Abstract
Purpose
This study was aimed to investigate the effect of pseudolaric acid B (PAB) on proliferation, invasion and epithelial-to-mesenchymal transition (EMT) in pancreatic cancer cells and to explore the possible mechanism.
Materials and Methods
The pancreatic cancer cell line SW1990 was cultured and treated with PAB dose- and time-dependent manners. Cell proliferation and invasion ability were measured by MTT assay and Matrigel/Transwell test, respectively. Semi-quantitative real-time polymerase chain reaction and Western blotting were conducted to detect the expression of EMT markers and the key molecules. Finally, nude mice subcutaneous transplantation tumor model was used to confirm the therapy efficacy of PAB.
Results
PAB could inhibit SW1990 cell proliferation and invasion in time- and dose-dependent manners. Vimentin, fibronectin, N-cadherin, Snail, Slug, YAP, TEAD1, and Survivin were down-regulated (p<0.01), while E-cadherin, caspase-9, MST1, and pYAP were up-regulated (p<0.05). Combined PAB and gemcitabine treatment markedly restricted the tumor growth compared with gencitabin or PAB alone groups.
Conclusion
PAB could inhibit the proliferation and invasion ability of pancreatic cancer cells through activating Hippo-YAP pathway and inhibiting the process of EMT.
Keywords: PAB, PDAC, EMT, cell proliferation, Hippo-YAP pathway
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and lethal types of cancers with a 5-year overall survival rate of only 5%, which is the second common causes of cancer-related mortality in gastroenterology.1 In recent years, the worldwide incidence of pancreatic cancer has steadily increased. In China, the incidence of pancreatic cancer also indicates an increasing tendency: the morbidity is 7.28/100000 (the 7th in malignant cancers) and the mortality is 6.61/100000 (the 6th in malignant cancers).2 However, radical surgery is not possible in the majority of pancreatic cancer patients, because of ambiguous clinical symptoms and early metastasis to the adjacent tissues and lymph glands. Only 39.7% of cases were histologically verified (surgery with histologic diagnosis 31.0%, cytological diagnosis 8.7%, surgery without histologic diagnosis 12.1%, and clinical diagnosis 48.2%). Overall, 30.0% of patients undergo curative-intent operation, and only 9.8% of patients receive comprehensive treatment.3 Even after successful removal of the tumor, the median survival varies between 18 and 25 months. Early invasion and dissemination of tumor cells are well-known factors contributing to this outcome. It is, therefore, extremely significant to identify the complex mechanism on the pancreatic carcinogenesis and develope new therapeutic approaches.
Epithelial-to-mesenchymal transition (EMT) is a cellular process during which epithelial cells lose their polarized organization and cell-cell junctions, resulting in changes of cell shape and cytoskeletal organization and acquire mesenchymal characteristics, such as fibroblast-like cell morphology and increased cell migration and invasion.4 EMT plays an important role in cancer metastasis in several human malignancies, including pancreatic cancer. The genetic and epigenetic changes typical for cancer cells, such as the activation of oncogenes and the inactivation of tumor suppressors, can act in concert with the EMT program, which is of potential importance for this rapid tumor progression negatively affecting the overall survival of pancreatic cancer patients.5 Therefore, inhibition of EMT in pancreatic cancer cells may facilitate the development of an effective strategy in the prevention and/or treatment of metastatic pancreatic cancer.
Pseudolaric acid B (PAB) is a diterpene acid isolated from the root and trunk bark of Pseudolarix kaempferi Gord. It has many functions, such as antifungal, antifertility, and cytotoxic activities. Recent studies have demonstrated its potent inhibition of cell growth of cancer cells, such as lung cancer,6 breast cancer,7 thyroid squamous cell carcinoma8 and gastric cancer.9 The anti-tumor action of PAB involves the induction of senescence through activation of the p53 pathway6,7 and autophagy,8 and via the Cox-2/PKC-α/P-gp pathway.9 However, there are few researches on the effect of PAB on pancreatic cancer cells. Thus, the aim of the present study was to investigate the antitumor effect and mechanism of PAB on pancreatic cancer.
