Abstract
Arrhythmias in the neonatal period are not uncommon, and may occur in neonates with a normal heart or in those with structural heart disease. Neonatal arrhythmias are classified as either benign or nonbenign. Benign arrhythmias include sinus arrhythmia, premature atrial contraction, premature ventricular contraction, and junctional rhythm; these arrhythmias have no clinical significance and do not need therapy. Supraventricular tachycardia, ventricular tachycardia, atrioventricular conduction abnormalities, and genetic arrhythmia such as congenital long-QT syndrome are classified as nonbenign arrhythmias. Although most neonatal arrhythmias are asymptomatic and rarely life-threatening, the prognosis depends on the early recognition and proper management of the condition in some serious cases. Precise diagnosis with risk stratification of patients with nonbenign neonatal arrhythmia is needed to reduce morbidity and mortality. In this article, I review the current understanding of the common clinical presentation, etiology, natural history, and management of neonatal arrhythmias in the absence of an underlying congenital heart disease.
Keywords: Arrhythmias, Neonate
Introduction
Arrhythmias in the neonatal period are not uncommon, and may occur in neonates with a normal heart or in those with structural heart disease. The incidence of neonatal arrhythmia is reported to be 1%–5% in all neonates1,2,3). The clinical manifestation is variable. Neonatal arrhythmias are classified as either benign or nonbenign. Benign arrhythmias include sinus arrhythmia, premature atrial contraction (PAC), premature ventricular contraction (PVC), and junctional rhythm; these arrhythmias have no clinical significance and do not need therapy. Supraventricular tachycardia (SVT), ventricular tachycardia (VT), atrioventricular (AV) conduction abnormalities, and genetic arrhythmias such as congenital long-QT syndrome (LQTS) are classified as nonbenign arrhythmias1,2,3,4,5). Congenital heart disease (CHD) not only affects the anatomical defect but also causes electrical changes, that induce various arrhythmias. In addition, surgical correction may also be related to the cause of arrhythmia in patients with CHD. Previous studies reported that arrhythmic substrates predispose patients to CHD-related arrhythmia, and the clinical manifestations and treatment differ between patients with a normal heart and those with CHD. In this article, I review the current understanding of the common clinical presentations, etiology, natural history, and management of neonatal arrhythmias in patients without underlying CHD.
Premature contraction or extrasystoles
Premature atrial and ventricular complexes are common arrhythmias and may occur in patterns of bigeminy, trigemini, or couplets.
1. Premature atrial contractions
PACs are common in the neonatal period and manifest as irregular heartbeats. In a previous study, PACs were detected on Holter electrocardiogram (ECG) in 51% of normal newborns6).
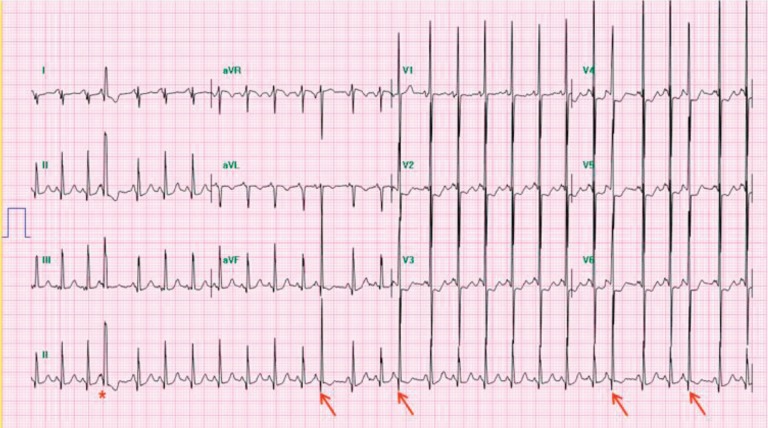
A premature P wave superimposed on the previous T wave can cause deformation of the T wave. Nonconduction of PACs can sometimes occur and be misdiagnosed as sinus bradycardia in neonatal intensive care units. The QRS morphology is similar to that of sinus rhythm. However, the aberrant conduction of PACs causes a different QRS morphology (Fig. 1). Isolated PACs in neonates are associated with electrolyte abnormalities, hypoglycemia, hypoxia, and hyperthyroidism. PACs are generally benign and usually do not need treatment5).
Fig. 1. Premature atrial complexes. A premature and unrecognized P wave occurs with the previous T wave, and the QRS complex has a similar morphology to the sinus rhythm. This indicates premature atrial complexes (arrows). The asterisk indicates aberrant ventricular conduction.
2. Premature ventricular contractions
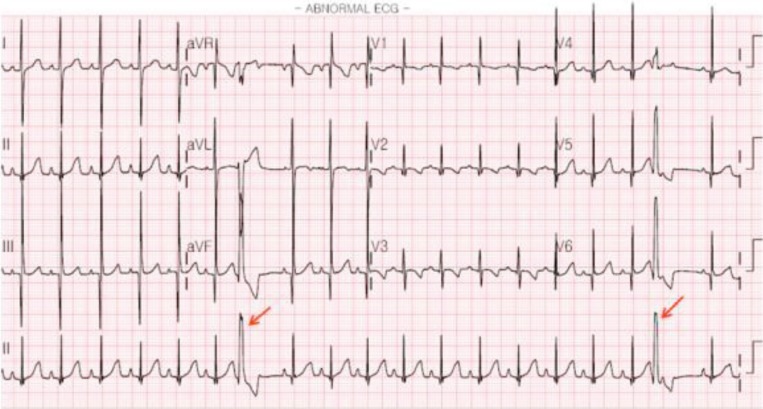
PVCs are relatively uncommon compared with PACs in the fetal and neonatal periods. In a previous study, PVCs were detected on Holter ECG in 18% of normal neonates6). In PVCs, there is no preceding P wave and premature QRS complex showing different morphology from sinus rhythm (Fig. 2).
