Abstract
Purpose
To investigate the association of cancer stem-cell markers [octamer-binding transcription factor 4 (OCT4), sex determining region Y-box 2 (SOX2), and Nanog homebox (NANOG)] expression with clinicopathological properties and overall survival (OS) in operative rectal cancer (RC) patients receiving adjuvant therapy.
Materials and Methods
153 patients with primary RC receiving surgery were enrolled. Tumor tissue and paired adjacent normal tissue sample were collected, and OCT4, SOX2, and NANOG expressions were assessed by immunofluorescent staining. The median follow-up duration was 5.2 years, and the last follow-up date was August 2016.
Results
Tumor tissue OCT4 (p<0.001), SOX2 (p=0.003), and NANOG (p<0.001) expressions were higher than those in adjacent tissue. OCT4 expression was positively correlated with pathological grade (R=0.185, p=0.022), tumor size (R=0.224, p=0.005), and N stage (R=0.170, p=0.036). NANOG expression was positively associated with tumor size (R=0.169, p=0.036). Kaplan-Meier suggested that OCT4+ was associated with worse OS compared with OCT4− (p<0.001), while no association of SOX2 (p=0.121) and NANOG expressions (p=0.195) with OS was uncovered. Compared with one or no positive marker, at least two positive markers were associated with shorter OS (p<0.001), while all three positive markers were correlated with worse OS compared with two or less positive markers (p<0.001). Multivariate Cox's analysis revealed that OCT4+ (p<0.001) and N stage (p=0.046) were independent factors for shorter OS.
Conclusion
Tumor tissue OCT4 expression was correlated with poor differentiation, tumor size, and N stage, and it can serve as an independent prognostic biomarker in operative patients with RC receiving adjuvant therapy.
Keywords: OCT4, SOX2, NANOG, prognosis, rectal cancer
INTRODUCTION
Rectal cancer (RC), one of the most commonly diagnosed malignant tumors, is a crucial threat imperiling human health worldwide.1 2016 cancer statistics report indicated approximately 39220 new RC cases in the United States and estimated incidence of 11 per 100000 people in the developing countries.2,3 Despite of great improvement in early diagnosis, operative treatment, adjuvant therapies (such as chemotherapy, radiotherapy and hormonotherapy) and integrated patients' care, the prognosis of RC patients remains far from satisfaction.4
Cancer stem cells (CSCs), a small subset of tumor cells, are characterized by self-renewing capacity, heterogeneity and resistance to several therapies (such as chemotherapy and radiotherapy), and contribute to the process of tumor development, infiltration, metastasis and recurrence.5 As common stem related transcription factors, octamer-binding transcription factor 4 (OCT4), sex determining region Y-box 2 (SOX2), and Nanog homebox (NANOG) are considered as critical regulators of self-renewal and pluripotency of embryonic stem, mediating tumor proliferation and tumor differentiation. And they have been identified as predictive biomarkers for poor prognosis in several carcinomas including hepatocellular cancer, esophageal squamous cell carcinoma (ESCC) and ovarian cancer.6,7,8,9 Several earlier studies indicate that OCT4, SOX2, and NANOG are frequently involved in digestive diseases, even though few studies have explored7,10 the effects of OCT4, SOX2, and NANOG expression on the prognosis in RC patients. Therefore, the purpose of this study was to investigate the association of cancer stem-cell marker (OCT4, SOX2, and NANOG) expressions with clinicopathological properties and overall survival (OS) in operative RC patients receiving adjuvant therapy.
MATERIALS AND METHODS
Participants
153 patients with primary RC receiving surgery at Department of General Surgery in the Second Affiliated Hospital of Harbin Medical University between December 2009 and December 2010 were consecutively enrolled in this cohort study. All patients were diagnosed by the validation of clinical properties, imageological examination and pathologic analysis. Patients who had received pre-surgery neo-adjuvant therapies, or were complicated with or history of other tumors, hematological malignancies or severe infection were excluded from this study. This study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University with approval No. 2009PHB012-01. All the patients signed the informed consents.
