Abstract
Objective
To investigate the influence of stereotactic radiosurgery on the risk of hemorrhage from brain metastases from malignant melanoma.
Methods
A cohort of 110 patients treated with stereotactic radiosurgery (SRS) for 358 melanoma brain metastases was identified. The incidence of hemorrhage before and after SRS was determined by review of serial MRI scans. Statistical analysis was performed to determine the influence of SRS on rate of hemorrhage. Overall survival (OS) and local control (LC) were assessed and prognostic factors, including hemorrhage pre- or post-SRS were analyzed.
Results
At presentation 83 of 358 (23.2%) melanoma metastases had hemorrhaged in 44 patients. Following SRS, 73 hemorrhages occurred in 358 treated tumors (20.4%). These rates were not significantly different; p=0.362, HR=0.846 (95% CI 0.591-1.211). The risk of post-SRS hemorrhage in patients was statistically significantly linked to previous hemorrhage. Fourteen of 65 patients (21.5%) who presented without hemorrhage prior to SRS subsequently demonstrated hemorrhage. Twenty-four of 44 patients (54.5%) who presented with hemorrhage went on to demonstrate further hemorrhage following SRS; p=0.005, HR 2.47 (95% CI 1.24-11.3). Mixed effects logistic regression modeling showed no influence of SRS on the risk of hemorrhage of a given lesion (p=0.99).
OS at 1 year was better for patients presenting with a single metastasis (41.2%) compared to multiple metastases (20.3%, p=0.009). LC was 60.4% at 1 year following SRS. LC was significantly lower for metastases demonstrating hemorrhage either pre-SRS (51.7% vs 64.9%, p=0.03) or post-SRS (32.7% vs. 67.8%, p<0.001).
Conclusions
The current data show that SRS does not alter the risk of subsequent hemorrhage of treated metastases. However, hemorrhage may complicate follow-up assessment of response and LC following SRS. Careful assessment of imaging following SRS should include awareness that hemorrhage may mimic treatment failure in these patients.
Keywords: Brain metastases, hemorrhage, radiosurgery, melanoma
INTRODUCTION
In the United States, at least 170,000 and up to 500,000 patients develop brain metastases comprising 10 to 30% of cancer patients in the United States.1-3 The incidence of brain metastases has been increasing partly due to improved imaging techniques as well as prolonged survival with effective systemic agents.4-6 Stereotactic radiosurgery (SRS) has provided an additional modality by which to treat patients with brain metastases and has been incorporated into randomized controlled trials.7-10 SRS offers a conformal treatment option which may limit toxicities associated with whole brain radiotherapy in patients with brain metastases.7 Hemorrhagic brain metastases provide a particular challenge. The pre-treatment hemorrhage rate of melanoma brain metastases ranges from 9% to 30%.11-15 Post-treatment hemorrhage has been noted in 15% to 25% of patients resulting in a craniotomy in 25% due to the hemorrhage.13, 15 It is unclear whether SRS influences the risk of subsequent hemorrhage. In this report, we investigate the influence of SRS on the risk of hemorrhage from brain metastases from malignant melanoma.
PATIENTS and METHODS
Between January 2000 and June 2011, 110 consecutive patients with 358 melanoma brain metastases were treated at the Huntsman Cancer Institute, University of Utah with SRS (Novalis, BrainLAB, Heimstetten, Germany) with available follow-up imaging. IRB-approved patient databases maintained by the Department of Radiation Oncology were used to identify the patients and provide clinical information for follow-up.
SRS treatment planning
The total dose delivered during SRS was dependent on the size of the metastatic lesions, as previously described by Shaw et al.16 Lesion diameter was determined as the diameter of a sphere that yielded the same volume as the measured lesion volume. In the majority of cases, dose was prescribed per RTOG 9005 guidelines, with lesions 3.01-4.00 cm in diameter treated to 15 Gy, lesions 2.01-3.00 cm treated to 18 Gy; however, variation did occur with lesions ≤ 2 cm, where in the earlier years of the study, these lesions were prescribed 20 Gy, and during the last year of the study they were prescribed 24 Gy. The prescription isodose line was identified utilizing a method previously described.17 Patients were treated with dynamic conformal arcs. Both frame-based and frameless techniques were utilized. Details regarding the frame-based technique have been previously reported.18 The frameless technique utilizes a thermal plastic mask in conjunction with the ExacTrac/Novalis image guidance system.19 This technique has been verified with quality assurance testing.20 When treating a hemorrhagic lesion, the entire lesion was included in the target.
