations: RN= radiation necrosis; WBRT= whole brain radiotherapy; SRS= stereotactic radiosurgery
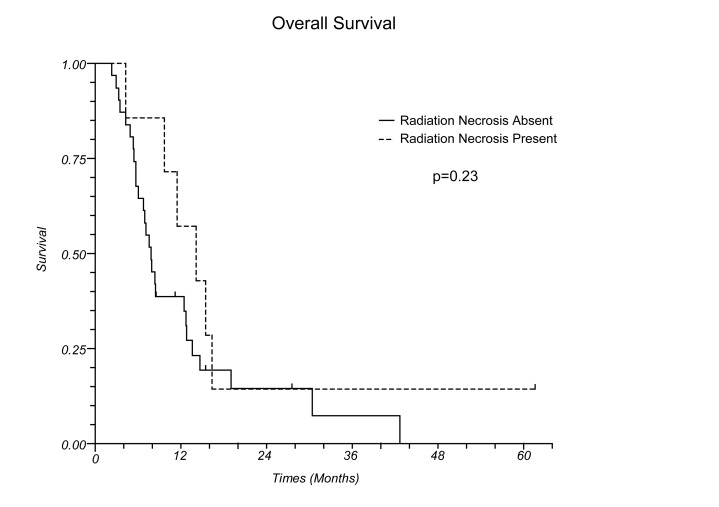
Figure 1 (b).
Overall survival for patients with and without radiation necrosis
Median follow up was 7 months (range 1-45 months). Of lesions without RN (n=36), 17 (47%) were stable, 7 (19%) had a PR, 1 (3%) had a CR, and 11 (31%) had PD after the second course of SRS. Using a competing risk model for analysis, with death as the competing risk, the incidence of local failure was 16.7% at 6 months and 19.4% at 12 months. Disease control according to histology and treatment parameters are outlined in Table 4.
Table 4.
Local control of metastases without radiation necrosis
| N | SD | CR/PR | PD | |
| (n=36) | (n=17) | (n=8) | (n=11) | |
| Histology | ||||
| Melanoma | 16 | 5 (31%) | 4 (25%) | 7 (44%) |
| Lung | 8 | 5 (63%) | 1 (13%) | 2 (25%) |
| Breast | 7 | 2 (29%) | 3 (43%) | 2 (29%) |
| Sarcoma | 2 | 2 (100%) | - | - |
| Renal cell | 3 | 3 (100%) | - | - |
| Median radiation dose (dose range) | ||||
| 1st SRS course | 20 Gy | 20 Gy | 20 Gy | |
| (18-24 Gy) | (15-22.5 Gy) | (17-22 Gy) | ||
| 2nd SRS course | 18 Gy | 20 Gy | 20 Gy | |
| (14-24 Gy) | (15-20 Gy) | (15-24 Gy) | ||
| Mean number of months between courses | 9.2 | 10.4 | 9.8 | |
| Mean tumor size before 2nd course (mm) | 15.6 | 13.8 | 14.0 |
Abbreviations: RN= radiation necrosis; SD= stable disease; PR= partial response; CR= complete response; PD= progressive disease; SRS= stereotactic radiosurgery
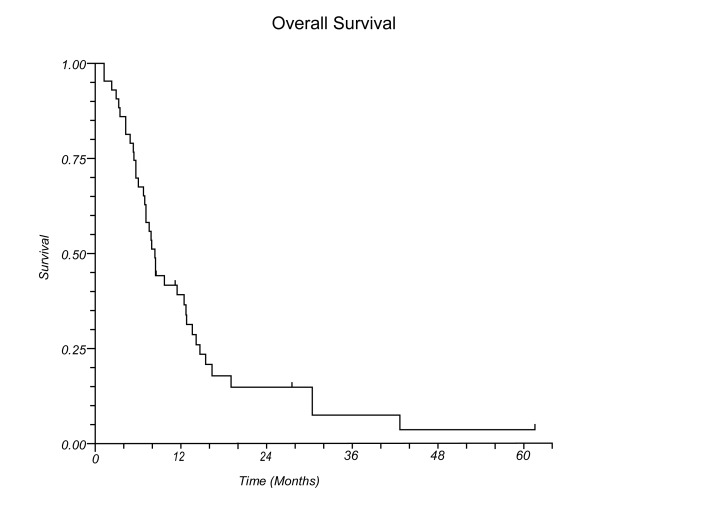
At the time of this review, 32 patients had died and 5 were alive with disease. Median survival after the second course of SRS for all patients was 8.3 months (Figure 1a), and median survival for those with and without RN was 14.1 and 7.7 months, respectively (p=0.23, Figure 1b).
Figure 1 (a).
