Abstract
Background
Prolonged hepatic warm ischemia during surgery remains a significant problem, particularly in the setting of liver resection and reduced remaining liver mass. The goal of the present study is to evaluate the effect of passive cooling caused by exposure to ambient conditions on hepatic injury in rats during warm ischemia followed by hepatectomy.
Methods
The left and median lobes of male rats were exposed to 75 min of ischemia under either normothermic (37°C) or mildly hypothermic (34°C) conditions. After 75 min of ischemia, the right lobe was resected, leaving the animal with only the remaining ischemic lobes. Animals were allowed to survive indefinitely or sacrificed at 4 h after reperfusion for determination of injury and inflammatory gene expression.
Results
Survival was already markedly higher in mildly hypothermic rats than normothermic rats at 24 h. Short passive cooling for the time course of the ischemic event significantly increased the hepatic induction of heat shock proteins 70 and 32 (both 3-fold versus normothermia, P < 0.05) in response to ischemia/reperfusion whereas it significantly decreased the induction of tumor necrosis factor-α (TNF-α) and macrophage inflammatory protein-2 (MIP-2) in the liver. Biochemical markers of hepatic injury were significantly lower in the passive cooling group than in normothermic animals: aspartate aminotransferase (AST) serum concentrations were 9277 ± 3461IU/L versus 15106 ± 4104IU/L (P<0.01), and alanine aminotransferase (ALT) levels 5986 ± 2246IU/L versus 9429 ± 3643IU/L (P<0.01).
Conclusion
We demonstrated in a clinically relevant model of hepatic ischemia/reperfusion that mild hypothermia significantly reduces hepatic injury and improves survival.
Keywords: warm ischemia, rats, hypothermic preconditioning, hepatectomy, hepatic injury, survival
INTRODUCTION
Hepatic resection frequently requires partial or complete occlusion of vascular inflow to achieve adequate control of bleeding. The severity of hepatic ischemia/reperfusion injury after unclamping hepatic vessels is a direct function of the length of time of occlusion as well as the underlying status of the liver. Partial or complete hepatic vascular occlusion is frequently used to decrease blood loss and allow for extensive resections. However, significant hepatic injury occurs in the setting of prolonged warm ischemia. Deliberate hypothermia has received significant attention as a means of protecting other organs against ischemia/reperfusion (I/R) injury. Specifically, induction of hypothermia is used to protect the brain in situations of out-of-hospital cardiac arrest and head trauma [1, 2], and mild hypothermia improves survival in experimental animals models of hemorrhagic shock [3, 4].
We and others have previously demonstrated that mild hypothermia is highly protective during warm ischemia and reperfusion of the liver [5, 6]. Some of the beneficial effects of hypothermia are preservation of hepatic metabolism during the ischemic phase and protection against oxidative stress and microcirculatory injury during the reperfusion phase. Of great importance and high clinical relevance is the observation that hypothermia has consistently been shown to provide protection against hepatic injury not only in several animal models [5–8], but also in humans [9–11].
In our previous report, we demonstrated that mild hypothermia provided a significant survival benefit to both lean and obese Zucker rats subjected to warm hepatic ischemia. In addition, we demonstrated that mild hypothermia (34°C) is sufficient to reduce I/R injury by inhibiting the inflammatory response as determined by myeloperoxidase (MPO) [6]. Further cooling to 31°C did not seem to provide any additional protective effect. Notably, in the previous studies, rats underwent 70% ischemia while the right lobe of the liver remained perfused. In the current study, the non-ischemic lobe was resected at the end of the warm ischemic period to exclude any contribution it might have to survival. Using this combined procedure, survival depends entirely on the functional integrity of the ischemic lobe. This has been described as a model of total hepatic ischemia [12], and is an excellent means for monitoring the protective effect of hypothermia. Complete clamping of the portal vein and hepatic artery in rats is problematic in that it induces significant bowel edema, and may confound the findings with respect to survival and inflammatory mediators that may be observed with bacterial translocation and bowel ischemia. Partial ischemia of the liver combined with hepatectomy, therefore, provides a cleaner model of pure hepatic ischemia.
One of the most potent mechanisms of organ protection during stress is the induction of the heat shock protein family, particularly heat shock proteins 70 (HSP70) and 32 (HSP32), or heme oxygenase-1 (HO-1), which can subsequently down-regulate inflammatory pathways known to influence ischemia reperfusion injury. HSPs can be induced in the liver in several ways, including heat exposure and expression via gene transfection [13]. Using a well-established warm I/R model with subsequent hepatectomy in rats, it was our goal to investigate the effect of clinically relevant mild hypothermia on heat shock protein factors, hepatic injury, and survival.
MATERIALS AND METHODS
Animal Model
All animal experiments were carried out at the University of California at San Francisco (UCSF) with approval by the UCSF Committee on Animal Research. Animals were treated in accordance with the National Institutes of Health guidelines for ethical research (NIH publication no. 80-123, revised 1985). Lean Zucker rats (Harlan, Indianapolis, IN) were used for this study. Upon arrival at our facility, rats weighed 275 to 325 grams and were 10 to 12 wk old. Animals had access to standard laboratory diet and were maintained on a 12-h light-dark cycle. Animals were acclimatized to their environment for at least 7 d before surgery. One group of normothermic and mildly hypothermic animals was enrolled in the survival study with 7 d survival as the anticipated endpoint. A second set of normothermic and mildly hypothermic animals was sacrificed 4 h after reperfusion for assessment of changes in acute phase proteins. Animals were anesthetized with isoflurane and the liver was exposed through a midline incision. Approximately 30 min were allowed to pass between the induction of anesthesia and the onset of ischemia. Normothermic rats were actively warmed with heat pads and heat lamps to maintain a body temperature of greater than 37°C as determined by continuous rectal temperature monitoring (Fig. 1). Anesthesia and surgery were performed in sham controls identical to those animals subjected to liver I/R except induction of ischemia. In the mild hypothermia group, rats were exposed to ambient room temperature. Animals were allowed to cool spontaneously. When temperatures fell below 34°C, animals were actively warmed to maintain rectal temperature approximately at 34°C (Fig. 1). This temperature is clinically acceptable and frequently deliberately used during neurosurgical procedures [14, 15]. In control animals, the body temperature of the animals was allowed to cool down spontaneously and maintained at 34°C in a heating pad for 75 min. In the 4-h reperfusion group, animals were allowed to wake up and then re-anesthetized and sacrificed for tissue collection.