Outcomes and definitions
Serial post-SRS, post-contrast T1-weighted MRI scans were analyzed. Evidence of hemorrhage was based on non-contrast T1-weighted MRI sequences and diffusion-weighted MRI sequences in addition to radiology reports. Failure was defined as re-treatment of a given lesion or a sustained increase in the diameter sum of 20% following SRS. If re-treatment was not performed, and there existed a sustained increase in the diameter sum of 20%, a neurosurgeon (R.L.J.) reviewed the case to verify failure. The neurosurgeon established failure if the volume increase was clearly due to a progression of tumor rather than pure hemorrhage. Survival was defined as the time from SRS to death or the last clinical follow-up. The institutional follow-up policy includes an initial post-radiosurgery MRI scan at 4 to 6 weeks followed by surveillance MRI scans every 8 to 12 weeks thereafter.
Statistical analysis
Kaplan-Meier (K-M) analyses were used to describe the local control, freedom from hemorrhage and survival for the study cohort while the log-rank test was used to test the difference in local control and survival among subgroups defined by a single risk factor. A mixed effects logistic regression model was used to evaluate for a possible correlation between SRS of a given lesion and its risk for subsequent hemorrhage. A random effect was included to account for possible correlation between multiple lesions in the same patient. A p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed using Stata Version 11 (Stata Inc, College Station, TX) or R version 2.15.0 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Between January 2000 and June 2011, follow-up imaging was available for 110 patients with 358 melanoma metastases treated with SRS at the Huntsman Cancer Institute at the University of Utah. Patient characteristics are displayed in Table 1. Most patients had RPA Class II disease (90.9%) and 46.4% presented with one brain metastasis. Most patients underwent a single course of SRS (64.5%) and WBRT was not utilized in 57.3% of patients. Planned WBRT was utilized in 27.2% of patients. The most common primary site was head and neck (33.6%). Salvage WBRT was utilized in 25.5% of cases. Metastasis characteristics are displayed in Table 2. The vast majority of metastases were less than 2 cm in size (80.7%).
Table 1.
Patient characteristics
| Patients | |||
| n | % | ||
| Total | 110 | ||
| Age (yrs)(range) | 55.9 (19.7 – 89.0) | ||
| Gender | Male | 76 | 69.1% |
| RPA | 1 | 9 | 12.0% |
| 2 | 100 | 73.8% | |
| 3 | 1 | 1.3% | |
| Primary site | |||
| Head and neck | 37 | 33.6% | |
| Trunk | 29 | 26.4% | |
| Extremity | 20 | 18.2% | |
| GI | 3 | 2.7% | |
| GU | 4 | 3.6% | |
| Unknown | 17 | 15.5% | |
| Single met initially | 1 | 51 | 46.4% |
| #courses | 1 | 71 | 64.5% |
| 2 | 28 | 25.5% | |
| 3 | 6 | 5.5% | |
| 4 | 1 | 0.9% | |
| 5 | 2 | 1.8% | |
| Pre-treatment hemorrhage | 44 | 40.0% | |
| Post-treatment hemorrhage | 38 | 34.5% | |
| WBRT | None | 63 | 57.3% |
| After SRS unplanned | 28 | 25.5% |
Table 2.
Metastases characteristics
| Metastases | |||
| n | % | ||
| Total | 358 | ||
| Median follow-up (months) | 3.8 | ||
| Median dose (Gy)(range) | 20 (12-24) | ||
| Size | <2 cm | 289 | 80.7% |
| 2 to 3 cm | 52 | 14.5% | |
| >3 cm | 17 | 4.7% | |
| Pre-tx hemorrhage | |||
| Yes | 78 | 21.8% | |
| No | 270 | 75.4% | |
| Unknown | 10 | 2.8% | |
| Post-tx hemorrhage | |||
| Yes | 74 | 20.7% | |
| No | 284 | 79.3% | |
| Local failures | 79 | 22.1% | |
| Response to local failures | Crani | 30 | 38.0% |
| WBRT | 9 | 11.4% | |
| Re-SRS | 7 | 8.9% | |
| Supportive | 33 | 41.8% | |
| Response to post-treatment hemorrhage | Crani | 15 | 20.3% |
| WBRT | 4 | 5.4% | |
| Re-SRS | 1 | 1.4% | |
| Supportive | 54 | 73.0% |
Pre-treatment hemorrhage was noted in 21.8% of metastases. Following SRS, post-treatment hemorrhage was noted in 20.7%. These rates were not significantly different (p=0.362, HR=0.846, 95% CI 0.591-1.211). The one year Kaplan-Meier rate of post-treatment hemorrhage was 32.6%. Utilizing a mixed effects logistic regression model to assess for a possible correlation between SRS and the risk of subsequent hemorrhage of a given lesion, we found no evidence for an influence of SRS on hemorrhage risk (p=0.99).