Fig. 2. Premature ventricular complexes. A wide and premature QRS complex without a preceding P wave indicates premature ventricular complexes (arrows). It is followed by a compensatory pause, not owing to the retrograde reset of the sinoatrial node.
Ventricular arrhythmia may occur in CHD, cardiomyopathy, inflammatory myocardial disease, metabolic disease, electrolyte disturbance and LQTS7,8). However, the immaturity of cardiac conduction tissue and the autonomic nerve system may be the main causes of frequent premature beats in newborns5). Isolated PVCs usually spontaneously resolve, and no treatment is required in neonates with a normal heart5,7).
In the quantitative assessment of PVCs, frequent PVCs are defined as >60 beats/hr in a child8). Although PVC burden is significantly associated with ventricular dysfunction in adults, asymptomatic frequent PVCs in newborns have a good long-term prognosis8).
Neonatal tachyarrhythmias
1. Supraventricular tachycardias
SVT is the most common type of tachyarrhythmia observed in pediatric patients, especially in the neonatal period. SVT is defined as tachycardia resulting from an abnormal mechanism involving the heart structures proximal to the bifurcation of the bundle of His, and that does not have the morphology of atrial flutter (AFL) on the surface ECG. SVT is classified into 2 types; real SVT and atrial tachycardia. Real SVT includes accessory pathway-related AV reentrant tachycardia (AVRT), AV nodal reentrant tachycardia (AVNRT), permanent form of junctional reciprocating tachycardia (PJRT), and junctional ectopic tachycardia (JET). The mechanism of tachycardia is associated with reentry, automaticity enhancement, and triggered activity. The most common mechanism is reentry. Accessory pathway-related AVRT, AVNRT, and PJRT can be classified as reentry tachycardias. Another mechanism of tachycardia is increased automaticity, which includes sinus tachycardia, ectopic atrial tachycardia (EAT), and JET. A rare mechanism of tachycardia is triggered activity, which includes atrial fibrillation (AF)4,5,9,10,11).
1) Atrioventricular reentrant tachycardia
Accessory pathway-related AVRT is commonly associated with Wolff-Parkinson-White (WPW) syndrome in neonates. WPW syndrome is defined as a congenital condition involving an abnormal electrical conduction of the accessory pathway. The accessory pathway connecting impulses between the atrium and the ventricle can be seen at any site in the AV groove. Interestingly, the presence of WPW was seen only in term infants, not in preterm infants, and it may account for differences in the clinical course between preterm and term infants12).
AVRT is initiated paroxysmally with PACs and terminates with AV block. There are 2 different mechanisms of accessory pathway-related reentrant SVT. The usual reentrant circuits for AVRT are the AV node for anterograde conduction and the accessory pathway for retrograde conduction (called “orthodromic” AVRT). During tachycardia, a narrow QRS complex with a retrograde P wave immediately following QRS is seen on the ECG (Fig. 3). Less commonly, “antidromic” tachycardia is the other mechanism of AVRT. Antergrade AV conduction occurs over the accessory pathway and retrograde conduction occurs over the AV node. In this case, a wide-complex rhythm may be observed.
Fig. 3. Atrioventricular reentrant tachycardia. Narrow QRS tachycardia with a ventricular rate of 245 beats/min is shown. The short RP interval is typical in atrioventricular reentrant tachycardia. Retrograde P waves (arrows) are shown in the ST segment in lead II.
Adenosine triphosphate is the first choice of drug for acute termination of AVRT, and the mechanism of the drug is related to AV node block13). To prevent recurrence, antiarrhythmic prophylaxis is recommended during the first year of life. Digoxin or propranolol is generally considered as the initial antiarrhythmic therapy. In a recent study, there was no difference in AVRT recurrence in infants <4 months old treated with an initial therapy of digoxin versus those treated with propranolol14). In case of failure of these first-line drugs, class IA (procainamide or quinidine), class IC (flecainide), or class III (amiodarone or sotalol) drugs can be considered15).
2) Atrioventricular nodal reentrant tachycardia
AVNRT is uncommon in small children, occurring in up to 3%–13% of infants with SVT. The reentrant circuit involves the fast pathway, which enters the compact AV node from the anterior septal region close to the compact AV node, and the slow pathway, which is located in the posterior septal region. In the common type of AVNRT, anterograde conduction occurs down the slow pathway and retrograde conduction occurs up the fast pathway11).
3) Permanent form of junctional reciprocating tachycardia
PJRT is a less common form of AVRT with a slow conducting retrograde accessory pathway. It presents with long RP tachycardia with inverted P waves in the inferior leads (II, III, and aVF). The typical characteristics are incessant tachycardia and slower ventricular rate (about 200 beats/min) than in other tachycardias16). Adenosine transiently terminates PJRT, which usually recurs after adenosine or direct current (DC) cardioversion. Therefore, maintenance therapy with antiarrhythmic drugs and radiofrequency catheter ablation are always required.