MATERIALS AND METHODS
Cell culture
The pancreatic cancer cell line SW1990 was maintained in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin G and 100 µg/mL streptomycin and cultured in a humidified atmosphere of 5% CO2 at 37℃. In the logarithmic growth phase, these cells were collected and were prepared as cell suspensions at a density of 5×104 cells/mL. They were then cultured in a six-well plate with 3 mL per well. When cells grew to 80–90% confluent, the experimental groups were treated with PAB (Medicine College of the Ocean University, Qingdao, China) at 0.5, 1, 2, 4, and 8 µmol/L for 24 h. Other experimental groups were treated with PAB at 4 µmol/L for 24, 48, and 72 h, respectively. Cell population was determined by MTT assay. In the control group, cells were incubated in the absence of PAB.
Cell proliferation analysis
MTT method was used to evaluate cell proliferation. Cells treated with PAB at 0.5, 1, 2, 4, and 8 µmol/L for 0, 24, 48, and 72 h were seeded into 96-well plate at a density of 1×104 cells/well in 200 µL of medium. At each time point, the culture medium was replaced with fresh medium containing MTT [5 mg/mL in phosphate-buffered saline (PBS), 200 µL/well] and incubated for an additional 4 h. Following incubation, medium was discarded and dimethyl sulfoxide (150 µL/well; Sigma-Aldrich) was added to each well for 10 min. The results were analyzed by an automated plate reader at 490 nm (Bio-Rad, Hercules, CA, USA).
Cell invasion assays
Cell invasion was measured by Matrigel-precoated Transwell inserts (8.0 mm pore size with polyethylene terephthalate membrane) according to the manufacturer's protocol. Briefly, cells treated with PAB at 0.5, 2, 4, and 8 µmol/L were seeded in the upper chamber, and DMEM medium with 10% FBS was added into the lower chamber. After 24 h of incubation, cells that passed through the lower side of the membrane were stained with crystal violet for 15 min, and quantified by counting six high-powered fields in the center of each well. The cells that invaded through the matrigel and reached the lower surface of the filter were quantified by counting the number of cells that migrated in three random microscopic fields per filter at a magnification of ×200 (Olympus Corporation, Tokyo, Japan).
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) amplification was carried out using the Power SYBR Green PCR Master Mix (TaKaRa, Ohtsu, Japan) in ABI PRISM 7900 Sequence detection system. Total mRNA from SW1990 cells incubated with PAB in different doses and times was extracted using Trizol (Invitrogen) according to the manufacturer's protocol. Reverse transcriptions were carried out using 500 ng of RNA and High Capacity RNA-to-cDNA kit (TaKaRa, Japan).
After treated with PAB at 0.5, 1, 2, 4, and 8 µmol/L for 24 h and treated with PAB at 4 µmol/L for 24, 48, and 72 h, the markers of EMT, such as, E-cadherin, N-cadherin, vimentin, Snail, Slug, and the key molecules of Hippo-YAP signaling pathway and Hedgehog signaling pathway (British Abcam company, Cambridge, UK) were tested. The primer sequences used for PCR amplification were shown in Table 1. The relative expression of mRNAs was normalized to GAPDH. The qRT-PCR reaction system was as follows: cDNA, 1 µL; double-distilled water, 3.4 µL; Tip Green qPCR SuperMix, 5 µL; passive reference dye (×50), 0.2 µL; forward primer (10 µmol/L), 0.2 µL; reverse primer (10 µmol/L), 0.2 µL. The total volume of the system was 10 µL. The reaction conditions were 95℃ for 30 s first, followed by 95℃ for 5 s, and finally 60℃ for 30 s, in all 40 cycles. Meanwhile, a 65℃ to 95℃ solubility curve was constructed. The results were expressed as mean±standard deviation (SD) and the comparative quantitative threshold cycle (ΔΔCt) method.