The Kaplan-Meier estimated 1-year overall survival was 30% and median survival was 7.5 months. Patients with a single brain metastasis at presentation had improved 1 year actuarial overall survival (41.2% vs. 20.3%; HR 0.60, 95% CI [0.41-0.89]) (Figure 1). Overall survival was not influenced by hemorrhage either pre- or post- SRS.
Figure 1.
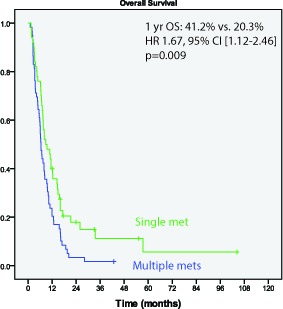
Overall survival is improved in patients with a single brain metastasis (p=0.009)
Local control at 1 year following SRS was 60.4%. Metastases with pre-treatment hemorrhage had worse 1 year actuarial local control than those without pre-treatment hemorrhage (51.7% vs. 64.9%; HR 1.90, 95% CI [1.07-3.37]); p=0.03)) (Figure 2). The crude post-treatment hemorrhage rate was 20.7% of metastases. The 1 year actuarial hemorrhage free survival was 67.9%. Metastases achieving a complete response following SRS (n=62) had a lower rate of hemorrhage post-SRS than those not achieving complete response (HR 0.19, p=0.02). Metastases with post-treatment hemorrhage had worse 1 year actuarial local control than those without post-treatment hemorrhage (32.7% versus 67.8% (HR 3.48; 95% CI [1.94-6.25], p<0.001))(Figure 3). Craniotomy was performed in 20.3% of those lesions while no intervention was performed in 73.0% of lesions after post-treatment hemorrhage.
Figure 2.
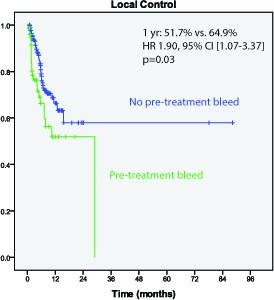
Local control is worse for metastases with pre-treatment hemorrhage (p=0.003)
Figure 3.
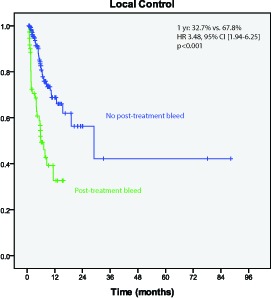
Local control is worse for matastases with post-treatment hemorrhage (p<0.001)
DISCUSSION
Historical data have shown that fractionated whole brain radiation therapy for melanoma brain metastases leads to no neurological improvement in 60% of cases for whom the median survival is 17 days.21 WBRT alone has been well documented as relatively ineffective at achieving local control in patients with metastatic melanoma leading to the suggestion that it is a “radioresistant” histology.22-28 SRS has improved the local control of melanoma cerebral metastases though it is controversial as to whether melanoma brain metastases have poorer local control following SRS treatment than non-melanoma brain metastases. Some retrospective studies have shown a lower rate of local control for melanoma brain metastases29-33 though others have shown no difference in local control rates of melanoma relative to other histologies.34-39 We report a 1 year local control rate for melanoma metastases following SRS of 60.4%.
We report a pre-treatment hemorrhage incidence of 21.8% compared with a post-treatment hemorrhage incidence of 20.7%. We also report no correlation between the use of SRS and hemorrhage risk of a treated lesion. This is consistent with the existing literature. Redmond et al.15 reported their institutional experience in treating melanoma brain metastases with Gamma Knife. They reported a pretreatment hemorrhage rate of 23.7% and post treatment hemorrhage rate of 15.2%. They also conclude that Gamma Knife does not appear to increase the rate of hemorrhage.
We found that metastases with pre-treatment hemorrhage have worse local control than those without pre-treatment hemorrhage. This is consistent with previous studies of hemorrhagic brain metatases treated with SRS in which pre-treatment hemorrhage and size correlated with worse local control.13, 29, 38 This may be due to a larger target (and subsequently lower dose delivered). In this report, we also note a higher likelihood for developing post-treatment hemorrhage if pre-treatment hemorrhage is noted. Previous studies have not demonstrated a relationship between pre-treatment hemorrhage and post-treatment hemorrhage.15
This analysis is limited by the single-institution retrospective design which predisposes it to selection biases. The limited median follow-up related to the patient population is a further drawback. Moreover, the patient population is restricted to those with follow-up imaging lending itself to further selection bias. There is no “control” group of patients with untreated intracranial melanoma metastases to ascertain a baseline risk of hemorrhage over time. As such, a random effects logistic regression model was utilized to estimate the influence of the given treatment (i.e., SRS) on hemorrhage risk using a given metastasis as its own control.