Treatment after surgery
In these 153 RC patients, 20 RC patients who were in TNM Stage I didn't take adjuvant treatment after surgery, while 131 patients who in TNM Stage II and Stage III received 5-fluorouracil based chemotherapy, among which 31% patients received radiotherapy at the same time, but others did not. Two Stage IV patients gave up chemotherapy or radiotherapy due to poor outcomes of surgery.
Data collection
Baseline demographic, pathological and clinical characteristics of all patients, including age, gender, pathological grade, tumor size, positive lymph nodes, distant metastasis, and TNM stage, were collected. TNM stage was assessed according to the 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual and the pathological grade was divided into well differentiation, moderate differentiation, and poor differentiation.
Follow-ups
Patients were intensively followed up during the first half year post-operation; once a month, followed by once every 3−9 months. The median follow-up duration was 5.2 years, and the last follow-up date was August 2016. OS was calculated from the time of the surgery to date of death from any cause or last follow-up.
Sample collection
Tumor tissue samples and paired adjacent normal tissue were obtained from all patients during the surgery, and they were subsequently fixed in formalin and embedded in paraffin for further determination.
Immunofluorescent staining
The formalin-fixed, paraffin-embedded sections (5 µm thick) were deparaffined and dehydrated at 65℃ for 3 h. After permeabilizaion in polybutylene terephthalate (PBST) [phosphate-buffered saline (PBS), 1% bovine serum albumin (BSA), 0.1% Triton] overnight, the sections were then treated with methanol containing 0.3% hydrogen peroxide and autoclaved at 121℃ for 10 min for antigen retrieval. After blocking with 10% goat serum, one section was incubated at 4℃ overnight with rabbit antibody against OCT4 with dilution 1:1600 (C52G3, CST, Boston, MA, USA) and mouse antibody against SOX2 with dilution 1:400 (L1D6A2, CST), while another section was incubated with mouse antibody against NANOG with dilution 1:2000 (1E6C4, CST). After washing with PBS three times, Alexa Fluor® 488 Conjugate labelled (green) antibody against rabbit IgG with dilution 1:500 (4412, CST) and Alexa Fluor® 594 Conjugate labelled (red) antibody against mouse IgG with dilution 1:500 (8890, CST) were added as secondary antibodies. After staining, the sections were counterstained with Hoechst 33342.
Histological score (HSCORE) ranged from 0 (no staining) to 4 (maximal staining), which was calculated to assess immunofluorescent staining, and the computational formula as follows: HSCORE=ΣPi(i+1). HSCORE 0.7 was considered as a threshold to distinguish positivity and negativity of immunofluorescent staining.11
Statistics
Statistics was carried out using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and 2010 office software (Microsoft, Boston, MA, USA). Data were presented as mean±standard deviation, count (percentage), or p value. OCT4, SOX2, and NANOG expressions in tumor tissues and paired adjacent tissues were compared by chi-square test. Correlation of expressions of these three factors with each other in patients was determined by Spearman test. Kaplan-Meier was drawn and log-rank test was used to investigate the correlation of OCT4, SOX2, and NANOG expressions with OS. Univariate Cox proportional hazard regression was performed to analyze the factors affecting OS, while factors with a p value <0.1 was further analyzed by multivariate Cox proportional hazard regression. p value <0.05 was considered significant.