Table 1. Primers of the Markers of Epithelial-to-Mesenchymal Transition and Hippo-YAP Signal Pathway.
| E-cadherin | Forward: 5'GCCTCCTCAAAAGAGAGTGGAAG3' |
| Reverse: 5'TGGCAGTGTCTCTCCAAATCCG3' | |
| N-cadherin | Forward: 5' GCCGTGAGAAGCTTTCCACTC3' |
| Reverse: 5'TTAAGGTTGGCTTCAGGCTCAA3' | |
| Fibronectin | Forward: 5'ACAACACCGAGGTGACTGAGAC3' |
| Reverse: 5'GGACACAACGATGCTTCCTGAG3' | |
| Vimentin | Forward: 5'AGGCAAAGCAGGAGTCCACTGA3' |
| Reverse: 5'ATCTGGCGTTCCAGGGACTCAT3' | |
| Snail | Forward: 5' AGCAGGAAGGACCCCACATC 3' |
| Reverse: 5' GCAGAGGACACAGAACCAGAAA3' | |
| Slug | Forward: 5'ATCTGCGGCAAGGCGTTTTCCA3' |
| Reverse: 5'GAGCCCTCAGATTTGACCTGTC3' | |
| YAP | Forward: 5' TGAACAAACGTCCAGCAAGATAC 3' |
| Reverse: 5'CAGCCCCCAAAATGAACAGTAG3' | |
| MST1 | Forward: 5' AGCCGCAGTTCACGTTTACCT3' |
| Reverse:5'GATCCACCCTCTTGCCACACT3' | |
| TEAD1 | Forward: 5'CAAGGTTTGAGAATGGCCGAT3' |
| Reverse:5'AAACACACAGGCCATGCAGAG3' | |
| Smoothened | Forward: 5'CCATTCCTCGACTGCCTCAG3' |
| Reverse:5'TCCGAGTCTGCATCCATGAGT3' | |
| GLI1 | Forward: 5'TATGGACCTGGCTTTGGA3' |
| Reverse:5'CCTATGTGAGCCCTATTTGC3' | |
| Sip1 | Forward: 5'GAGTTGATGCCTCGGCTATTGC3' |
| Reverse:5'CTGGACATTGAGCTCCTTCGATC3' | |
| GAPDH | Forward: 5'ACGGATTTGGTCGTATTGGG3' |
| Reverse: 5'TGATTTTGGAGGGATCTCGC3' |
Western blot analysis
After treated with PAB at 4 µmol/L for 24, 48, and 72 h, cell lysates were harvested using RIPA buffer and proteins were resolved in 10% SDS-polyacrylamide gels, followed by electro-phoretical transfer to polyvinylidene difluoride membranes. After blocking, the membrane was probed with rabbit polyclonal antibodes specific for human E-cadherin, N-cadherin, vimentin, fibronection, Snail and Slug (British Abcam company, 1:1000 dilution) at 1:1000 dilution, and human YAP, pYAP, and GAPDH (Santa Cruz, CA, USA, 1:1000) antibodies overnight at 4℃. After incubation with horseradish peroxidase-conjugated anti-human secondary antibodies (Santa Cruz, 1:5000) for 1 h at room temperature, blots were visualized by using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Detection of GAPDH on the same membrane was used as a loading control. After chemiluminescence with a commercial assay, the target bands were detected with chemiluminescence imaging system and analyzed by gel image-processing system. The protein levels were normalized against GAPDH.