In summary, we report no influence of SRS on the risk of hemorrhage in melanoma brain metastases. Pre-treatment hemorrhage as well as post-treatment hemorrhage correlates to poorer local control within this subset of patients. Hemorrhage must be taken into account when reporting local control data following SRS for melanoma brain metastases.
REFERENCES
- 1. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005; 23(25): 6207-6219. [DOI] [PubMed] [Google Scholar]
- 2. Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol 1978; 19: 579-592. [PubMed] [Google Scholar]
- 3. Sheehan J, Niranjan A, Flickinger JC, Kondziolka D, Lunsford LD. The expanding role of neurosurgeons in the management of brain metastases. Surg Neurol 2004; 62(1): 32-40; discussion 40-1. [DOI] [PubMed] [Google Scholar]
- 4. Paterson AH, Agarwal M, Lees A, Hanson J, Szafran O. Brain metastases in breast cancer patients receiving adjuvant chemotherapy. Cancer 1982; 49(4): 651-4. [DOI] [PubMed] [Google Scholar]
- 5. Sundermeyer ML, Meropol NJ, Rogatko A, Wang H, Cohen SJ. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer 2005; 5(2): 108-113. [DOI] [PubMed] [Google Scholar]
- 6. Davis PC, Hudgins PA, Peterman SB, Hoffman JC., Jr Diagnosis of cerebral metastases: double-dose delayed CT vs contrast-enhanced MR imaging. AJNR J Am Neuroradiol 1991; 12(2): 293-300. [PMC free article] [PubMed] [Google Scholar]
- 7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009; 10(11): 1037-1044. [DOI] [PubMed] [Google Scholar]
- 8. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004; 363(9422): 1665-1672. [DOI] [PubMed] [Google Scholar]
- 9. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006; 295(21): 2483-2491. [DOI] [PubMed] [Google Scholar]
- 10. Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999; 45(2): 427-434. [DOI] [PubMed] [Google Scholar]
- 11. Byrne TN, Cascino TL, Posner JB. Brain metastasis from melanoma. J Neurooncol 1983; 1(4): 313-317. [DOI] [PubMed] [Google Scholar]
- 12. Enzmann DR, Kramer R, Norman D, Pollock J. Malignant melanoma metastatic to the central nervous system. Radiology 1978; 127(1): 177-180. [DOI] [PubMed] [Google Scholar]
- 13. Liew DN, Kano H, Kondziolka D, et al. Outcome predictors of Gamma Knife surgery for melanoma brain metastases. Clinical article. J Neurosurg 2011; 114(3): 769-779. [DOI] [PubMed] [Google Scholar]
- 14. Radbill AE, Fiveash JF, Falkenberg ET, et al. Initial treatment of melanoma brain metastases using gamma knife radiosurgery: an evaluation of efficacy and toxicity. Cancer 2004; 101(4): 825-833. [DOI] [PubMed] [Google Scholar]
- 15. Redmond AJ, Diluna ML, Hebert R, et al. Gamma Knife surgery for the treatment of melanoma metastases: the effect of intratumoral hemorrhage on survival. J Neurosurg 2008; 109 Suppl: 99-105. [DOI] [PubMed] [Google Scholar]
- 16. Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 2000; 47(2): 291-298. [DOI] [PubMed] [Google Scholar]
- 17. Hazard LJ, Wang B, Skidmore TB, et al. Conformity of LINAC-based stereotactic radiosurgery using dynamic conformal arcs and micro-multileaf collimator. Int J Radiat Oncol Biol Phys 2009; 73(2): 562-570. [DOI] [PubMed] [Google Scholar]
- 18. Samlowski WE, Watson GA, Wang M, et al. Multimodality treatment of melanoma brain metastases incorporating stereotactic radiosurgery (SRS). Cancer 2007; 109(9): 1855-1862. [DOI] [PubMed] [Google Scholar]
- 19. Jin JY, Ryu S, Faber K, et al. 2D/3D image fusion for accurate target localization and evaluation of a mask based stereotactic system in fractionated stereotactic radiotherapy of cranial lesions. Med Phys 2006; 33(12): 4557-4566. [DOI] [PubMed] [Google Scholar]
- 20. Solberg TD, Medin PM, Mullins J, S Li. Quality assurance of immobilization and target localization systems for frameless stereotactic cranial and extracranial hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys 2008; 71(1 Suppl): S131-135. [DOI] [PubMed] [Google Scholar]