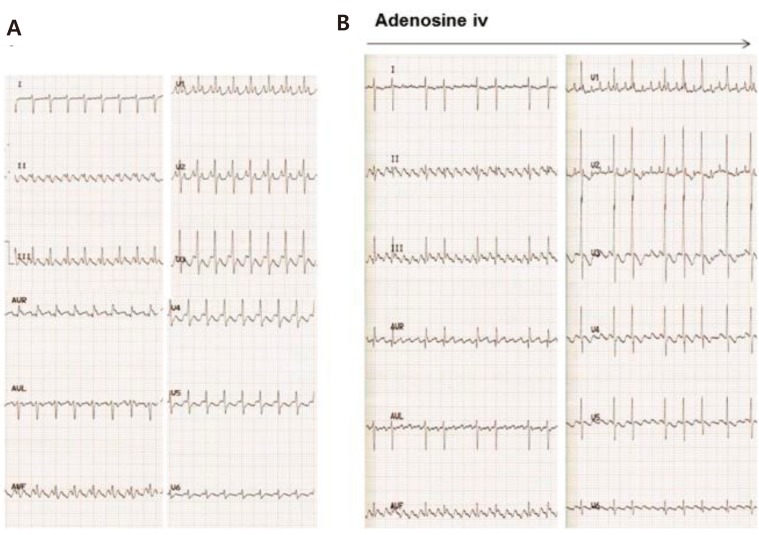
4) Ectopic atrial tachycardia
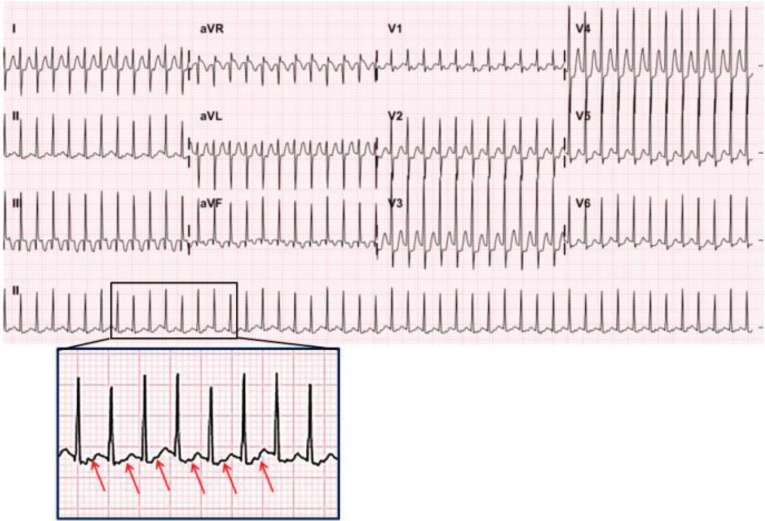
EAT has relevance to increased automaticity of the atria. It shows an abnormal P wave axis with incessant tachycardia. Therefore, “warm-up” and “cool-down” phenomena can be observed. It usually shows 1:1 AV conduction, although a variable ventricular rate is also possible (Fig. 4). This tachycardia may not be responsive to adenosine or DC cardioversion. Moreover it can be resistant to antiarrhythmic drugs. EAT can lead to tachycardia-induced cardiomyopathy if not properly controlled17,18). The combination of flecainide and amiodarone or flecainide and sotalol or high-dose sotalol therapy can control refractory SVT in infants19,20,21). In addition, younger patients (<3 years old) are more likely to respond to antiarrhythmic therapy and have a high incidence of EAT resolution than children ≥3 years old22).
Fig. 4. Ectopic atrial tachycardia. (A) Narrow QRS tachycardia with a ventricular rate of 180 beats/min showing incessant ectopic atrial tachycardia. The abnormal P wave is unrecognizable because it is buried in the previous T wave. (B) Arrows indicate the appearance of an abnormal ectopic P wave in lead II during adenosine injection.
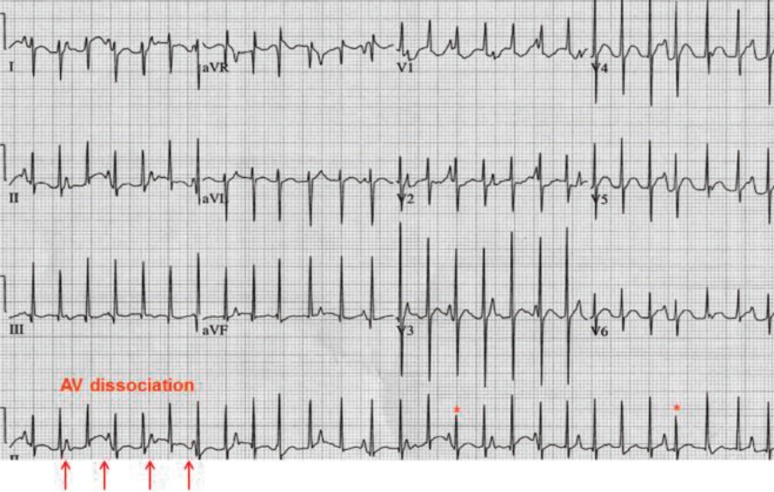
5) Junctional ectopic tachycardia
JET is also characterized by incessant tachycardia with a single ectopic focus initiating at or near the AV node. It usually shows AV dissociation with an atrial slower than the ventricular rate (Fig. 5). The ventricular rate often ranges between 160 beats/min to as high as 280 beats/min. The mechanism of tachycardia is related to the enhanced automaticity of the AV junction. JET is usually observed in postoperative patients with CHD within the first several days after cardiopulmonary bypass. JET not associated with cardiac surgery is a rare arrhythmia in newborns. Congenital JET is highly associated with morbidity and mortality in neonates23,24). Patents presenting symptoms at age <6 months are more likely to have incessant JET; however, patient outcomes considerably improve with amiodarone therapy, permanent pacing, radiofrequency catheter ablation, and cryoablation, although congenital JET remains difficult to treat25).