RESULTS
Baseline characteristics
In the present study, the mean age of RC patients was 65.97± 11.59 years, and male and female were 83 and 70, respectively (Table 1). The number of RC patients with well, moderate, and poor differentiation was 23 (15%), 109 (71%), and 21 (14%), respectively. The mean of tumor size was 4.71±1.22 cm, and 79 (52%) patients were with tumor size <5 cm, while 74 (48%) patients were with tumor size ≥5 cm. There were 3 (2%) patients in T1 stage, 17 (11%) patients in T2 stage, 131 (86%) patients in T3 stage and 2 (1%) patients in T4a stage. As for N stage, 90 (59%) patients were in N0, 24 (16%) patients in N1a, 15 (10%) patients in N1b, 3 (2%) patients in N1c, 12 (8%) patients in N2a, and 9 (6%) patients in N2b. One hundred and fifty one (99%) patients were in M0 stage, and 2 (1%) patients were in M1a stage. According to the T, N, and M stage, 20 (13%), 68 (44%), 52 (34%), 11 (7%), and 2 (1%) patients were in TNM stage I, stage IIA, stage IIIB, stage IIIC, and stage IVA, respectively.
Table 1. Baseline Characteristics of RC Patients.
| Parameters | RC patients (n=153) |
|---|---|
| Age (yrs) | 65.97±11.59 |
| Gender (male/female) | 83/70 |
| Pathological grade, n (%) | |
| Well differentiation | 23 (15) |
| Moderate differentiation | 109 (71) |
| Poor differentiation | 21 (14) |
| Tumor size (cm) | 4.71±1.22 |
| <5 | 79 (52) |
| ≥5 | 74 (48) |
| T stage, n (%) | |
| T1 | 3 (2) |
| T2 | 17 (11) |
| T3 | 131 (86) |
| T4a | 2 (1) |
| N stage, n (%) | |
| N0 | 90 (59) |
| N1a | 24 (16) |
| N1b | 15 (10) |
| N1c | 3 (2) |
| N2a | 12 (8) |
| N2b | 9 (6) |
| M stage, n (%) | |
| M0 | 151 (99) |
| M1a | 2 (1) |
| TNM stage, n (%) | |
| I | 20 (13) |
| IIA | 68 (44) |
| IIIB | 52 (34) |
| IIIC | 11 (7) |
| IVA | 2 (1) |
RC, rectal cancer.
Data was presented as mean value±standard deviation or counts (with or without percentage).
OCT4, SOX2, and NANOG expression in tumor tissues and paired adjacent normal tissues
OCT4, SOX2 and NANOG expressions were mainly expressed on the cytoplasm of cell (Fig. 1). As shown in Table 2, OCT4 (p<0.001), SOX2 (p=0.003), and NANOG (p<0.001) expressions in tumor tissue were higher than those in adjacent tissue. Co-expressions status of OCT4, SOX2, and NANOG in tumor tissues was evaluated, and Table 3 shows that there were 31 (20%) patients with no marker positive, 50 (33%) patients with only one marker positive, 48 (31%) patients with two markers positive [including: 1) OCT4+ and SOX2+ group (N=28, 18%); 2) OCT4+ and NANOG+ group (N=16, 10%); and 3) SOX2+ and NANOG+ group (N=4, 3%)], and 24 (16%) patients with all three markers positive.
Fig. 1. Immunohistochemical staining for OCT4, SOX2, and NANOG expressions in tumor tissues and paired adjacent normal tissues. Immunohistochemical staining for OCT4− (A), OCT4+ (B), SOX2− (C), SOX2+ (D), NANOG− (E), and NANOG+ (F). OCT4, SOX2, and NANOG expressions were mainly found on cell membrane. OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; NANOG, Nanog homebox.