Overall survival for all patients
4. DISCUSSION
The American College of Radiology recently published Appropriateness Criteria for retreatment of BMs [12]. Options for re-treatment include WBRT, SRS, surgery, chemotherapy, and supportive care. Several institutions have described the use of repeat WBRT for progressive metastases after an initial course of WBRT [1,2,3], and in our review, this was the initial treatment for the 17 patients who had received prior WBRT. The Mayo Clinic reported on 86 patients who underwent repeat WBRT with a median dose of 30 Gy for the first course and 20 Gy for the second course [3]. Partial improvement or complete resolution of symptoms was seen in 70% of patients. Median survival after reirradiation was 4 months. Son et al. evaluated 17 patients who underwent repeat WBRT with a median dose of 35 Gy for the first course and a median dose of 21.6 Gy for the second course [2]. Seventeen patients received repeat treatment, and of 10 patients with complete follow-up data, 8 experienced complete or partial symptom resolution. Median survival after retreatment was 5.2 months. Sadikov et al. [1] evaluated 72 patients treated with repeat WBRT and found a 4.1 month median survival after the second course of treatment. The most common fractionation regimens were 20 Gy in 5 fractions for the first course and 25 Gy in 10 fractions for the second course. Among 55 patients with evaluable follow-up, 67% had stable or improved disease after reirradiation. Sadikov et al reviewed WBRT reirradiation studies; after retreatment, median survival ranged from 2-4 months, and 27-75% of patients had stable or improved disease. These studies have demonstrated that repeat WBRT is feasible and has the potential to provide disease control.
Unfortunately, WBRT is associated with neurocognitive toxicity [13,14], and a standard two to three week course of daily treatment affects quality of life in patients with metastatic disease Neurocognitive toxicity after a second course of WBRT is not well documented but a repeat course of WBRT is likely to result in neurologic compromise. When possible, it is preferable to deliver SRS to patients with limited intracranial and stable extracranial disease in an effort to both increase the likelihood of disease control and avoid the burden of WBRT. As systemic therapies improve, more patients will live with BMs, and treatment modalities which increase the therapeutic ratio will become increasingly important. Therefore, SRS has been utilized as salvage treatment after WBRT in select patients [4,5,6]. The one-year local control rate after salvage SRS ranges from 65-91%, and the median survival ranges from 7-10 months [6]. Chao et al. [4] reported on 111 patients who underwent WBRT (median dose 37.5 Gy), followed by salvage SRS (dose range 15-24 Gy). Local control after SRS was achieved in 75% of patients. Median survival after SRS was 9.9 months for the entire group and was longer in patients with a >6 month interval between WBRT and salvage SRS (median survival 12.3 months for > 6 months versus 6.8 months for ≤6 months). In a French study involving 54 patients treated with salvage SRS after WBRT, the 1 and 2 year local control rates were 91.3% and 84%, respectively, and the median survival was 7.8 months [5]. On multivariate analysis, Recursive Partitioning Analysis (RPA) class and interval between WBRT and SRS predicted for overall survival and brain-disease free survival.
In the salvage setting, most patients are treated with repeat WBRT or with salvage WBRT or with salvage SRS, whichever treatment modality was not previously utilized. In select patients with a good performance status, controlled extracranial disease, and limited intracranial disease, a repeat course of SRS for progression of a lesion previously treated with SRS is a potential treatment option. Outcomes after treatment of a brain metastasis with two courses of SRS are not well documented. Most salvage SRS series include both retreatment of progressive lesions as well as treatment of new, distant sites in the brain. We found that retreatment with SRS is feasible and effective in controlling BMs in approximately two-thirds of patients. Kwon et al. reported a 6 month local control rate of 90.7% in 43 patients who underwent a second course of gamma knife radiosurgery as salvage treatment for progressive BMs [15]. In this study, 30 patients received retreatment to a previously treated lesion. Median survival after the second course of SRS was approximately 8 months. We report a similar median survival of 8.3 months, and in our series, patients with RN had a longer median survival than those without RN. It can be postulated that patients with longer survival have more time in which to develop RN, however we found that the median time to RN development was only 2.8 months while the MS for this group was 14.1 months. The exact cause of the lengthened survival in the RN is difficult to ascertain, however, at a minimum, it appears that RN does not result in a survival detriment.
Toxicity, including RN, after reirradiation, with either WBRT or SRS is difficult to measure and is often not well documented [1,2]. In patients who received two courses of WBRT, Kurup et al. [16] reported 1 case of RN in 56 patients while Hazuka and Kinzie [17] found 3 cases of RN on 8 autopsy specimens. Wong et al identified 5 out of 86 patients who had radiographic abnormalities consistent with radiation-related changes, however in 4 cases, these changes were present after the first course of WBRT [3]. In patients treated with SRS salvage after WBRT, the rate of RN ranges from 2-11% [4,6,10]. In RTOG 9005 [10] patients were treated with SRS as salvage treatment for progression of disease after WBRT. In total, 64% of patients had BMs, and the incidence of RN was 11% at 2 years. Chao et al. found 2 cases (2%) of RN in 111 patients treated with salvage SRS after WBRT [4]. One case was diagnosed on positron emission tomography and one case was confirmed on pathology after surgical resection. An Italian study including 69 patients who underwent WBRT followed by salvage SRS documented 4 cases (6%) of RN suspected by MRI and confirmed by SPECT-CT [6]. No symptomatic treatment was necessary in these 4 patients.