Fig. 5. Junctional ectopic tachycardia. The ratio of P wave to the QRS complex is ≤1.0, which indicates frequent atrioventricular (AV) dissociation in lead II (arrows). Intermittent shortening of the RR interval is manifested as sinus capture (asterisks).
2. Atrial flutter
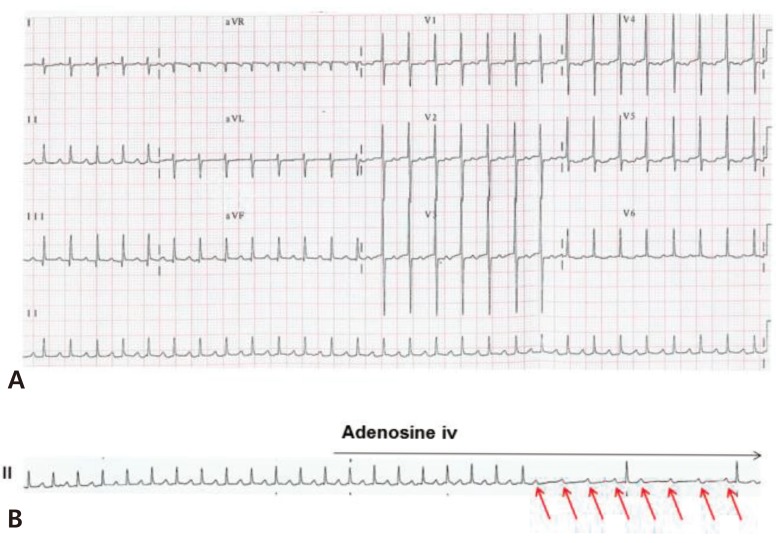
AFL is characterized by saw-tooth flutter waves with an atrial rate of up to 500 beats/min, and is frequently associated with 2:1 AV conduction in neonates (Fig. 6A). Variable AV conduction is also common. The mechanism of AFL is macroreentry within the atrial wall.
Fig. 6. Atrial flutter. (A) Atrial flutter with 2:1 atrioventricular conduction resulting a ventricular rate of 198 beats/ min is detected on 12-lead electrocardiogram. Saw-tooth flutter waves are identified in leads II, III, and aVF. (B) After the adenosine injection, atrial flutter with variable atrioventricular conduction is shown and the atrial rate was 370 beats/min.
AV block does not terminate AFL because the AV node is not involved the reentrant circuit. For this reason, adenosine cannot terminate AFL but unmasks the flutter wave by causing AV block (Fig. 6B). Fetal AFL can cause hydrops fetalis or fetal heart failure. After birth, AFL is usually treated with DC cardioversion or transesophageal pacing in patients with hemodynamically unstable AFL. In some patients with AFL, antiarrhythmic therapy such as digoxin and propranolol can be used as maintenance therapy. Although refractory AFL is a critical condition in neonates, recurrence of AFL is uncommon and long-term drug therapy is rarely required26,27).
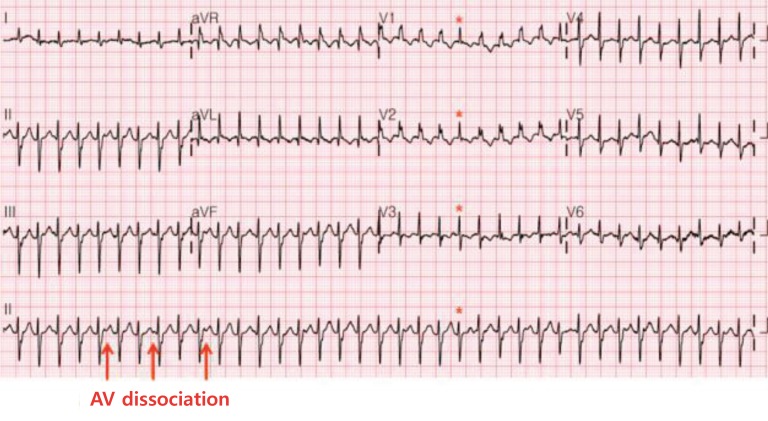
3. Ventricular tachycardias
Wide QRS complex tachycardias should be assumed to be VT in pediatric patients. However, SVT with aberrancy, SVT with the presence of an underlying bundle branch block or ventricular preexcitation, or SVT in an antidromic tachycardia circuit can result in wide QRS complex tachycardias.