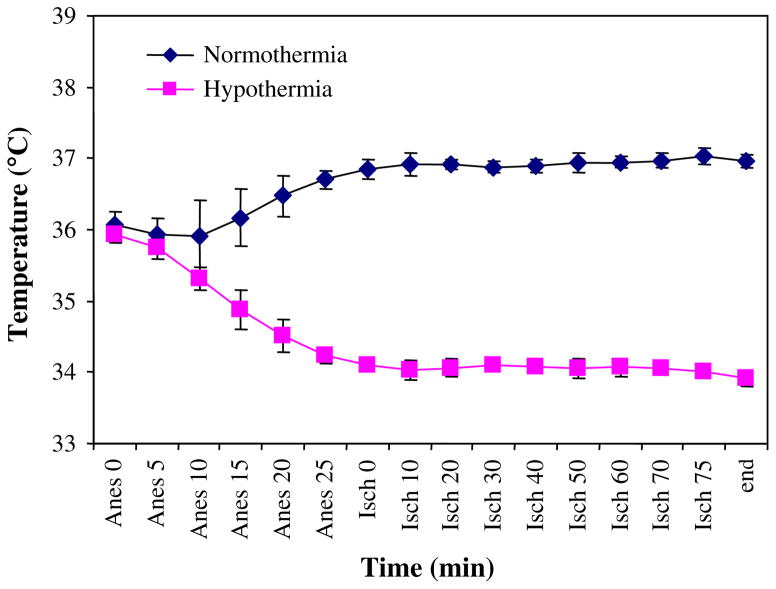
FIG. 1.
Temperature characteristics during the course of the experiment of the groups undergoing liver ischemia/reperfusion. Temperature was held constant at 37°C (normothermia) or allowed to fall and then held constant at 34°C (hypothermia). (Color version of Figure is available online.)
We selected 70% liver ischemia since this is a well-established and reproducible model in our laboratory and in the literature. In detail, vascular structures supplying the left and median lobes were identified and clamped for 75 min using a bulldog clamp. To confirm the appropriate placement of the clamps, the left and median lobes of the liver were inspected for ischemic color change. The perfused right and caudate lobes allowed outflow from the splanchnic circulation, avoiding venous congestion. During ischemia, the abdomen was lightly packed with moist sponges and the incision approximated with clamps. At the end of ischemia, the right and caudate lobe (nonischemic) was excised and the clamps were removed to allow reperfusion. Animals received approximately 5 mL of normal saline intraperitoneally before closure of the incision.
Animal Recovery and Survival
Postoperative recovery was closely monitored. Animal behavior and appearance were assessed every 30 min for 4 h. Animals were then sacrificed in the short-term reperfusion groups. In the survival groups, animal behavior and appearance were assessed every 30 min for 6 h before being returned to the animal facility at night. Animals were specifically evaluated for signs of poor clinical condition such as lethargy, ruffled fur, and guarding upon abdominal palpation, lack of grooming, and decreased food intake. Animals that exhibited signs of pain or suffering were sacrificed during the observation period. Upon sacrifice, all animals underwent necropsy. Animals with intra-abdominal pathology such as peritoneal hemorrhage were excluded from the study.
Markers of Hepatocellular Injury
Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured in all rats assigned to the 4-h reperfusion group and, when possible, in the 7-d reperfusion group (IDDEX Veterinary Services, Sacramento, CA).
Evaluation of Liver Histology
The remaining hepatic lobes were excised, fixed in 10% formalin, and then embedded in paraffin for light microscopy. Sections were cut at 5 μm and stained with hematoxylin and eosin for histological examination. All specimens in the 4-h reperfusion group were evaluated by a pathologist who was blinded to normothermia or hypothermia condition. Tissues were inspected at ×4, ×10, and ×40 magnification. Hepatic necrosis was assessed as percent of total tissue area on the following scale: <5%, 5%–25%, 25%–50%, 50%–75%, and>75%.
Real-Time Quantitative PCR
Total RNA was isolated from liver tissues using TRIzol reagent (Invitrogen, Carlsbad, CA). One μg total RNA was reverse-transcribed to cDNA according to manufacturer’s protocol using 200 U MMLV-Reverse Transcriptase with 250 ng of random primers (Invitrogen, Carlsbad, CA). The expression of several genes including tumor necrosis factor-α (TNF-α), macrophage inflammatory protein-2 (MIP-2), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), IL-6, and monocyte chemoattractant protein-1 (MCP-1) was assessed via quantitative real-time PCR using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Table 1 shows primers and 5′-FAM/3′ Black Hole Quencher-labeled TaqMan probes designed with ABI Primer Express 2.0 software and synthesized by Integrated DNA Technologies (Coralville, IA). Each primer-probe combination was validated to within the accepted 95% to 105% efficiency. Fifty ng of reverse-transcribed cDNA was used as template along with 500 nM forward and reverse primers, 200 nM TaqMan probe, 200 nM dNTPs (Allstar Scientific, Sunnyvale, CA), 5.5 mM MgCl2, and TaqMan buffer (Applied Biosystems, Foster City, CA) for PCR amplification. Fluorescence was measured after each PCR cycle (initial one-time 10 min denaturation at 95°C, followed by 40 amplification cycles with 15 s denaturation at 95°C and 1 min annealing-extension at 60°C). Each sample was measured in triplicate and all genes were normalized to the endogenous control β-actin. Negative controls processed without reverse transcriptase were run in duplicate and found to lack contamination by genomic DNA. Target gene expression was normalized to that of sham controls by the comparative C(t) method using threshold cycle C(t) values determined on ABI GeneAmp software.