Animal studies
The animal experiments were performed in accordance with China Animal Protection Law, and the protocols were approved by the Animal Care and Use Committee of the Affiliated Hospital of Qingdao University. Female BALB/c mice (nu/nu; 4 weeks old, 18−22 g weight) were purchased from the Shanghai SLAC Animal Center (Shanghai, China). Total 200 µL of SW1990 cell suspension at a density of 5×106 cells/mL were injected beneath the axillary dorsal skin to establish the subcutaneously transplanted tumor animal model. After pathological confirmation and the longest diameter of the subcutaneous tumor had attained a size of 0.5 cm, 96 subcutaneous tumor-burdened nude mice were randomly divided into the following four groups and they then received subcutaneous multipoint injections: 1) the control group, which received 2 mg/mL PBS (0.9% sodium chloride); 2) the PAB group, which received 4 µmol/L PAB dissolved in PBS; 3) the gemcitabine group, which received 125 mg/kg gemcitabine dissolved in PBS; and 4) the combination group, which received both PAB (4 µmol/L) and gemcitabine (125 mg/kg) three times per week for at least 8 weeks. The drug toxicity was evaluated by the inhibition of tumor volume. Tumor volume (V) was calculated by the formula: V=0.5×length×width2. Inhibitory rate was calculated using tumor mass volume and weight.
Statistical analysis
All data were obtained from at least three independent experiments. Values were expressed as means±SD. Statistics were calculated with SPSS 19.0 (IBM Corp., Armonk, NY, USA). Multiple comparisons between control group and groups treated at different concentrations of PAB or on different time points were assessed by one-way ANOVA and Fisher protected least significant difference tests. The difference between groups was considered statistically significant with a value of p<0.05.
RESULTS
PAB inhibited the proliferation and invasion ability of SW1990 cells in vitro
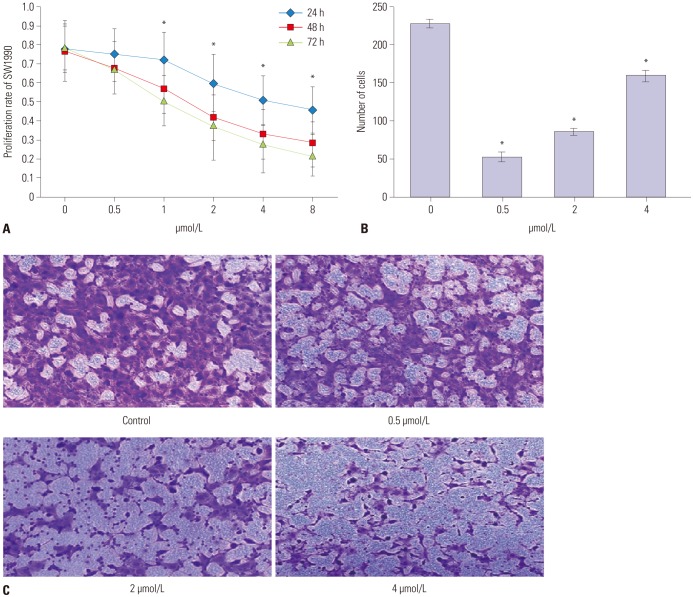
To investigate the effect of PAB on SW1990 cells, the proliferation of SW1990 cells was detected by MTT. The results showed that the proliferation of this cell line was markedly reduced with the increase of dose and treated time of PAB (p<0.01), demonstrating that PAB played a negative role in the regulation of SW1990 cell proliferation in a dose-dependent and time-dependent manner (Fig. 1A). Potential impact of PAB on pancreatic cancer cells invasion was researched. As shown in Fig. 1B and C, there were 228±5 SW1990 cells in the control group passed through the Transwell micropores after 24 h. After treatment with PAB at different concentrations (0.5, 2, and 4 µmol/L), the mean number of SW1990 cells passed through the Transwell micropores were 53±6, 86±8, and 159±7, respectively, after 24 h (p<0.01). These data revealed a novel mechanism that PAB inhibited SW1990 cell invasion in a dose-dependent manner.
Fig. 1. The effect of PAB on proliferation and invasion ability of pancreatic cancer cells. (A) The effect of PAB on proliferation rate of SW1990 cells. The proliferation of pancreatic cancer cell line was markedly reduced by PAB in dose- and time-dependent manners. (B) The number of cells passed through the Transwell micropores. The number of cells was significantly decreased when treated with PAB. (C) The cell morphology detected by crystal violet staining (×100). *p<0.01 when compared to the control group. PAB, pseudolaric acid B.