Repeat treatment with SRS after an initial course of SRS to a single lesion has a greater potential to result in RN given the high radiation doses used for SRS. In our series, a second course of SRS lead to RN in 16% of patients. RN did not appear to be associated with SRS dose or use of WBRT but may have been associated with the time interval between courses of SRS, size of the treated lesion, and use of surgery. Similarly, Kwon et al. identified 8 cases of RN in 43 patients (18.6%), 30 of whom underwent 2 courses of SRS to the same lesion [15]. Chin et al. found 17 cases of symptomatic RN amongst 243 patients (n=157 metastases, 17/157 (11%)) treated with GKS for brain tumors [18]. Of these 17 patients, 4 (23.5%) had undergone 2 or more radiosurgical treatments to the same tumor. Therefore, Chin et al. cautioned against the use of repeat SRS for progression BMs. Yamaka et al. evaluated outcomes in 41 patients treated with repeat gamma knife radiosurgery for new or progressive BMs [19]. In four cases in which SRS was performed for tumor progression, 1 developed RN. Other investigators have reported lower rates of RN after retreatment with a second course of SRS. Mariya et al. reported a RTOG/EORTC grade 4 CNS toxicity in two out of two patients who underwent three courses of SRS to a single lesion but no grade 4 toxicity in 6 patients who underwent only two courses of SRS [20]. Repeat SRS outcomes for 27 patients with benign and malignant tumors (4 cases of BMs) were evaluated by Bhatnagar et al. [21]. In this series, there were 3 patients with radiographic evidence RN, all of which were clinically occult.
Due to the low rate of RN after RT, we were unable to identify variables associated with RN. Potential predictors of RN include large tumor size, high doses of RT, and short duration between SRS courses. In our series, the median dose for patients with RN was 18 Gy versus a median dose of 20 Gy for patients without RN. This is possibly attributable to the larger mean maximum tumor dimension in the RN group versus the group without RN. As expected, the mean number of days between treatment courses was fewer for patients with RN versus patients without RN. Surgical resection of the target lesion and WBRT were performed, either before or between courses of SRS, in a greater number of patients with RN versus without RN.
Limitations of this study include the challenge in identifying and distinguishing RN versus tumor progression on routine imaging. In addition, while this series, to our knowledge, represents the largest reported series of patients retreated with a second course of SRS to the same lesion, the small sample size and limited number of events prohibits a robust statistical analysis of predictors of local control and RN. Finally, our patient population is comprised of a relatively high percentage of melanoma patients, and most lesions were small (≤2cm) at the time of retreatment, and so these outcome data may not be generalizable to all patients.
CONCLUSIONS
Retreatment with SRS to a single lesion is associated with a relatively low risk of RN, and reirradiation with SRS provides high rates of tumor control thereby allowing the majority of patients to avoid the toxicities and inconveniences associated with WBRT. Repeat SRS for progressive BMs should be considered in patients with a good performance status, a limited number of intracranial metastases, and with controlled extra-cranial disease.
Acknowledgements
No specific funding was obtained for this project.