VT is rare in the neonatal period. It is defined as three or more PVCs in a row at a rate faster than 120 bpm/min and has a wide QRS complex with AV dissociation during the tachycardia. Capture beats or fusion beats are diagnostic markers in VT (Fig. 7). Nonsustained VT is defined as three or more consecutive PVCs with a duration of <30 seconds. The possible cause of VT may be associated with myocarditis, cardiac tumor, CHD, hereditary cardiomyopathy, LQTS, and electrolyte abnormalities in neonates. Idiopathic VT is defined as VT without an identified underlying cause28,29).
Fig. 7. Ventricular tachycardia. A wide QRS compex with right bundle branch block pattern ventricular tachycardia is shown. A high ventricular rate of 270 beats/min is dissociated from the atrial rate (arrows). A capture beat (asterisks), which is completely conducted by the sinus beat, is a hallmark of ventricular tachycardia.
There are 2 benign forms of VT according to the bundle branch block pattern of QRS morphology that suggests the origin of VT; right ventricular outflow tract VT (left bundle branch block pattern with inferior axis) and idiopathic fascicular VT (right bundle branch block pattern QRS morphology with superior axis). The initial selection of the drug is rather different. In patients with idiopathic fascicular VT, verapamil is the first-choice antiarrhythmic drug. The mechanism of VT is reentry within the posterior fascicle of the left ventricle. In case of right ventricular outflow tract VT, beta-blocker is the first-choice drug. The assumed mechanism of VT is triggered activity30).
If hemodynamically unstable VT develops, DC cardioversion should be performed. However, intravenous lidocaine can be administered in patients with hemodynamically stable VT.
Neonatal bradyarrhythmias
1. Sinus bradycardia
Sinus bradycardia is defined as a sinus rate slower than normal for age, and is frequently detected in the neonatal intensive unit. Transient sinus bradycardia in newborns is benign and usually resolves within 48–72 hours. Furthermore, it may occur after a stressful labor or delivery. In premature babies, apnea is associated with intermittent bradycardia. Sinus bradycardia is mostly associated with general condition abnormalities such as hypoxia, acidosis, hypoglycemia, and increased intracranial pressure4,5,10). Therefore, physicians should make an effort to find the etiology and treat the underlying cause.
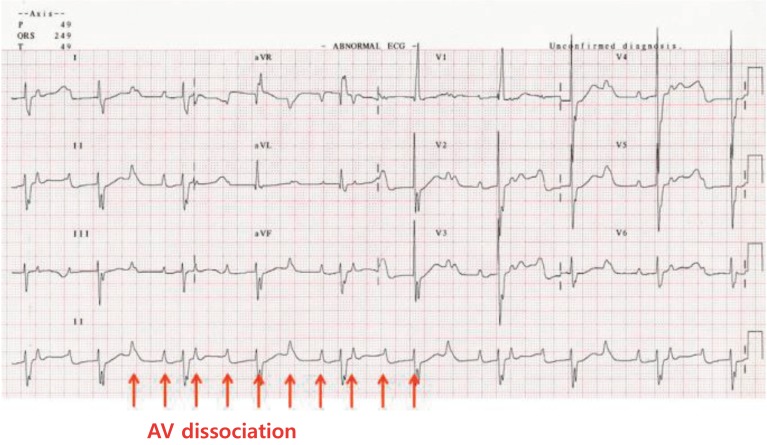
2. Complete AV block
AV block is defined as a conduction disturbance of an impulse from the atria to the ventricle and classified as either first-, second-, or third-degree (or complete) AV block. Among these types, complete AV block of the AV node results in AV dissociation between atrial and ventricular activity. There is no relationship between the P wave and QRS complexes (Fig. 8). Ventricular escape rhythm can occur anywhere from the AV node to the Purkinje system.
Fig. 8. Complete atrioventricular block. The atrial rate is 150 beats/ min and the wide QRS complex ventricular rate is 55 beats/min. The atrioventricular (AV) dissociation (arrows) with slow ventricular rate indicates complete heart block. The escape beats may be ventricular in origin because the QRS complex is wide and bizarre.
Congenital complete AV block in a normal structural heart may occur in infants born to mothers with connective tissue disorders such as systemic lupus erythematosus. This accounts for 90% of the cases. The maternal autoimmune antibody crosses the placenta and damages the AV node of the fetus31,32). Among cases of congenital complete AV block, only 10% are considered idiopathic heart block33). Recently, it has been recognized that the prognosis of congenital complete AV block differs depending on whether the block is identified in a fetus, newborn, or older child32,33).
Permanent pacemaker implantation is indicated in newborns or infants with complete heart block with a ventricular rate of <55 beats/min or in those with CHD and a ventricular rate of <70 beats/min. Another indication is complete heart block with a wide complex escape rhythm, complex ventricular ectopy, or ventricular dysfunction34).
Genetic arrhythmias
Cardiac channelopathy represents arrhythmic conditions related to mutations in genes encoding the critical ion channels of the heart. It includes congenital LQTS, short-QT syndrome (SQTS), catecholaminergic polymorphic VT (CPVT), and Brugada syndrome.