TABLE 1.
Sequences of Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Primers and TaqMan Probes
| Gene | GenBank Accession no. | Sequence (5′ –> 3′) | |
|---|---|---|---|
| β-actin | NM_031144 | Forward | CTGGCTCCTAGCACCATGAAG |
| Reverse | GAGCCACCAATCCACACAGA | ||
| Probe | TCAAGATCAT TGCTCCTCCT GAGCG | ||
| TNFα | NM_012675 | Forward | CCA AAT GGG CTC CCT CTC AT |
| Reverse | GGG CTA CGG GCT TGT CAC T | ||
| Probe | TGG CCC AGA CCC TCA CAC TCA GAT CA | ||
| MIP2 | NM_053647 | Forward | GAA GCC CCC TTG GTT CAG A |
| Reverse | GCC CAT GTT CTT CCT TTC CA | ||
| Probe | TCC AAA AGA TAC TGA ACA AAG GCA AGG CTA ACT | ||
| MCP-1 | NM_031530 | Forward | CTG TCT CAG CCA GAT GCA GTT AA |
| Reverse | AGC CGA CTC ATT GGG ATC AT | ||
| Probe | CCC CAC TCA CCT GCT GCT ACT CAT TCA C | ||
| IL-6 | NM_012589 | Forward | ATATGTTCTCAGGGAGATCTTGGAA |
| Reverse | TGCATCATCGCTGTTCATACAA | ||
| Probe | TGAGAAAAGAGTTGTGCAATGGCAATTCTG | ||
| ICAM-1 | NM_012967 | Forward | CAC AAG GGC TGT CAC TGT TCA |
| Reverse | CCC TAG TCG GAA GAT CGA AAG TC | ||
| Probe | AAT GTC TCC GAG GTC AGG CAG CTC C | ||
| VCAM-1 | NM_012889 | Forward | GAA GGA AAC TGG AGA AGA CAA TCC |
| Reverse | TGT ACA AGT GGT CCA CTT ATT TCA ATT | ||
| Probe | TGG AGG TCT ACT CAT TCC CTG AAG ACC CA |
Western Immunoblotting
Liver tissues were homogenized in a buffer containing 50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 0.15 M NaCl, 0.25 M sucrose, 1 mM DTT, and protease inhibitor, and centrifuged at 2000 g for 20 minutes at 4°C. The supernatant was aliquoted, snap-frozen and stored at −80°C. Liver homogenates were assayed for protein concentration by the Pierce BCA protein assay (Pierce Biotechnology, Rockford, IL) with BSA as the standard. Fifty μg of liver protein were separated on a NOVEX-NuPAGE 10% Bis-Tris SDS-PAGE gel (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membrane using the XCell SureLock system (Invitrogen, Carlsbad, CA). Mouse anti-HSP70 monoclonal antibody, goat anti-TNFα polyclonal antibody (SC-1349), goat anti-MIP2 polyclonal antibody (SC-1388), and goat anti-actin antibody (SC-1616) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). In addition, mouse anti-HSP32 monoclonal antibody was purchased from Stressgen (Ann Arbor, MI) and rabbit anti-cleaved caspase-3 monoclonal antibody was purchased from Cell Signaling (Danvers, MA). The membranes were incubated with a 1:100 dilution of anti-TNFα and anti-MIP2 antibody, 1:500 dilution of anti-HSP70 and anti-actin antibody, and 1:1000 dilution of anti-HSP32 and cleaved caspase-3 antibody followed by a 1:10,000- fold dilution of a secondary anti-mouse or anti-goat IgG from Santa Cruz Biotechnology (Santa Cruz, CA). Immunoreactive proteins were developed using SuperSignal West Dura (Pierce Biotechnology, Rockford, IL) and visualized using FluorChem system from Alpha Innotech (San Leandro, CA). Band intensities were quantified via spot densitometry and first normalized to actin and then normalized to sham control.
Statistical Analysis
All data are presented as mean±SD. Data obtained by real-time quantitative PCR and western immunoblotting were compared by analysis of variance with post hoc Dunnett’s test using a sham group as the control. Experimental groups were compared by unpaired two tailed t-test. Survival groups were compared using the Fisher exact test. P values < 0.05 were considered statistically significant.
RESULTS
Animal Recovery after Surgery and Survival
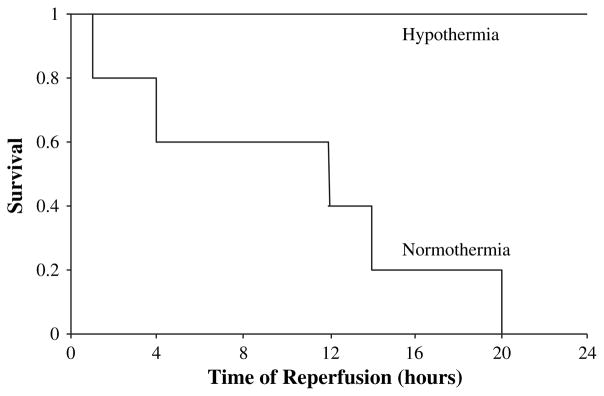
All animals (n = 10 normothermic and n = 10 hypothermic) in the 4-h reperfusion study survived the observation period. Temperature curves are depicted in Fig. 1. None of the animals in the normothermic I/R group (n = 5) survived the first 24 h of reperfusion. In contrast, after 24 h of reperfusion, all animals survived in the hypothermic group (n = 5) and were in good clinical condition (Fig. 2, P < 0.01).
FIG. 2.
After 24 h of reperfusion, all five hypothermic rats were alive and in good condition. In the normothermic rat group, none of the five rats survived 24 h of reperfusion. Survival was significantly improved in mildly hypothermic rats when compared to normothermic rats. *P < 0.01.