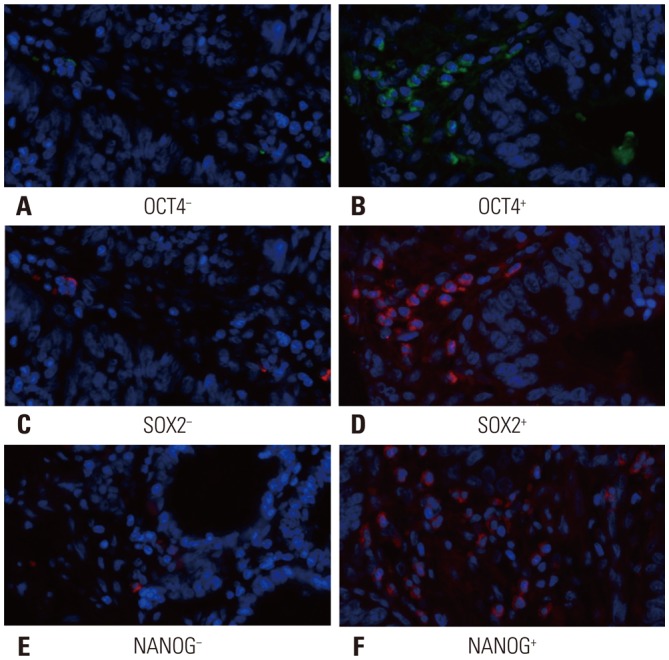
Table 2. OCT4, SOX2, and NANOG Expression in Tumor Tissues and Paired Adjacent Tissues.
| Parameters (n=153) | OCT4 | SOX2 | NANOG | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| Tumor tissue (%) | 85 (56) | 68 (44) | 72 (47) | 81 (53) | 61 (40) | 92 (60) |
| Adjacent tissue (%) | 34 (22) | 119 (78) | 47 (31) | 106 (69) | 28 (18) | 125 (82) |
| p value | <0.001* | 0.003* | <0.001* | |||
OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; NANOG, Nanog homebox.
Data was presented as counts (percentage). Comparison was determined by chi-square test.
*p<0.05 was considered significant.
Table 3. Co-expression of OCT4, SOX2, and NANOG in Tumor Tissues.
| Parameters | Rectal cancer patients (n=153) |
|---|---|
| Patients with no marker positive (%) | 31 (20) |
| Patients with only one marker positive (%) | 50 (33) |
| Patients with two markers positive (%) | 48 (31) |
| OCT4+ and SOX2+ | 28 (18) |
| OCT4+ and NANOG+ | 16 (10) |
| SOX2+ and NANOG+ | 4 (3) |
| Patients with all three markers positive (%) | 24 (16) |
OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; NANOG, Nanog homebox.
Data was presented by counts (percentage).
Correlations of OCT4, SOX2, and NANOG expressions
Correlations of expressions of these three factors with each other in patients were assessed, and Table 4 shows that OCT4 expression was positively correlated with expression of SOX2 (R=0.316, p<0.001) and NANOG (R=0.164, p=0.043), while no correlation between SOX2 and NANOG expressions was found (R=-0.019, p=0.817).
Table 4. Correlation of OCT4, SOX2, and NANOG Expression.
| OCT4 | SOX2 | NANOG | |
|---|---|---|---|
| OCT4 | |||
| Coefficient R | 1.000 | - | - |
| p value | - | - | - |
| SOX2 | |||
| Coefficient R | 0.316 | 1.000 | - |
| p value | <0.001* | - | - |
| NANOG | |||
| Coefficient R | 0.164 | −0.019 | 1.000 |
| p value | 0.043* | 0.817 | - |
OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; NANOG, Nanog homebox.
Correlation between each two continuous variables was determined by Spearman test.
*p<0.05 was considered significant.
Correlations of OCT4, SOX2, and NANOG expressions with clinical and pathological features of RC patients
Correlations of OCT4, SOX2, and NANOG expressions with clinical and pathological features of RC patients were evaluated. As shown in Table 5, OCT4 expression was positively correlated with pathological grade (R=0.185, p=0.022), tumor size (R=0.224, p=0.005), and N stage (R=0.170, p=0.036), whereas NANOG expression was positively associated with tumor size (R=0.169, p=0.036). No correlation of OCT4, SOX2, and NANOG expressions with other clinical and pathological features was found in RC patients (Table 5).