PAB inhibited the EMT in pancreatic cancer cells
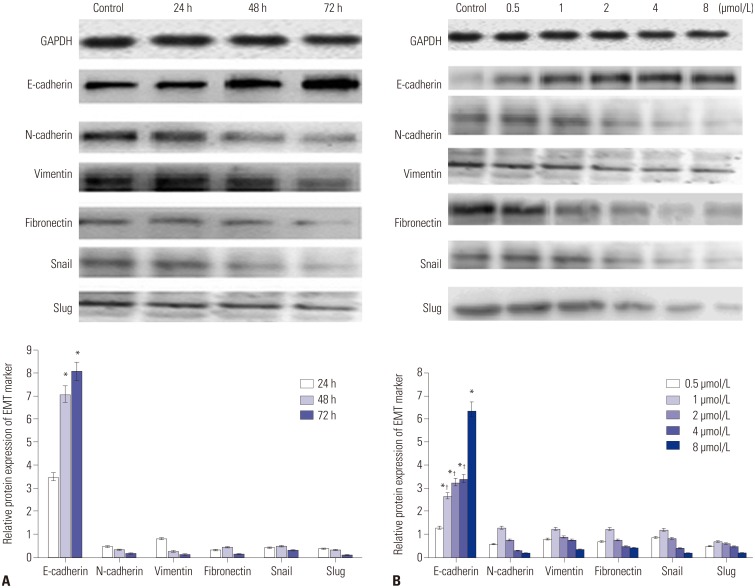
RT-PCR analysis showed that the EMT marker mRNA expression level changed significantly after treatment with 4 µmol/L of PAB for different times (Fig. 2A). With the prolongation of time, PAB could markedly decrease the expression of vimentin, fibronectin, N-cadherin, Snail, and Slug, whereas the expression of E-cadherin was increased. Similar finding was also obtained after treatment with different concentrations of PAB (0.5, 1, 2, 4, and 8 µmol/L) for 24 h; the mRNA levels of vimentin, fibronectin, N-cadherin, Snail, and Slug were reduced, while the mRNA level of E-cadherin increased with the increasing concentration of PAB (Fig. 2B). The same tendency was demonstrated by western blotting result.
Fig. 2. The effect of PAB on the expression levels of EMT markers (vimentin, fibronectin, N-cadherin, Snail, Slug, and E-cadherin). (A) PAB down-regulated the expression levels of vimentin, fibronectin, N-cadherin, Snail, and Slug, and up-regulated the expression level of E-cadherin time-dependent manner. (B) Different concentrations of PAB down-regulated the expression levels of vimentin, fibronectin, N-cadherin, Snail, and Slug, and up-regulated the expression level of E-cadherin. *p<0.01 when compared to that of 24 h, or compared to that of 0.5 µmol/L, †p<0.01 level when compared to that of 8 µmol/L. PAB, pseudolaric acid B; EMT, epithelial-to-mesenchymal transition.