Hepatocellular Injury
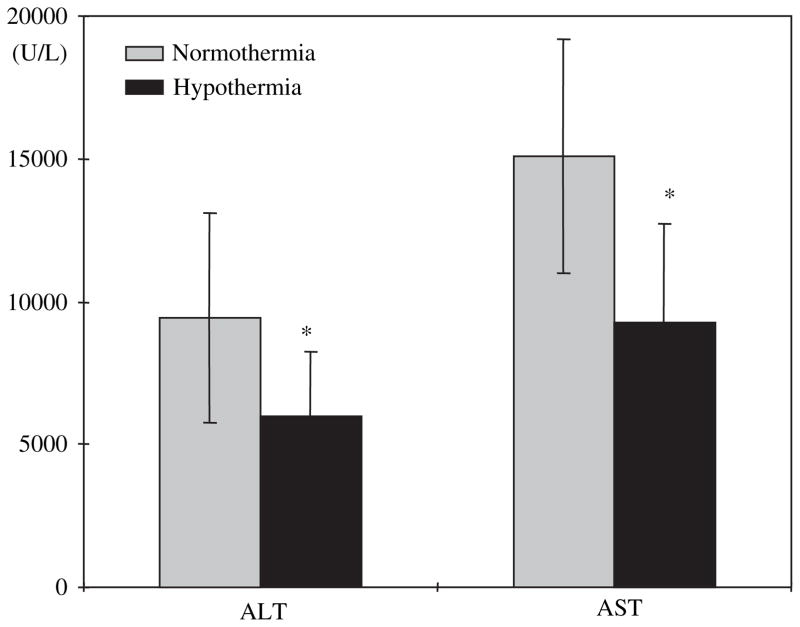
After 4 h of reperfusion, mildly hypothermic rats (n = 10) had significantly lower levels of serum AST than normothermic rats (n = 10) (9277±3461 IU/L versus 15106±4104 IU/L, P < 0.01, Fig. 3). Serum ALT levels followed the same pattern as AST (5986±2246 IU/L in mildly hypothermic rats versus 9429±3643 IU/L in normothermic rats, P < 0.01, Fig. 3).
FIG. 3.
Serum AST and ALT measured after 75 min of ischemia under normothermic or mildly hypothermic conditions, followed by 4 h of reperfusion. Mildly hypothermic rats had significantly lower AST and ALT levels than normothermic rats. Values represent mean±SD for n = 10. *P < 0.05.
Histological Evaluation
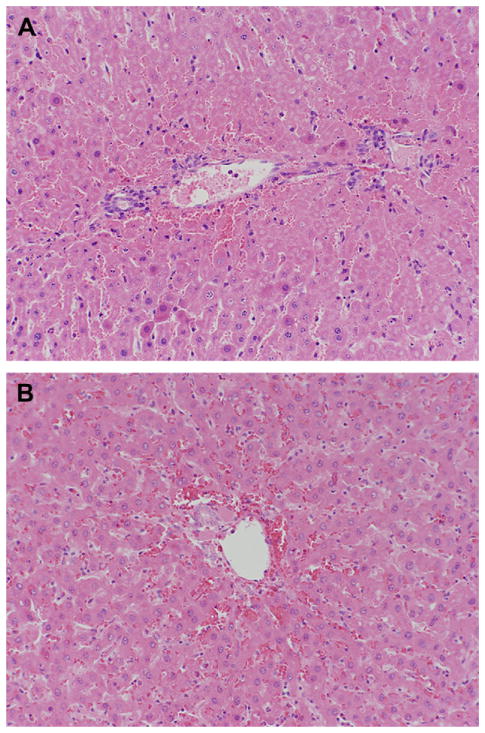
Both normothermic and mildly hypothermic rats demonstrated moderate to severe hepatic congestion on histological evaluation at 4 h of reperfusion. Histologic necrosis at 4 h accounted for less than 15% of the total liver area in all rats, regardless of normo- or hypothermia (Fig. 4A and 4B). This small percentage of necrosis is consistent with the current understanding that hepatic necrosis is still evolving at 4 h of reperfusion. After 24 h of reperfusion, liver tissue could not be examined histologically because none of the animals in the normothermic group survived.
FIG. 4.
Representative liver histology of liver samples after 75 min of ischemia under normothermic or mildly hypothermic conditions, followed by 4 h of reperfusion. (A) Illustrates a liver exposed to normothermic I/R. (B) Illustrates a liver exposed to hypothermic I/R. Necrosis accounted for less than 15% of the total liver area in normo- as well as hypothermic rats. Sections were cut at 5 μm and stained with hematoxylin and eosin for histological examination at magnification ×20. (Color version of Figure is available online.)
Cleaved Caspase-3 Immunoblotting
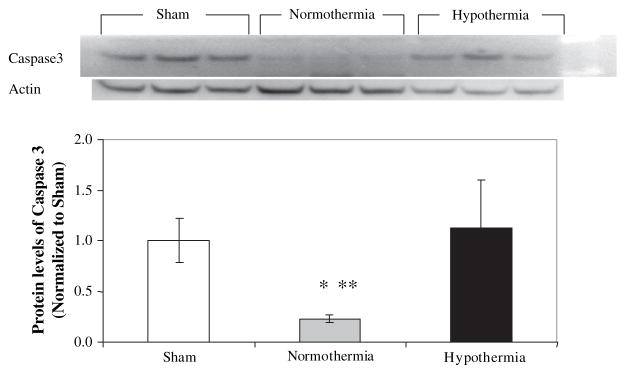
Cleaved caspase-3 levels were not different in the livers from hypothermic animals compared with the protein levels in the livers from sham control animals. However, the protein levels of the cleaved caspase-3 were significantly lower by more than 4-fold in the livers from normothermic animals (P < 0.05) compared with either sham control or hypothermic animals (Fig. 5).
FIG. 5.
Apoptosis was reduced in normothermic rats. Representative immunoblot for cleaved caspase-3 in livers after I/R along with densitometric analysis. Cleaved caspase-3 protein levels were measured by Western blot in 50 μg of liver homogenates. Densitometric values were normalized to actin and are expressed as a ratio of sham±SD with n = 3 in each group. * P < 0.01 versus sham and **P < 0.01 versus normothermia.