Table 5. Correlation of OCT4, SOX2, and NANOG Expression with Clinicopathological Features.
| Parameters | Age | Gender (male) | Pathological grade | Tumor size | T stage | N stage | M stage | TNM stage |
|---|---|---|---|---|---|---|---|---|
| OCT4 expression | ||||||||
| R | −0.122 | 0.155 | 0.185* | 0.224* | 0.143 | 0.170* | −0.013 | 0.091 |
| p value | 0.133 | 0.055 | 0.022* | 0.005* | 0.077 | 0.036* | 0.875 | 0.264 |
| SOX2 expression | ||||||||
| R | −0.023 | −0.054 | 0.079 | 0.047 | −0.126 | 0.045 | −0.109 | −0.113 |
| p value | 0.774 | 0.506 | 0.329 | 0.561 | 0.121 | 0.583 | 0.182 | 0.163 |
| NANOG expression | ||||||||
| R | −0.012 | −0.056 | −0.124 | 0.169* | −0.051 | 0.139 | −0.094 | 0.062 |
| p value | 0.887 | 0.491 | 0.128 | 0.036* | 0.534 | 0.087 | 0.249 | 0.450 |
OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; NANOG, Nanog homebox.
Data was presented as correlation index R and p value. Correlation was determined by Pearson test.
*p<0.05 was considered significant.
Correlations of OCT4, SOX2, and NANOG expressions with OS
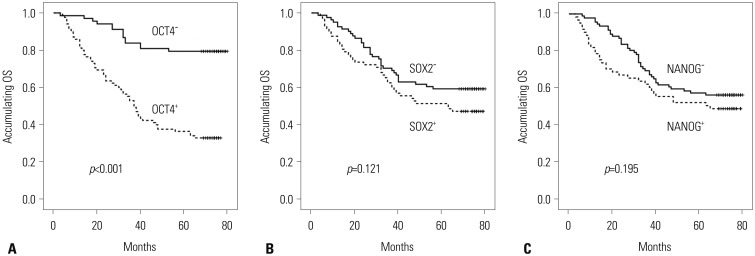
The mean OS was 56 months, and the 5-year survival was 53.6% in these RC patients. Correlations of OCT4, SOX2, and NANOG expressions with OS were assessed. Fig. 2 shows that OCT4+ was associated with worse OS compared with OCT4– in RC patients (p<0.001) (Fig. 2A), while no association of SOX2 (p=0.121) (Fig. 2B) and NANOG expressions (p=0.195) (Fig. 2C) with OS was observed in the present study.
Fig. 2. The correlations of OCT4, SOX2, and NANOG expressions with OS. (A) Correlation of OCT4+ and OCT4− with OS. (B) Correlation of SOX2+ and SOX2− with OS. (C) Correlation of NANOG+ and NANOG− with OS. Kaplan-Meier curves were used to analyse the correlation of OCT4, SOX2, and NANOG expressions with OS. Comparison of two groups was made by log-rank test. p<0.05 was considered significant. OS, overall survival; OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; NANOG, Nanog homebox.
Correlation of number of positive markers with OS
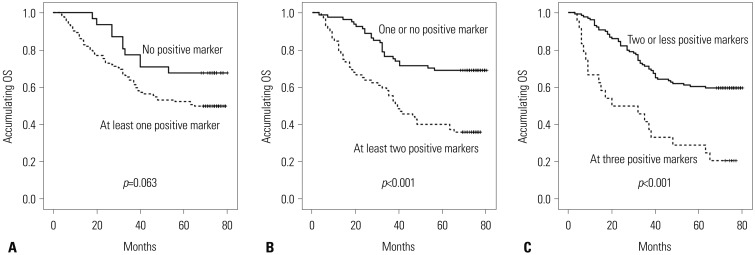
Compared with one or no positive marker, at least two positive markers were associated with shorter OS (p<0.001) (Fig. 3B), while all three positive markers were correlated with even worse OS compared with two or less positive markers (p< 0.001) (Fig. 3C). No correlation of no positive marker and at least one positive marker with OS was found (p=0.063) (Fig. 3A).