PAB suppressed the EMT of pancreatic cancer cells through Hippo-YAP pathway
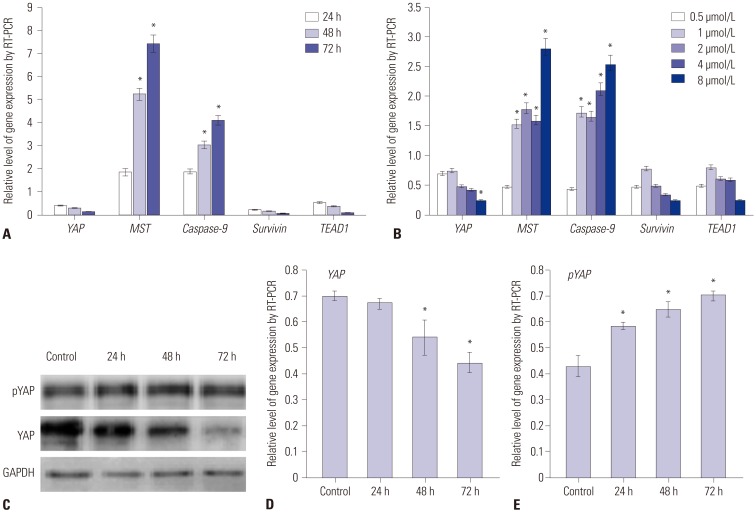
To explore the potential mechanism of PAB in suppressing the proliferation and invasion of pancreatic cancer cells, we also screened Hippo-YAP signaling pathway potential target genes. The results indicated that YAP and its downstream genes, TEAD1, were significantly decreased by PAB in time- and dose dependent manners, whereas the expression levels of the Hippo upstream gene, MST1, were prominently increased (p< 0.01) (Fig. 3A and B), respectively. We further confirmed the protein levels of YAP and pYAP by Western-blotting (Fig. 3C). After treatment by PAB, the protein level of YAP was decreased (Fig. 3D), whereas that of pYAP was increased (Fig. 3E) (p< 0.01). These results suggested that up-regulation of PAB suppressed the EMT of pancreatic cancer cells through Hippo-YAP pathway.
Fig. 3. The effect of PAB on Hippo-YAP pathway and synergized with gemcitabine against pancreatic cancer. (A) The relative level of Hippo-YAP pathway-related genes at different time points. Significant differences of MST and Caspase-9 were found at different treated time points. (B) The relative level of Hippo-YAP pathway-related genes when treated with various concentrations of PAB. (C) Expression level of YAP and pYAP detected by Western blotting. Significant differences of MST and Caspase-9 were found by different concentrations of PAB. (D) Expression level of YAP detected by RT-PCR. (E) Expression level of pYAP detected by RT-PCR. *p<0.01 when compared to the control group. PAB, pseudolaric acid B; RT-PCR, realtime polymerase chain reaction.
PAB sensitized pancreatic cancer cells to chemotherapeutic drugs in vivo
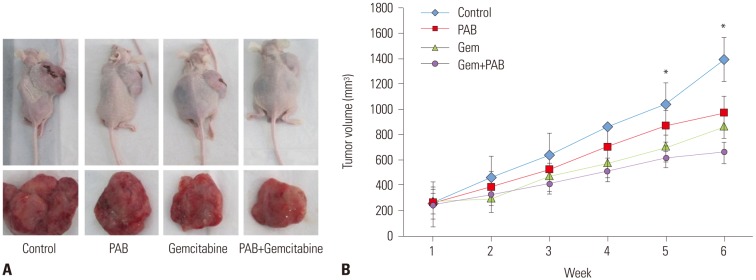
Based on the aforementioned results, we further evaluated the therapeutic effect of PAB plus gemcitabine treatment using an in vivo xenograft mouse model. There were no significant differences among the groups prior to treatment. The inhibitory rates of PAB, gemcitabine, and combination groups were 36.9, 37.4, and 85.2% respectively, which were much more than that of control group (p<0.01). Furthermore, treatment with PAB plus gemcitabine resulted in significantly smaller tumors than in control mice (p<0.05), or mice treated with gemcitabine or PAB alone (p<0.05) detected by tumor morphology (Fig. 4A) and tumor volume (Fig. 4B). These results suggested that PAB dramatically sensitized gemcitabine resistance. Taken together, these findings suggested that PAB sensitizeed pancreatic cancer cells to gemcitabine in vivo.
Fig. 4. The effect of PAB and gemcitabine on tumor volume. (A) Tumor morphology. (B) Tumor volume. *p<0.05 when compared to the control group. PAB, pseudolaric acid B.
DISCUSSION
In the present study, we found that PAB inhibited the proliferation and invasion of pancreatic cancer cell line SW1990. By PAB, the expressions of vimentin, fibronectin, N-cadherin, Snail, and Slug were significantly down-regulated, while E-cadherin expression was significantly up-regulated. Accompanied with the change of EMT markers, Hippo pathway target genes YAP, TEAD1, and apoptin Survivin were down-regulated, while caspase-9 and MST1 were up-regulated in a time-dependent manner. These results together identified that PAB inhibits cell proliferation and invasion through activating Hippo-YAP pathway and inhibiting the process of EMT, which may be an effective strategy in the prevention and/or treatment of pancreatic cancer.