Nomenclature
Brain metastases BMs
Centimeters cm
Complete response CR
Magnetic resonance imaging MRI
Partial response PR
Progressive disease PD
Radiation necrosis RN
Spoiled gradient echo SPGR
Stable disease SD
Stereotactic radiosurgery SRS
Whole brain radiotherapy WBRT
References
- 1. Sadikov E, Bezjak A, QL Yi, Wells W, Dawson L, Millar B, Laperriere N. Value of whole brain re-irradiation for brain metastases-single centre experience. Clin Oncol (R Coll Radiol). 2007; 19(7): 532-538. [DOI] [PubMed] [Google Scholar]
- 2. Son CH, Jimenez R, Niemierko A, Loeffler J, K Oh, Shih H. Outcomes after whole brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012; 82(2): e167-172. [DOI] [PubMed] [Google Scholar]
- 3. Wong WW, Schild SE, Sawyer TE, Shaw E. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996; 34(3): 585-590. [DOI] [PubMed] [Google Scholar]
- 4. Chao S, Barnett G, VogelbaumM Angelov L, Weil R, Neyman G, Reuther A, Suh J. Salvage stereotactic radiosurgery effectively treats recurrences from whole-brain radiation therapy. Cancer. 2008; 113(8): 2198-2204. [DOI] [PubMed] [Google Scholar]
- 5. Noel G, Proudhom MA, Valery CA, Cornu P, Boisseri G, Hasboun D, Simon J, Feuvret L, Duffau H, Tep B, Delattre JY, Marsault C, Philippon J, Fohanno D, Baillet F, Mazeron JJ. Radiosurgery for the re-irradiation of brain metastasis: results in 54 patients. Radiother Oncol. 2001; 60:61-67. [DOI] [PubMed] [Google Scholar]
- 6. Maranzano E, Trippa F, Casale M, Costantini S, Anselmo P, Carletti S, Principi M, Caserta C, Loreti F, Giorgi C. Reirradiation of brain metastases with radiosurgery. Radiother Oncol. 2012; 102(2): 192-197. [DOI] [PubMed] [Google Scholar]
- 7. Loeffler J, Kooy H, Wen P, Fine H, Cheng CW, Mannarino E, Tsai J, Alexander E. The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol. 1990; 8(4): 576-582. [DOI] [PubMed] [Google Scholar]
- 8. Davey P, O’Brien PF, Schwartz ML, Cooper PW. A phase I/II study of salvage radiosurgery in the treatment of recurrent brain metastases. J Br Neurosurg. 1994; 8(6): 717-723. [DOI] [PubMed] [Google Scholar]
- 9. Combs S, Schulz-Ertner D, Thilmann C, Edler L, Debus J. Treatment of cerebral metastases from breast cancer with stereotactic radiosurgery. Strahlenther Onkol. 2004; 180(9): 590-596. [DOI] [PubMed] [Google Scholar]
- 10. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000; 47(2): 291-298. [DOI] [PubMed] [Google Scholar]
- 11. Hazard LJ, Wang B, Skidmore TB, Chern SS, Salter BJ, Jensen RL, Shrieve DS. Conformity of LINAC-based stereotactic radiosurgery using dynamic conformal arcs and micro-multileaf collimator. Int J Radiat Oncol Biol Phys. 2009;73(2):562-570. [DOI] [PubMed] [Google Scholar]
- 12. Patel S, Robbins J, Gore E, Bradley J, Gaspar L, Germano I, Ghafoori P, Henderson M, Lutz S, McDermott M, Patchell R, Robins H, Vassil A, Wippold F, Videtic G. ACR Appropriateness Criteria; Follow-up and Retreatment of Brain Metastases. J Am Clin Oncol. 2012; 35(3): 302-305. [DOI] [PubMed] [Google Scholar]
- 13. Chang E, Wefel J, Hess K, Allen P, Lang F, Kornguth D, Arbuckle R, Swint M, Shiu A, Maor M, Meyers C. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009; 10(11): 1037-1044. [DOI] [PubMed] [Google Scholar]
- 14. Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S, Shioura H, Inomata T, Kunieda E, Hayakawa K, Nakagawa K, Kobashi G, Shirato H. Neurocognitive function of patients with brain metastases who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007; 68(5): 1388-1395. [DOI] [PubMed] [Google Scholar]
- 15. Kwon KY, Kong DS, Lee JI, Nam DH, Park K, Hyun Kim J. Outcome of repeated radiosurgery for recurrent metastatic brain tumors. Clin Neurol Neurosurg. 2007; 109(2): 132-137. [DOI] [PubMed] [Google Scholar]
- 16. Kurup P, Reddy S, Hendrikson F. Results of reirradiation for cerebral metastases. Cancer. 1980; 46: 2587-2589. [DOI] [PubMed] [Google Scholar]
- 17. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988; 15(2): 433-437. [DOI] [PubMed] [Google Scholar]
- 18. Chin L, L Ma, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001; 94(6): 899-904. [DOI] [PubMed] [Google Scholar]
- 19. Yamanaka K, Iwai Y, Yasui T, Nakajima H, Komiyama M, Nishikawa M, Morikawa T, Kishi H. Gamma knife radiosurgery for metastatic brain tumor: the usefulness of repeated gamma knife radiosurgery for recurrent cases. Stereotact Funct Neurosurg. 1999; 72(suppl 1): 73-80. [DOI] [PubMed] [Google Scholar]
- 20. Mariya Y, Sekizawa G, Matsuoka Y, Seki H, Sugawara T, Sasaki Y. Repeat stereotactic radiosurgery in the management of brain metastases from non-small cell lung cancer. Yohoku J. Esp. Med. 2011; 223(2): 125-131. [DOI] [PubMed] [Google Scholar]
- 21. Bhatnagar A, Heron D, Kondziolka D, Lunsford D, Flickinger J. Analysis of repeat stereotactic radiosurgery for progressive primary and metastatic CNS tumors. Int J Radiat Oncol Biol Phys. 2002; 53(3): 527-532. [DOI] [PubMed] [Google Scholar]