Fig. 3. The correlation of number of positive markers with OS. (A) Association of no positive marker and at least one positive marker with OS. (B) Association of one or no positive marker and at least two positive markers with OS. (C) Association of two or less positive markers and all three positive markers with OS. Kaplan-Meier curves were used to evaluate the correlation of number of positive markers with OS. Comparison of two groups was made by log-rank test. p<0.05 was considered significant. OS, overall survival.
Analysis of factors affecting OS by univariate and multivariate model
Univariate Cox's proportional hazards regression was used to evaluate the factors affecting OS in RC patients. Table 6 indicates that OCT4+ (p<0.001), poor differentiation (p<0.001), T stage (p=0.033), N stage (p<0.001), and TNM stage (p<0.001) were correlated with worse OS in RC patients. All factors with a p value <0.1 were further analysed by the multivariate Cox's proportional hazards regression model. The result indicated that the OCT4+ (p<0.001) and N stage (p=0.046) were independent factors for shorter OS.
Table 6. Univariate and Multivariate Cox Analysis of the Factors Affecting OS in Rectal Cancer Patients.
| Univariate Cox | Multivariate Cox | |||||||
|---|---|---|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | HR | 95% CI | |||
| Lower | Higher | Lower | Higher | |||||
| OCT4+ (vs. OCT4−) | <0.001* | 4.724 | 2.626 | 8.496 | <0.001* | 4.071 | 2.210 | 7.498 |
| SOX2+ (vs. SOX2−) | 0.126 | 1.440 | 0.903 | 2.295 | - | - | - | - |
| NANOG+ (vs. NANOG−) | 0.199 | 1.360 | 0.850 | 2.174 | - | - | - | - |
| Age | 0.269 | 0.988 | 0.968 | 1.009 | - | - | - | - |
| Gender (male) | 0.261 | 1.310 | 0.818 | 2.098 | - | - | - | - |
| Tumor size ≥5 cm (vs. <5 cm) | 0.125 | 1.158 | 0.960 | 1.397 | - | - | - | - |
| Pathological grade | <0.001* | 2.273 | 1.450 | 3.563 | 0.264 | 1.470 | 0.748 | 2.890 |
| T stage | 0.033* | 1.961 | 1.057 | 3.641 | 0.728 | 1.187 | 0.452 | 3.114 |
| N stage | <0.001* | 2.011 | 1.479 | 2.736 | 0.046* | 1.685 | 1.010 | 2.813 |
| M stage | 0.140 | 2.910 | 0.705 | 12.007 | - | - | - | - |
| TNM stage | <0.001* | 1.861 | 1.314 | 2.636 | 0.264 | 1.470 | 0.748 | 2.890 |
OS, overall survival; HR, hazard ratio; CI, confidence interval; OCT4, octamer-binding transcription factor 4; SOX2, sex determining region Y-box 2; NANOG, Nanog homebox.
Data was presented as p value, HR, and 95% CI. Univariate Cox proportional hazard regression was performed to analyze the factors affecting OS, while factors with p value <0.1 was further analyzed by multivariate Cox proportional hazard regression. Pathological grade was scored as 1-well differentiation, 2-moderate differentiation, 3-poor differentiation; T stage was scored as 1-T1, 2-T2, 3-T3, and 4-T4; N stage was scored as 0-N0, 1-N1, and 2-N2; M stage was scored as 0-M0 and 1-M1; TNM stage was scored as 1-Stage I, 2-Stage II, 3-Stage III, and 4-Stage IV. Based on these definitions, Cox proportional hazard regressions analysis was performed.
*p value <0.05 was considered significant.
DISCUSSION
The present study found that: 1) Stronger expressions of OCT4, SOX2, and NANOG were found in tumor tissue than those in paired adjacent tissue; 2) OCT4 expression was positively correlated with poor differentiation, tumor size and N stage, while NANOG expression was positively associated with tumor size; 3) OCT4+ was correlated with worse OS than OCT4− in RC patients, and OCT4+ and N stage could independently predict worse OS; and 4) All three positive markers seem to have greater value in predicting worse OS compared with only one or two positive markers in RC patients.