1. Long-QT syndrome
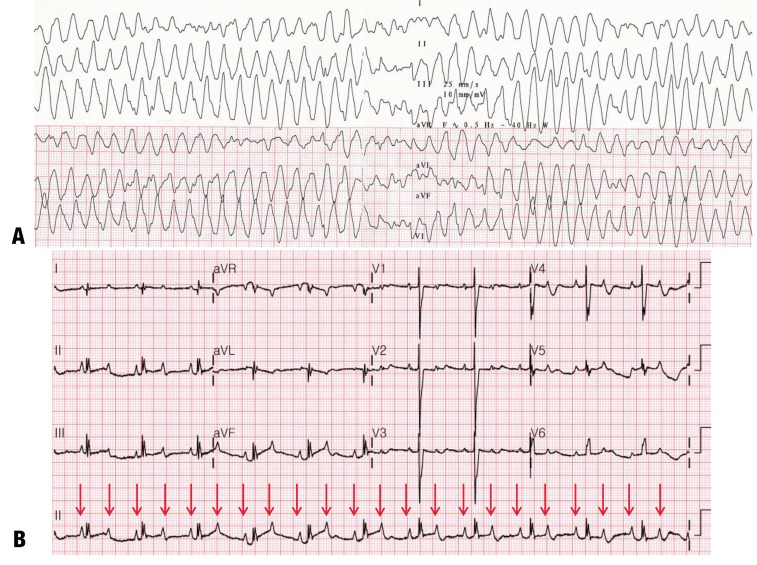
Congenital LQTS is a channelopathy due to a gene mutation of the ion channel associated with ventricular repolarization. It presents with syncope and sudden cardiac death, and is characterized by polymorphic VT or torsades de pointes (Fig. 9A). Approximately 75% of clinically definite LQTS cases are caused by mutations in three genes: KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3). To date, ≥600 mutations have been identified in 14 LQTS-susceptibility genes35,36,37). During the neonatal period, congenital LQTS is usually diagnosed with QT prolongation combined with sinus bradycardia or 2:1 AV block (Fig. 9B)38). Moreover, a beta-blocking agent is considered the initial therapy in newborns. In congenital LQTS, implantable cardioverter defibrillator (ICD) implantation is recommended for patients with a family history of sudden death, intolerance to medication, or recurrent syncope on full doses of a beta-blocker34).
Fig. 9. Congenital long QT syndrome. (A) Electrocardiogram of a newborn with a history of fetal bradycardia showing polymorphic ventricular tachycardia with undulating and sinusoidal appearance of the axis of the QRS complex. This indicates torsade de pointes. (B) The underlying rhythm is QT prolongation with 2:1 atrioventricular block (arrows).
2. Catecholaminergic polymorphic VT
CPVT is characterized by exercise- or stress-induced syncope or sudden death. Patients have CPVT have a family history of juvenile sudden death. CPVT clinically mimics LQTS but is much more malignant than concealed LQTS. The age at presentation can vary from infancy to 40 years. The hallmark ECG feature of CPVT is exercise- or isoproterenol-induced ventricular arrhythmia, particularly bidirectional VT. Beta-blocker is first therapy for patients with symptomatic CPVT39,40).
3. Short-QT syndrome
Patients with SQTS present with sudden death, syncope, palpitation and paroxysmal AF.
Sudden death occurs in patients aged from 3 months to 70 years. The characteristic ECG finding is a short QT interval (QTc≤330 ms), with tall, symmetrical, peaked T waves. Most patients have potential ventricular fibrillation. The therapy of choice is ICD implantation. Furthermore, adjunctive antiarrhythmic therapy with propafenone, quinidine, dofetilide, or sotalol may decrease the risk for ventricular fibrillation41,42).
Conclusion
The most common benign arrhythmia in newborns is PAC, which does not require treatment. AVRT, which is the most frequent tachyarrhythmia in neonates can be properly controlled with antiarrhythmic drug therapy. Complete AV block is the most common cause of nonbenign bradyarrhythmia, for which permanent pacemaker implantation should be performed. Although the frequency of nonbenign arrhythmias is not high, the prognosis depends on the early recognition and proper management of the condition in some serious neonatal cases. Precise diagnosis with risk stratification of patients with nonbenign neonatal arrhythmia is needed to reduce morbidity and mortality.