Quantitative Real-Time PCR and Western Blot Analysis
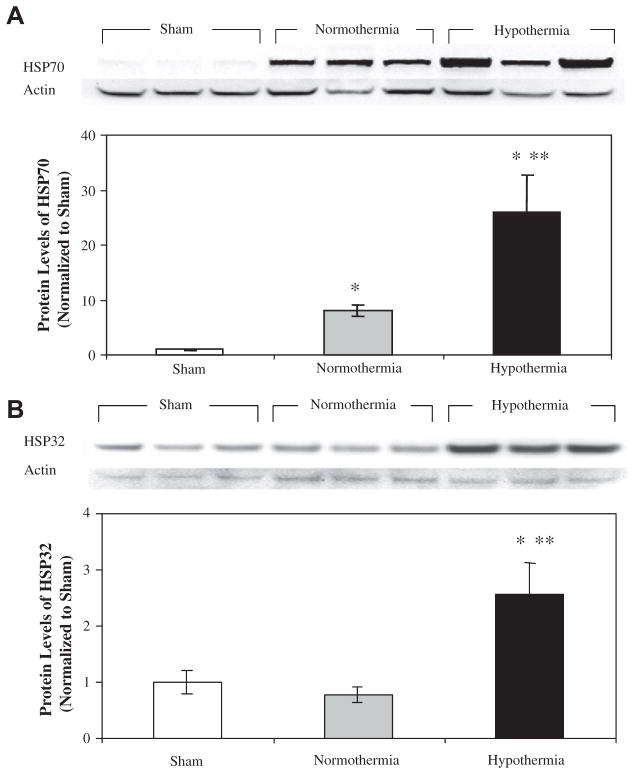
HSP70 and HSP32 were measured in the livers of sham, normothermic I/R and hypothermic I/R animals. HSP70 levels were 8-fold higher in normothermic I/R and 26-fold higher in hypothermic I/R animals than sham controls (P < 0.01, Fig. 6A). HSP70 was 3-fold higher in the hypothermic I/R group than in the normothermic I/R group (P < 0.01). HSP32 levels were almost 3-fold higher in the hypothermic I/R group (P < 0.01) than the normothermic I/R and sham groups (Fig. 6B). There was no significant difference between the normothermic I/R and sham groups. To determine whether passive cooling itself without surgical stress could induce HSP70 and HSP32, animals were cooled but not subjected to ischemia or hepatectomy. Neither HSP70 nor HSP32 was elevated in this control group (Fig. 6C). Thus, passive cooling alone does not induce these particular HSPs.
FIG. 6.
HSP70 and HSP32 in rat liver after sham operation or hepatic I/R. HSP70 (A) and HSP32 (B) were measured by Western blot after sham operation or 75 min of ischemia and 4 h of reperfusion; 50 μg of liver homogenate were analyzed; immunoreactive bands were quantitated by densitometry, normalized to actin, and expressed as mean±S.D. for n = 3. HSP70 and HSP32 levels were both induced by hepatic I/R. Protein levels were significantly higher in hypothermic livers than normothermic livers. *P < 0.01 versus sham and **P < 0.01 versus normothermia.
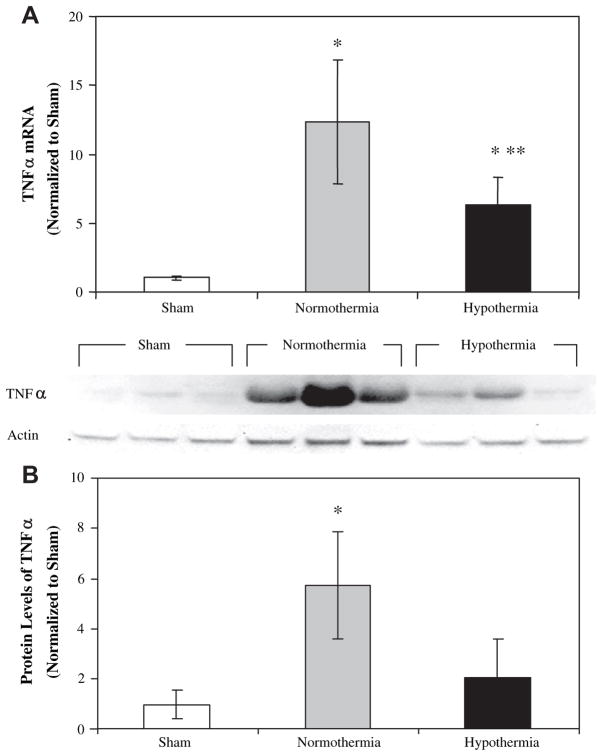
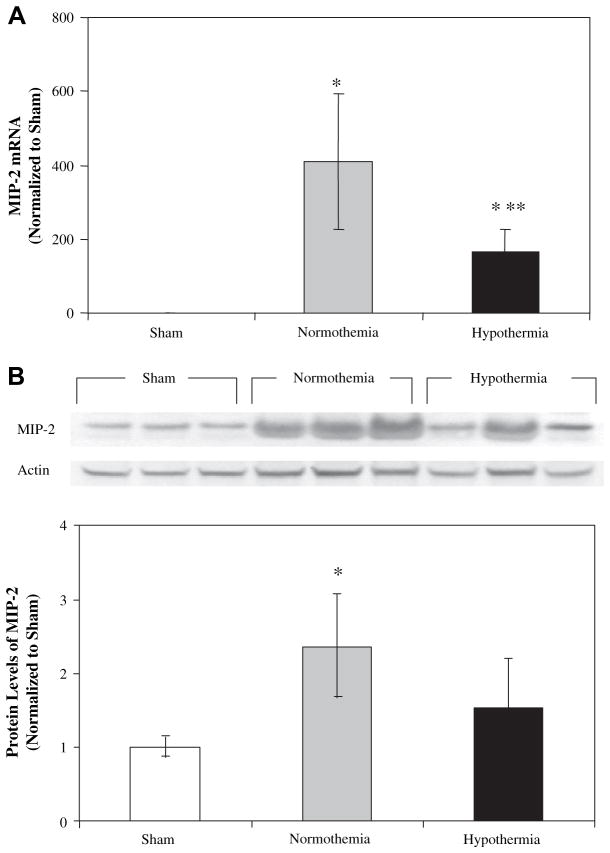
TNFα mRNA was significantly elevated after both normothermic and hypothermic I/R relative to sham (P < 0.01), and was nearly 2-fold higher in normothermic animals than hypothermic animals (P < 0.05) (Fig. 7A). Accordingly, TNF-α was induced by I/R in the same pattern. Hepatic TNF-α levels after I/R were almost 3-fold higher in the normothermic group than the hypothermic group (Fig. 7B). Like TNF-α, MIP-2 mRNA was induced in response to I/R in both normothermic and hypothermic groups (P < 0.01) (Fig. 8A). MIP-2 mRNA levels were 2.5-fold higher in normothermic animals than hypothermic animals (P < 0.05). MIP-2 levels roughly paralleled MIP-2 gene expression in the liver (Fig. 8B). Levels were highest in the normothermic I/R group (P < 0.05 versus sham) followed by the hypothermic I/R and sham groups.