OCT4, located in 6 chromosomes (6p21.31), is one of the Pit-Oct-Unc (POU) transcription factor family members, which plays a vital role in maintaining stem cell self-renewal, pluripotency, and differentiation.12 Upregulating OCT4 expression induces the proliferation and metastasis of epithelial ovarian carcinoma cells by regulating mircoRNA (miRNA)-26b/karyopherin α2 (KPNA2) axis,8 and stimulates cervical cancer cell proliferation and represses its apoptosis by mediating miR-125b/BRI1-Associated Receptor Kinase 1 (BAK1) pathway.13 Also, OCT4 expression targets phosphatase and tensin homolog (PTEN) and Tenascin-C (TNC) genes, increasing the migration of lung cancer cells,14 and inhibits differentiation by targeting miR-145 in endometrial carcinoma cells.15 As described above, a large amount of evidence indicate that OCT4 expression acts as a tumor promotor in the tumorigenesis and malignant transformation of various carcinomas.
As for patients with renal cell carcinoma, high expression of tissue OCT4 is positively associated with histological grade, TNM stage, and lymphatic metastasis.16 Moreover, positive correlation of tissue OCT4 up-regulation with poorer differentiation and higher TNM stage was also found in patients with non-small-cell lung cancer (NSCLC).17 In line with previous findings in other tumors, we found that OCT4 expression in RC tissue was positively correlated with poor differentiation, tumor size, and N stage. It is highly likely that high expression of OCT4 might induce tumorigenesis and tumor metastasis by promoting cells proliferation/invasion and inhibiting apoptosis via mediating various genes and pathways.13,14,15
In terms of prognostic value, tumor tissue OCT4+ has been shown to be a potential biomarker forecasting poor prognosis in patients with several malignancies, such as ESSC, gastric cancer, oral squamous cell carcinoma, and bladder cancer.18,19,20,21 Partially in line with these results, our present study revealed that OCT4+ could independently predict worse OS in surgical RC patients receiving adjuvant therapy. There are two possible reasons. Firstly, acting as a CSCs biomarker, OCT4, could increase CSCs, resulting in the resistance of radiotherapy, chemotherapy, and conventional systemic therapy, thereby decreasing therapeutic effects and increasing recurrences in RC patients.22,23,24,25 Secondly, OCT4 expression regulates multiple signalling pathways, including p38 mitogen-activated protein kinase (MAPK)/caspase-3, Wnt/β-catenin, AKT, and Janus Kinase (JAK)/signal transducer and activator of transcription (STAT)3 signal pathways, thereby resulting in the promotion of tumorigenesis and malignant transformation, and increase of recurrences.26,27,28,29
Although resection surgery is considered as a golden standard for therapy in RC patients, endoscopy has rapidly evolved and widely been applied in RC patients over the last decade, which is helpful for early diagnosis and treatment for single and small tumors.30 It is of an interest to cite a previous report, which showed that OCT4 expression contributes to early detection and/or therapy in gastric cancer patients treated with endoscopy.31 However, the the best of our knowledge, this is the only study to explore the role of OCT4 in RC patients who received endoscopy. Therefore, the effect of OCT4 in tissue samples obtained from RC patients treated with endoscopy should be investigated.