PAB has been demonstrated to exert a potent antitumor effect on MCF7 human breast cancer cell,7 thyroid squamous cell carcinoma,8 and gastric cancer9 through activating autophagy, arresting cell cycle, and down-regulating the Cox-2/PKC-a/P-gp/mdr1 signaling pathway. However, there are few studies on pancreatic cancer cells. Our study found that PAB could inhibit pancreatic cancer cell proliferation and induce apoptosis time- and dose-dependently.
PDAC is the most common invasive cancer, which is very easy to metastasize even in an early stage. Numerous studies have suggested that EMT contributes to early-stage dissemination of cancer cells and is pivotal for invasion and metastasis of PDAC.10,11 The epithelial cells of pancreatic cancer acquire mesenchymal phenotype by EMT to increase the ability of anti-apoptosis, migration, and invasion. Suppression of EMT leads to an increase in cancer cell proliferation with enhanced expression of nucleoside transporters in tumors, contributing to enhanced sensitivity to gemcitabine treatment and increased overall survival of mice.11 In the pancreatic cancer tissue, the defect of E-cadherin is positively correlated with the differentiation of pancreatic cancer and lymph node metastasis. Increased fibronectin or vimentin and decreased E-cadherin correlated with high metastatic potential12 and poor survival.5 Our present study showed that PAB treatment in vitro significantly decreased invasive ability of SW1990 cells. The longer the PAB treatment, the stronger the ability to inhibit the SW1990 cells invasion. Meanwhile, we found that the expression of EMT markers exhibited corresponding changes; The expression of epithelial marker protein E-cadherin was significantly up-regulated, while the expressions of mesenchymal marker proteins, such as N-cadherin, vimentin, and fibronectin,13 were down-regulated. EMT has earlier been shown to be significantly inhibited by PAB. Therefore, we speculate that PAB inhibits the migration of pancreatic cancer cells by changing the EMT marker proteins.
Snail and Slug are the members of Snail superfamily and the transcriptional repressor of E-cadherin. Their increased expression facilitates the association with EMT and enhances invasion ability of the cancer cells.14 Up-regulation of Slug is significantly correlated with a higher tumor stage, and the E- to N-cadherin switch in bladder cancer cells and tissues promotes EMT, and increases cell invasiveness and chemoresistance.15
Our present experiment indicated that PAB down-regulated the expressions of Snail and Slug at protein and mRNA levels, suggesting that PAB inhibits the invasion ability of pancreatic cancer cells by down-regulating the transcription factor Snail and Slug, and up-regulating the expression of E-cadherin.
YAP is the key effect factor of Hippo signaling pathway and is a candidate oncogene implicated in the EMT and the metastatic potential of mammary epithelial cells.16 Overexpression of YAP induces EMT in different cell lines and anchorage-independent proliferation of pancreatic epithelial cell.17,18 Our results showed that, with the prolong of effected time of PAB, MST mRNA level was elevated and the expression levels of YAP mRNA and protein were declined gradually, while pYAP protein level increased and TEAD1 mRNA level decreased, suggesting that PAB could prompt YAP phosphorylation through MST. After phosphorylation, pYAP assembles in cytoplasm and cannot go into nucleus, thus weakening the effect of YAP as a kind of cancer gene, and inhibiting the transcription of downstream genes and the proliferation of pancreatic cancer cells.