Acknowledgments
I thank Dr. Min Jeong Cho (Department of Pediatrics, Pusan National University Hospital, Busan, Republic of Korea) for her support. She provided Figures 2, 3, 5, and 7.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Southall DP, Richards J, Mitchell P, Brown DJ, Johnston PG, Shinebourne EA. Study of cardiac rhythm in healthy newborn infants. Br Heart J. 1980;43:14–20. doi: 10.1136/hrt.43.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badrawi N, Hegazy RA, Tokovic E, Lotfy W, Mahmoud F, Aly H. Arrhythmia in the neonatal intensive care unit. Pediatr Cardiol. 2009;30:325–330. doi: 10.1007/s00246-008-9355-4. [DOI] [PubMed] [Google Scholar]
- 3.Kundak AA, Dilli D, Karagöl B, Karadağ N, Zenciroğlu A, Okumuş N, et al. Non benign neonatal arrhythmias observed in a tertiary neonatal intensive care unit. Indian J Pediatr. 2013;80:555–559. doi: 10.1007/s12098-012-0852-3. [DOI] [PubMed] [Google Scholar]
- 4.Doniger SJ, Sharieff GQ. Pediatric dysrhythmias. Pediatr Clin North Am. 2006;53:85–105. vi. doi: 10.1016/j.pcl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Jaeggi E, Öhman A. Fetal and neonatal arrhythmias. Clin Perinatol. 2016;43:99–112. doi: 10.1016/j.clp.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Nagashima M, Matsushima M, Ogawa A, Ohsuga A, Kaneko T, Yazaki T, et al. Cardiac arrhythmias in healthy children revealed by 24-hour ambulatory ECG monitoring. Pediatr Cardiol. 1987;8:103–108. doi: 10.1007/BF02079464. [DOI] [PubMed] [Google Scholar]
- 7.Batra A, J Silka M. Ventricular arrhythmias. Prog Pediatr Cardiol. 2000;11:39–45. doi: 10.1016/s1058-9813(00)00034-5. [DOI] [PubMed] [Google Scholar]
- 8.De Rosa G, Butera G, Chessa M, Pardeo M, Bria S, Buonuomo PS, et al. Outcome of newborns with asymptomatic monomorphic ventricular arrhythmia. Arch Dis Child Fetal Neonatal Ed. 2006;91:F419–F422. doi: 10.1136/adc.2005.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kothari DS, Skinner JR. Neonatal tachycardias: an update. Arch Dis Child Fetal Neonatal Ed. 2006;91:F136–F144. doi: 10.1136/adc.2004.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wren C. Cardiac arrhythmias in the fetus and newborn. Semin Fetal Neonatal Med. 2006;11:182–190. doi: 10.1016/j.siny.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Spearman AD, Williams P. Supraventricular tachycardia in infancy and childhood. Pediatr Ann. 2014;43:456–460. doi: 10.3928/00904481-20141022-13. [DOI] [PubMed] [Google Scholar]
- 12.Goldman LE, Boramanand NK, Acevedo V, Gallagher P, Nehgme R. Preterm infants with paroxysmal supraventricular tachycardia: presentation, response to therapy, and outcome. J Interv Card Electrophysiol. 2001;5:293–297. doi: 10.1023/a:1011468800418. [DOI] [PubMed] [Google Scholar]
- 13.Camm AJ, Garratt CJ. Adenosine and supraventricular tachycardia. N Engl J Med. 1991;325:1621–1629. doi: 10.1056/NEJM199112053252306. [DOI] [PubMed] [Google Scholar]
- 14.Sanatani S, Potts JE, Reed JH, Saul JP, Stephenson EA, Gibbs KA, et al. The study of antiarrhythmic medications in infancy (SAMIS): a multicenter, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants. Circ Arrhythm Electrophysiol. 2012;5:984–991. doi: 10.1161/CIRCEP.112.972620. [DOI] [PubMed] [Google Scholar]
- 15.Weindling SN, Saul JP, Walsh EP. Efficacy and risks of medical therapy for supraventricular tachycardia in neonates and infants. Am Heart J. 1996;131:66–72. doi: 10.1016/s0002-8703(96)90052-6. [DOI] [PubMed] [Google Scholar]
- 16.Dorostkar PC, Silka MJ, Morady F, Dick M., 2nd Clinical course of persistent junctional reciprocating tachycardia. J Am Coll Cardiol. 1999;33:366–375. doi: 10.1016/s0735-1097(98)00590-7. [DOI] [PubMed] [Google Scholar]
- 17.Mehta AV, Sanchez GR, Sacks EJ, Casta A, Dunn JM, Donner RM. Ectopic automatic atrial tachycardia in children: clinical characteristics, management and follow-up. J Am Coll Cardiol. 1988;11:379–385. doi: 10.1016/0735-1097(88)90106-4. [DOI] [PubMed] [Google Scholar]
- 18.Poutiainen AM, Koistinen MJ, Airaksinen KE, Hartikainen EK, Kettunen RV, Karjalainen JE, et al. Prevalence and natural course of ectopic atrial tachycardia. Eur Heart J. 1999;20:694–700. doi: 10.1053/euhj.1998.1313. [DOI] [PubMed] [Google Scholar]
- 19.Fenrich AL, Jr, Perry JC, Friedman RA. Flecainide and amiodarone: combined therapy for refractory tachyarrhythmias in infancy. J Am Coll Cardiol. 1995;25:1195–1198. doi: 10.1016/0735-1097(94)00513-p. [DOI] [PubMed] [Google Scholar]
- 20.Price JF, Kertesz NJ, Snyder CS, Friedman RA, Fenrich AL. Flecainide and sotalol: a new combination therapy for refractory supraventricular tachycardia in children <1 year of age. J Am Coll Cardiol. 2002;39:517–520. doi: 10.1016/s0735-1097(01)01773-9. [DOI] [PubMed] [Google Scholar]
- 21.Knudson JD, Cannon BC, Kim JJ, Moffett BS. High-dose sotalol is safe and effective in neonates and infants with refractory supraventricular tachyarrhythmias. Pediatr Cardiol. 2011;32:896–903. doi: 10.1007/s00246-011-0010-0. [DOI] [PubMed] [Google Scholar]