FIG. 7.
TNFα mRNA and protein in rat liver after sham operation or hepatic I/R. TNFα mRNA was measured by TaqMan qRT-PCR in liver samples after sham operation or 75 min of ischemia and 4 h of reperfusion (A). Values were normalized to β-actin and expressed as mean±SD for n = 3 in the sham group and n = 5 in the normothermic and hypothermic I/R groups. TNFα protein was measured by Western blot (B); 50 μg of liver homogenate were analyzed. Immunoreactive bands were quantitated by densitometry, normalized to actin and expressed as mean±SD for n = 3. *P < 0.05 versus sham and **P < 0.05 versus normothermia.
FIG. 8.
MIP-2 expression in rat liver after sham operation or hepatic I/R. MIP-2 mRNA was measured by TaqMan qRT-PCR in liver samples after sham operation or 75 min of ischemia and 4 h of reperfusion (A). Values were normalized to β-actin and expressed as mean±SD for n = 3 in sham control group and n = 5 each in the normothermic and hypothermic I/R groups. MIP-2 protein was measured by Western blot (B); 50 μg of liver homogenate were analyzed. Immunoreactive bands were quantitated by densitometry, normalized to actin and expressed as mean±SD for n = 3. * P < 0.05 versus sham and **P < 0.05 versus normothermia.
MCP-1 and IL-6 mRNA levels were significantly increased after I/R relative to sham, but there was no difference in gene expression between rats exposed to I/R under normothermic or hypothermic conditions (data not shown). ICAM-1 and VCAM-1 mRNA level was 2- to 3-fold higher in livers after I/R relative to sham (P < 0.05), but there was no significant difference between normothermic and hypothermic animals (data not shown).
DISCUSSION
In this study, we demonstrated in a warm ischemia model with subsequent resection of the nonischemic lobe that exposure to passive cooling due to anesthesia- induced vasodilatation confers significant protection against hepatic injury. Seventy-five minutes of warm ischemia was chosen because it causes noticeable hepatic injury in lean rats and has clinical relevance. The experimental model resulted in animals that were only left with hepatic lobes that were injured during warm ischemia, since the nonischemic right lobe was resected at the end of ischemia. Since rats can survive on remaining hepatic mass significantly less than 30%, the resection of the nonischemic lobes eliminates the possibility that animals in this study survived based on preserved function of the noninjured hepatic lobes. Our survival results impressively demonstrate this with a mortality of 100% in the normothermic group at 24 h reperfusion compared to a mortality of 0% in mildly hypothermic rats.
As already mentioned, at 4 h reperfusion, the injury is still evolving and the degree of injury may not yet reflect in the extent of necrosis. This is consistent with our findings that while hepatocytes appeared to be swollen in both groups, necrosis accounted for less than 15% of the total liver area in normo- as well as hypothermic rats. Subsequently, we examined the degree of apoptosis evaluated by cleaved caspase-3 levels. Interestingly, normothermia resulted in significant reduction of cleaved caspase-3 levels (Fig. 5). These results are surprising since hypothermia is generally thought to reduce apoptosis. A repeated assay performed by a different laboratory technician resulted in the same findings; hence results appear to be accurate and reproducible. There are several possible explanations. First, we were only able to sample at a very early time point (4 h of reperfusion) since none of the normothermic rats were alive at 24 h after reperfusion. Apoptosis is a dynamic ongoing process, which is likely not to be entirely completed at 4 h after reperfusion. Determination of cleaved caspase-3 at a later time point may have resulted in different findings. Secondly, while there are several different pathways that may induce apoptosis, increased apoptotic activity requires energy in order to undergo programmed cell death [16, 17]. In contrast, necrotic cell death is basically an energy independent process. In the present model, injury in the normothermic group is very severe, resulting in 100% mortality. In the normothermic group, it is possible that energy depletion was very profound and energy recovery incomplete. As a result, less hepatocytes entered the apoptotic cell death pathway in the normothermic group compared with the hypothermic group and, instead, entered the necrotic cell death pathway. Hepatic ischemia reperfusion is frequently divided in an acute phase occurring within 4 to 6 h and a sub-acute phase lasting up to 24 h. During the acute phase, increased expression of molecular chaperones such as HSP70 and HSP32 as well as hepatic induction of cytokines (e.g., TNF-α) and chemokines (e.g., MCP-1 or MIP-2) have been observed [18, 19].
In the sub-acute phase, leukocytes are an important component of the inflammatory response to injury [20]. Our group has previously demonstrated that mild hypothermia protects hepatic metabolism and oxidative phosphorylation [21], but it is very likely that the observed metabolic protection under mildly hypothermic conditions only partially explains the protective effect of mild hypothermia.
In this acute phase study of hepatic ischemia reperfusion injury, we demonstrated several significant differences between several transcription factors and downstream genes. Although some were predictable, others were novel.