In the present study, we provided evidence that PAB contributed to drug resistance in pancreatic cancer. To examine the antitumor effect of PAB in vivo, we treated pancreatic cancer mouse model with PAB, gemcitabine or a combination of both. Our results showed that the combination treatment led to a significant inhibition of tumor growth compared to treatment with either agent alone. Therefore, the combination of gemcitabine with PAB could achieve a greater therapeutic effect, a beneficial characteristic of combination therapy with two or more chemotherapeutics. EMT contributes to drug resistance in pancreatic cancer.19 Thus, PAB inhibited EMT in pancreatic cancer cells, thus potentially facilitating the development of an effective strategy in the prevention and/or treatment of metastatic pancreatic cancer.
In conclusion, we found that PAB inhibited pancreatic cancer cells proliferation in a dose- and time-dependent manners through activating Hippo-YAP pathway and inhibiting the process of EMT. PAB also synergized with gemcitabine against pancreatic cancer, implicating potential application of PAB in pancreatic cancer therapy. However, further studies, including preclinical studies and clinical trials, are needed to confirm our strategy for the treatment for pancreatic cancer.
ACKNOWLEDGEMENTS
This work was supported by grants from the Key Project of Chinese Ministry of Science and Technology (2012ZX10002007, 2013ZX1002001), Shandong Medical and Health Science and Technology Development Plan (2013WS0270), Natural and Science Funding of Shandong Province (ZR2014HM094), College and University Scientific Research Development Program of Shandong Province (J15LL5T), and Qingdao People's Livelihood Science and Technology Plan (14-2-3-8-nsh). In addition, this research was also supported by Chinese postdoctoral science foundation (2017M612221).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M, Liu L, et al. Cancer statistics: current diagnosis and treatment of pancreatic cancer in Shanghai, China. Cancer Lett. 2014;346:273–277. doi: 10.1016/j.canlet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Yang YM, Yang WX. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget. 2017;8:41679–41689. doi: 10.18632/oncotarget.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu W, Liu W, Shen X, Shi Y, Wang L, Liu H. Emergence of heterogeneous nuclear ribonucleoprotein A2/B1 vs loss of E-cadherin: their reciprocal immunoexpression profiles in human pancreatic cancer. Ann Diagn Pathol. 2013;17:14–17. doi: 10.1016/j.anndiagpath.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Yao GD, Yang J, Li Q, Zhang Y, Qi M, Fan SM, et al. Activation of p53 contributes to pseudolaric acid B-induced senescence in human lung cancer cells in vitro. Acta Pharmacol Sin. 2016;37:919–929. doi: 10.1038/aps.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Chen C, Xu T, Yan M, Xue B, Wang Y, et al. Pseudolaric acid B activates autophagy in MCF-7 human breast cancer cells to prevent cell death. Oncol Lett. 2016;11:1731–1737. doi: 10.3892/ol.2016.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Ren P, Zhong T, Wang Y, Yan M, Xue B, et al. Pseudolaric acid B inhibits proliferation in SW579 human thyroid squamous cell carcinoma. Mol Med Rep. 2015;12:7195–7202. doi: 10.3892/mmr.2015.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Q, Li Y. The inhibitory effect of pseudolaric acid B on gastric cancer and multidrug resistance via Cox-2/PKC-α/P-gp pathway. PLoS One. 2014;9:e107830. doi: 10.1371/journal.pone.0107830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009;137:361–371. doi: 10.1053/j.gastro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu K, Zeng J, Zhou J, Fan J, Chen Y, Wang Z, et al. Slug contributes to cadherin switch and malignant progression in muscle-invasive bladder cancer development. Urol Oncol. 2013;31:1751–1760. doi: 10.1016/j.urolonc.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Vesuna F, van Diest P, Chen JH, Raman V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun. 2008;367:235–241. doi: 10.1016/j.bbrc.2007.11.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang N, Morrison CD, Liu P, Miecznikowski J, Bshara W, Han S, et al. TAZ induces growth factor-independent proliferation through activation of EGFR ligand amphiregulin. Cell Cycle. 2012;11:2922–2930. doi: 10.4161/cc.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karamitopoulou E. Role of epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma: is tumor budding the missing link. Front Oncol. 2013;3:221. doi: 10.3389/fonc.2013.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]