- 21. Gottlieb JA, Frei E, 3rd, Luce JK. An evaluation of the management of patients with cerebral metastases from malignant melanoma. Cancer 1972; 29(3): 701-705. [DOI] [PubMed] [Google Scholar]
- 22. Doss LL, Memula N. The radioresponsiveness of melanoma. Int J Radiat Oncol Biol Phys 1982; 8(7): 1131-1134. [DOI] [PubMed] [Google Scholar]
- 23. Geara FB, Ang KK. Radiation therapy for malignant melanoma. Surg Clin North Am 1996; 76(6): 1383-1398. [DOI] [PubMed] [Google Scholar]
- 24. Isokangas OP, Muhonen T, Kajanti M, Pyrhonen S. Radiation therapy of intracranial malignant melanoma. Radiother Oncol 1996; 38(2): 139-144. [DOI] [PubMed] [Google Scholar]
- 25. Rades D, Heisterkamp C, Schild SE. Do patients receiving whole-brain radiotherapy for brain metastases from renal cell carcinoma benefit from escalation of the radiation dose? Int J Radiat Oncol Biol Phys 2010; 78(2): 398-403. [DOI] [PubMed] [Google Scholar]
- 26. Sampson JH, Carter JH, Jr., Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg 1998; 88(1): 11-20. [DOI] [PubMed] [Google Scholar]
- 27. Madajewicz S, Karakousis C, West CR, Caracandas J, Avellanosa AM. Malignant melanoma brain metastases. Review of Roswell Park Memorial Institute experience. Cancer 1984; 53(11): 2550-2552. [DOI] [PubMed] [Google Scholar]
- 28. Gupta G, Robertson AG, MacKie RM. Cerebral metastases of cutaneous melanoma. J Br Cancer 1997; 76(2): 256-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Selek U, Chang EL, Hassenbusch SJ, 3rd, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys 2004; 59(4): 1097-1106. [DOI] [PubMed] [Google Scholar]
- 30. Powell JW, Chung CT, Shah HR, et al. Gamma Knife surgery in the management of radioresistant brain metastases in high-risk patients with melanoma, renal cell carcinoma, and sarcoma. J Neurosurg 2008; 109 Suppl: 122-128. [DOI] [PubMed] [Google Scholar]
- 31. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: an alternative to whole brain radiotherapy? Neurosurgery 2003; 52(6): 1318-226; discussion 26. [DOI] [PubMed] [Google Scholar]
- 32. Brown PD, Brown CA, Pollock BE, Gorman DA, Foote RL. Stereotactic radiosurgery for patients with “radioresistant” brain metastases. Neurosurgery 2002; 51(3): 656-65; discussion 65-67. [PubMed] [Google Scholar]
- 33. Chang EL, Selek U, Hassenbusch SJ, 3rd, et al. Outcome variation among “radioresistant” brain metastases treated with stereotactic radiosurgery. Neurosurgery 2005; 56(5): 936-945; discussion 36-45. [PubMed] [Google Scholar]
- 34. Ulm AJ, Friedman WA, Bova FJ, Bradshaw P, Amdur RJ, Mendenhall WM. Linear accelerator radiosurgery in the treatment of brain metastases. Neurosurgery 2004; 55(5): 1076-1085. [DOI] [PubMed] [Google Scholar]
- 35. Shehata MK, Young B, Reid B, et al. Stereotatic radiosurgery of 468 brain metastases < or =2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys 2004; 59(1): 87-93. [DOI] [PubMed] [Google Scholar]
- 36. Mori Y, Kondziolka D, Flickinger JC, Kirkwood JM, Agarwala S, Lunsford LD. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys 1998; 42(3): 581-589. [DOI] [PubMed] [Google Scholar]
- 37. Lavine SD, Petrovich Z, Cohen-Gadol AA, et al. Gamma knife radiosurgery for metastatic melanoma: an analysis of survival, outcome, and complications. Neurosurgery 1999; 44(1): 59-64; discussion 64-66. [DOI] [PubMed] [Google Scholar]
- 38. Mathieu D, Kondziolka D, Cooper PB, et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery 2007; 60(3): 471-81; discussion 81-82. [DOI] [PubMed] [Google Scholar]
- 39. Grob JJ, Regis J, Laurans R, et al. Radiosurgery without whole brain radiotherapy in melanoma brain metastases. Club de Cancerologie Cutanee. Eur J Cancer 1998; 34(8): 1187-1192. [DOI] [PubMed] [Google Scholar]