To our knowledge, this is the first report in which mild hypothermia is associated with significant up-regulation of HSP70 and HSP32 during hepatic ischemia followed by resection of the nonischemic lobe. HSP70 and HSP32 have both been shown to be protective in other hepatic ischemia models [22, 23]. These proteins are believed to prevent liver injury by limiting oxidative stress and inflammatory response. HSP70 activation is known to reduce nuclear factor-kB activation in hepatocytes [24]. Induction of HSP70 by arsenite before partial hepatic ischemia in mice has been shown to result in a striking reduction of liver injury that was associated with reduced liver neutrophil recruitment and nuclear factor-κB (NFκB) activation [23]. The reduced NFκB activation led to reduced serum levels of TNFα and MIP-2, but increased the level of interleukin- 6. These findings on HSP70-induced chemokine induction match perfectly with the finding of our present study. Over-expression of HSP32 has been considered in part a response to variety of noxious stimuli [25], but also is considered to have anti-inflammatory properties [26]. Activation of HSP32 ameliorates liver IRI by down-regulating STAT-1 phosphorylation in the type-1 IFN pathway downstream of TLR4, with resultant inhibition of CXCL-10 [27]. The chemokine CXCL-10 is a key mediator in the neutrophil-mediated phase of hepatic inflammation and IRI.
Of interest is the fact that up-regulation of HSP70 and HSP32 was not observed in hypothermic sham animals. This may indicate that up-regulation of heat shock proteins in the hypothermic liver may be a result of reduced I/R injury in the hypothermic animals, allowing for a more robust recovery and increased protein synthesis (Fig. 6).
The importance of the cytokine TNF-α induction on liver injury is well established and confirmed in our study. TNF-α plays a crucial role in induction of inflammatory pathways and apoptosis through different membrane receptors TNF-R1 and TNF-R2 [28]. TNF-R1 is considered the primary signaling TNF receptor for both apoptosis and inflammation. When TNF-R1-deficient mice were subjected to I/R or when wild-type mice were pretreated with the TNF inhibitor pentoxifylline prior to I/R, postoperative liver injury was significantly diminished compared with that of control mice [12]. Of interest in the present study is that hepatoprotection was associated with significant suppression of hepatic TNF-α mRNA and protein. Oxidant stress is an important inducer of TNF-α. Several studies have demonstrated that modification of oxidative stress may change TNF-α expression [12]. In the context of this study, HSP32 may be limiting TNF-α induction by virtue of its ability to modify cellular response to oxidant stress. HSP32-mediated blunting of the rise in TNF-α after I/R can have downstream effects, such as suppression of the leukocyte chemoattractant MIP-2. This could lead to reduced inflammation in the late phase of hepatic I/R and ultimately lead to increased survival.
Concerns to clinicians are side effects of perioperative hypothermia such as cardiac arrhythmia, coagulopathy, and susceptibility to wound infection. It is important to note, however, that complications of hypothermia have typically been encountered in the setting of massive resuscitation following trauma in critically ill patients in the ICU. When performed in a controlled setting such as elective surgery, hypothermia is less of a concern. Indeed, mild intraoperative hypothermia (defined as core temperatures between 34 and 36°C) is commonly encountered even in the absence of purposeful cooling. This mild degree of hypothermia is not linked to adverse sequelae of hypothermia such as coagulopathy and cardiac arrhythmias. Although some may be cautious about considering hypothermia for hepatic surgery due to anticipated blood loss, it should be emphasized that refinement of surgical techniques and intraoperative-management make this less of a concern. Indeed, in the majority of liver resections, blood loss is less than 1 to 2 L [29, 30].
Hypothermia has been shown to provide protection against hepatic injury in several animal models and human studies. Methods of cooling have included total body cooling, hypothermic perfusion, extracorporeal cooling, and topical cooling. However, the temperatures used experimentally are often clinically unacceptable or require in situ manipulation with selective perfusion. In humans, several reports demonstrated that localized cooling (e.g., selective perfusion of the liver with cold solution) may decrease hepatic injury during warm ischemia [31–33]. Our data indicate that even simple spontaneous cooling as often seen after induction of anesthesia may be sufficient to confer a protective effect on the liver during hepatic resection.
In conclusion, we have demonstrated that anesthesia- induced mild hypothermia significantly reduces hepatic injury and improves survival in a warm I/R rat model. These findings extend our previous work in which we reported that hypothermia protects both normal and steatotic livers from I/R injury in the absence of hepatectomy. The reduced injury pattern and excellent survival in the hypothermic group was associated with significant up-regulation of cytoprotective HSP70 and HSP32 and concomitant suppression of the pro-inflammatory mediators TNF-α and MIP-2. The findings are of clinical relevance since anesthesia-induced mild hypothermia is a common occurrence in the operating room, unless patients are actively warmed. Our findings raise caution that active warming of patients during hepatic resections may have an adverse effect on postoperative outcome. At the same time, they indicate that a potentially life-saving maneuver (mild hypothermia) can be performed with no special instrumentation or set-up, and can be implemented on short notice as mandated by the clinical situation.
Acknowledgments
This study was in part supported by a grant from the Foundation of Anesthesia Research and Education (CUN), the Hellman Family Award (CUN), and the National Institutes of Health (DK68450, JJM), Cell and Tissue Biology Core Facility of the UCSF Liver Center (DK26743).