- 22.Salerno JC, Kertesz NJ, Friedman RA, Fenrich AL., Jr Clinical course of atrial ectopic tachycardia is age-dependent: results and treatment in children < 3 or > or =3 years of age. J Am Coll Cardiol. 2004;43:438–444. doi: 10.1016/j.jacc.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Sarubbi B, Vergara P, D'Alto M, Calabro R. Congenital junctional ectopic tachycardia: presentation and outcome. Indian Pacing Electrophysiol J. 2003;3:143–147. [PMC free article] [PubMed] [Google Scholar]
- 24.Villain E, Vetter VL, Garcia JM, Herre J, Cifarelli A, Garson A., Jr Evolving concepts in the management of congenital junctional ectopic tachycardia. A multicenter study. Circulation. 1990;81:1544–1549. doi: 10.1161/01.cir.81.5.1544. [DOI] [PubMed] [Google Scholar]
- 25.Collins KK, Van Hare GF, Kertesz NJ, Law IH, Bar-Cohen Y, Dubin AM, et al. Pediatric nonpost-operative junctional ectopic tachycardia medical management and interventional therapies. J Am Coll Cardiol. 2009;53:690–697. doi: 10.1016/j.jacc.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Casey FA, McCrindle BW, Hamilton RM, Gow RM. Neonatal atrial flutter: significant early morbidity and excellent long-term prognosis. Am Heart J. 1997;133:302–306. doi: 10.1016/s0002-8703(97)70224-2. [DOI] [PubMed] [Google Scholar]
- 27.Woo J, Khan O, Caldarelli L, Williams P. Tachycardia in the Neonate. Pediatr Ann. 2015;44:e247–e250. doi: 10.3928/00904481-20151012-09. [DOI] [PubMed] [Google Scholar]
- 28.Perry JC. Ventricular tachycardia in neonates. Pacing Clin Electrophysiol. 1997;20(8 Pt 2):2061–2064. doi: 10.1111/j.1540-8159.1997.tb03628.x. [DOI] [PubMed] [Google Scholar]
- 29.Davis AM, Gow RM, McCrindle BW, Hamilton RM. Clinical spectrum, therapeutic management, and follow-up of ventricular tachycardia in infants and young children. Am Heart J. 1996;131:186–191. doi: 10.1016/s0002-8703(96)90068-x. [DOI] [PubMed] [Google Scholar]
- 30.Lee SH, Chen SA, Tai CT, Chiang CE, Wu TJ, Cheng CC, et al. Electropharmacologic characteristics and radiofrequency catheter ablation of sustained ventricular tachycardia in patients without structural heart disease. Cardiology. 1996;87:33–41. doi: 10.1159/000177057. [DOI] [PubMed] [Google Scholar]
- 31.Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol. 2010;55:2778–2784. doi: 10.1016/j.jacc.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 32.Eliasson H, Sonesson SE, Sharland G, Granath F, Simpson JM, Carvalho JS, et al. Isolated atrioventricular block in the fetus: a retrospective, multinational, multicenter study of 175 patients. Circulation. 2011;124:1919–1926. doi: 10.1161/CIRCULATIONAHA.111.041970. [DOI] [PubMed] [Google Scholar]
- 33.Martin TA. Congenital heart block: current thoughts on management, morphologic spectrum, and role of intervention. Cardiol Young. 2014;24(Suppl 2):41–46. doi: 10.1017/S1047951114001358. [DOI] [PubMed] [Google Scholar]
- 34.Brugada J, Blom N, Sarquella-Brugada G, Blomstrom-Lundqvist C, Deanfield J, Janousek J, et al. Pharmacological and non-pharmacological therapy for arrhythmias in the pediatric population: EHRA and AEPC-Arrhythmia Working Group joint consensus statement. Europace. 2013;15:1337–1382. doi: 10.1093/europace/eut082. [DOI] [PubMed] [Google Scholar]
- 35.Camm AJ, Janse MJ, Roden DM, Rosen MR, Cinca J, Cobbe SM. Congenital and acquired long QT syndrome. Eur Heart J. 2000;21:1232–1237. doi: 10.1053/euhj.2000.2222. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51:2291–2300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 37.Tester DJ, Benton AJ, Train L, Deal B, Baudhuin LM, Ackerman MJ. Prevalence and spectrum of large deletions or duplications in the major long QT syndrome-susceptibility genes and implications for long QT syndrome genetic testing. Am J Cardiol. 2010;106:1124–1128. doi: 10.1016/j.amjcard.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horigome H, Nagashima M, Sumitomo N, Yoshinaga M, Ushinohama H, Iwamoto M, et al. Clinical characteristics and genetic background of congenital long-QT syndrome diagnosed in fetal, neonatal, and infantile life: a nationwide questionnaire survey in Japan. Circ Arrhythm Electrophysiol. 2010;3:10–17. doi: 10.1161/CIRCEP.109.882159. [DOI] [PubMed] [Google Scholar]
- 39.Sarkozy A, Brugada P. Sudden cardiac death and inherited arrhythmia syndromes. J Cardiovasc Electrophysiol. 2005;16(Suppl 1):S8–S20. doi: 10.1111/j.1540-8167.2005.50110.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu N, Ruan Y, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Prog Cardiovasc Dis. 2008;51:23–30. doi: 10.1016/j.pcad.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Rudic B, Schimpf R, Borggrefe M. Short QT syndrome: review of diagnosis and treatment. Arrhythm Electrophysiol Rev. 2014;3:76–79. doi: 10.15420/aer.2014.3.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira R, Campuzano O, Sarquella-Brugada G, Cesar S, Iglesias A, Brugada J, et al. Short QT syndrome in pediatrics. Clin Res Cardiol. 2017;106:393–400. doi: 10.1007/s00392-017-1094-1. [DOI] [PubMed] [Google Scholar]