References
- 1.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre LA, Fergusson DA, Hebert PC, et al. Prolonged therapeutic hypothermia after traumatic brain injury in adults: A systematic review. JAMA. 2003;289:2992. doi: 10.1001/jama.289.22.2992. [DOI] [PubMed] [Google Scholar]
- 3.Takasu A, Norio H, Gotoh Y, et al. Effect of induced-hypothermia on short-term survival after volume-controlled hemorrhage in pigs. Resuscitation. 2003;56:319. doi: 10.1016/s0300-9572(02)00405-7. [DOI] [PubMed] [Google Scholar]
- 4.Kentner R, Rollwagen FM, Prueckner S, et al. Effects of mild hypothermia on survival and serum cytokines in uncontrolled hemorrhagic shock in rats. Shock. 2002;17:521. doi: 10.1097/00024382-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Choi S, Noh J, Hirose R, et al. Mild hypothermia provides significant protection against ischemia/reperfusion injury in livers of obese and lean rats. Ann Surg. 2005;241:470. doi: 10.1097/01.sla.0000154259.73060.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrends M, Hirose R, Serkova NJ, et al. Mild hypothermia reduces the inflammatory response and hepatic ischemia/reperfusion injury in rats. Liver Int. 2006;26:734. doi: 10.1111/j.1478-3231.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 7.Biberthaler P, Luchting B, Massberg S, et al. The influence of organ temperature on hepatic ischemia-reperfusion injury: A systematic analysis. Transplantation. 2001;72:1486. doi: 10.1097/00007890-200111150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Heijnen BH, Straatsburg IH, Gouma DJ, et al. Decrease in core liver temperature with 10 degrees C by in situ hypothermic perfusion under total hepatic vascular exclusion reduces liver ischemia and reperfusion injury during partial hepatectomy in pigs. Surgery. 2003;134:806. doi: 10.1016/s0039-6060(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 9.Azoulay D, Eshkenazy R, Andreani P, et al. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg. 2005;241:277. doi: 10.1097/01.sla.0000152017.62778.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YI, Hwang YJ, Lee JW, et al. 101 hepatectomies under continuous inflow occlusion following simple in situ liver cooling in patients with chronic liver diseases. Hepatogastroenterology. 2004;51:1093. [PubMed] [Google Scholar]
- 11.Yamanaka N, Dai CL, Okamoto E. Historical evolution of hypothermic liver surgery. World J Surg. 1998;22:1104. doi: 10.1007/s002689900525. [DOI] [PubMed] [Google Scholar]
- 12.Rudiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 13.Coito AJ, Buelow R, Shen XD, et al. Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury. Transplantation. 2002;74:96. doi: 10.1097/00007890-200207150-00017. [DOI] [PubMed] [Google Scholar]
- 14.Leslie K, Bjorksten AR, Ugoni A, et al. Mild core hypothermia and anesthetic requirement for loss of responsiveness during propofol anesthesia for craniotomy. Anesth Analg. 2002;94:1298. doi: 10.1097/00000539-200205000-00045. [DOI] [PubMed] [Google Scholar]
- 15.Iwata T, Inoue S, Kawaguchi M, et al. Comparison of the effects of sevoflurane and propofol on cooling and rewarming during deliberate mild hypothermia for neurosurgery. Br J Anaesth. 2003;90:32. [PubMed] [Google Scholar]
- 16.Clavien PA, Rudiger HA, Selzner M. Mechanism of hepatocyte death after ischemia: Apoptosis versus necrosis. Hepatology. 2001;33:1555. doi: 10.1053/jhep.2001.0103306le02. [DOI] [PubMed] [Google Scholar]
- 17.Rudiger HA, Graf R, Clavien PA. Liver ischemia: Apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149. [PubMed] [Google Scholar]
- 18.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury–A fresh look. Exp Mol Pathol. 2003;74:86. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 21.Niemann CU, Choi S, Behrends M, et al. Mild hypothermia protects obese rats from fulminant hepatic necrosis induced by ischemia-reperfusion. Surgery. 2006;140:404. doi: 10.1016/j.surg.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Carini R, Albano E. Recent insights on the mechanisms of liver preconditioning. Gastroenterology. 2003;125:1480. doi: 10.1016/j.gastro.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Kuboki S, Schuster R, Blanchard J, et al. Role of heat shock protein 70 in hepatic ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1141. doi: 10.1152/ajpgi.00491.2006. [DOI] [PubMed] [Google Scholar]
- 24.Andres D, Diez-Fernandez C, Castrillo A, et al. Relationship between the activation of heat shock factor and the suppression of nuclear factor-kappaB activity in rat hepatocyte cultures treated with cyclosporine A. Biochem Pharmacol. 2002;64:247. doi: 10.1016/s0006-2952(02)01115-2. [DOI] [PubMed] [Google Scholar]
- 25.Bauer M, Bauer I. Heme oxygenase-1: Redox regulation and role in the hepatic response to oxidative stress. Antioxid Redox Signal. 2002;4:749. doi: 10.1089/152308602760598891. [DOI] [PubMed] [Google Scholar]
- 26.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchihashi S, Zhai Y, Bo Q, et al. Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: Inhibition of type-1 interferon signaling. Transplantation. 2007;83:1628. doi: 10.1097/01.tp.0000266917.39958.47. [DOI] [PubMed] [Google Scholar]
- 28.Schwabe RF, Brenner DA. Mechanisms of liver injury. I. TNF-alpha-induced liver injury: Role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 29.Smyrniotis V, Kostopanagiotou G, Theodoraki K, et al. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Otsubo T, Takasaki K, Yamamoto M, et al. Bleeding during hepatectomy can be reduced by clamping the inferior vena cava below the liver. Surgery. 2004;135:67. doi: 10.1016/s0039-6060(03)00343-x. [DOI] [PubMed] [Google Scholar]
- 31.Yamanaka N, Furukawa K, Tanaka T, et al. Topical cooling-assisted hepatic segmentectomy for cirrhotic liver with hepatocellular carcinoma. J Am Coll Surg. 1997;184:290. [PubMed] [Google Scholar]
- 32.Kim YI, Hiratsuka K, Kitano S, et al. Simple in situ hypothermia reduced ischaemic injury to human liver during hepatectomy. Eur J Surg. 1996;162:717. [PubMed] [Google Scholar]
- 33.Hannoun L, Delriviere L, Gibbs P, et al. Major extended hepatic resections in diseased livers using hypothermic protection: Preliminary results from the first 12 patients treated with this new technique. J Am Coll Surg. 1996;183:597. [PubMed] [Google Scholar]