SOX2 [located on chromosome 3 (3p181.71)] and NANOG [located on chromosome 12 (12p13.31)] are responsible for regulating the pluripotency and self-renewal of stem cells.32,33 Both of them have been identified to play critical roles in multiple processes during tumorigenesis and tumor development in various malignant tumors, such as lung cancer, breast cancer, and endometrial cancer.34,35 Whole blood SOX2 has been shown to be positively associated with lymph node metastasis, and SOX2 expression predicts poor progression-free survival and OS in small-cell lung cancer patients after first-line chemo-therapy.36,37 On the other hand, however, overexpression of tissue SOX2 has also been shown to predict better outcome in NSCLC patients.38 As for the NANOG expression, high expression of tissue NANOG was found in renal cancer patients, even though no association was found between NANOG expression and any clinicopathological features.16 The results, therefore, are not always consistent, and some controversies about the effects of SOX2 and NANOG expressions on the prognosis of tumors still exist.16,38,39 In the current study, we showed that NANOG expression was associated only with tumor size, however, no correlation of SOX2 and NANOG expressions with other clinicopathological properties as well as OS in operative RC patients receiving adjuvant therapy was observed.
We also found that the combination of all three positive markers seems to have even greater value in predicting worse OS compared with only one or two positive markers in RC patients. It is quite possible that CSCs markers (OCT4, SOX2, and NANOG expressions) are strongly associated with CSCs that are characterized by stem-cell properties. Therefore, all three positive markers could reflect high concentration of CSCs, which is related to the resistance of radio-, chemo-, and hormone-therapy, thereby increasing recurrences in RC patients.
Some limitations existed in this study: 1) Sample size was relatively small, possibly resulting in lower statistical efficiency compared with larger sample size studies. 2) There were two RC patients in stage IV enrolled in our study, which might affect the predictive value of tumor tissue OCT4, SOX2, and NANOG expressions on RC patients. In the present study, therefore, we excluded these two stage IV patients and analysed the correlations of OCT4, SOX2, and NANOG expressions with clinicopathological properties and OS in RC patients in stage I, II, and III. We found no difference in the prospective value between RC patients with or without stage IV patients. The effects of tumor tissue OCT4, SOX2, and NANOG expression in the stage IV patients should to be investigated in further study. 3) In the current study, OCT4, SOX2, and NANOG expressions in blood sample were not assessed, even though blood sample is more easily obtained because of its non-invasiveness, particularly the RC patients in stage IV who cannot undergo surgery or biopsy.
In conclusion, tumor tissue OCT4 expression was correlated with poor differentiation, tumor size, and N stage, and it could serve as an independent prognostic biomarker in operative patients with RC receiving adjuvant therapy.
Footnotes
The authors have no financial conflicts of interest.
ACKNOWLEDGEMENTS
This study was supported by Research and Development Project of Scientific Achievement Transformation of Harbin Medical University, the Mechanism of CircRNA in the Pathogenesis of Colorectal Cancer.
References
- 1.Li X, Lu H, Xu K, Wang H, Liang X, Hu Z. Negative lymph node count is an independent prognostic factor for patients with rectal cancer who received preoperative radiotherapy. BMC Cancer. 2017;17:227. doi: 10.1186/s12885-017-3222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Hoecht S, Hinkelbein W. Treatment of rectal cancer. N Engl J Med. 2006;355:2486. author reply 2487-8. [PubMed] [Google Scholar]
- 5.Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer. 2013;13:727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 6.Cao L, Li C, Shen S, Yan Y, Ji W, Wang J, et al. OCT4 increases BIRC5 and CCND1 expression and promotes cancer progression in hepatocellular carcinoma. BMC Cancer. 2013;13:82. doi: 10.1186/1471-2407-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Huang GR, Hu P. Over-expression of Oct4 in human esophageal squamous cell carcinoma. Mol Cells. 2011;32:39–45. doi: 10.1007/s10059-011-2315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J, Zhang L, Huang H, Huang Y, Huang L, Wang J, et al. MiR-26b/KPNA2 axis inhibits epithelial ovarian carcinoma proliferation and metastasis through downregulating OCT4. Oncotarget. 2015;6:23793–23806. doi: 10.18632/oncotarget.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumann J, Bahr F, Horst D, Kriegl L, Engel J, Luque RM, et al. SOX2 expression correlates with lymph-node metastases and distant spread in right-sided colon cancer. BMC Cancer. 2011;11:518. doi: 10.1186/1471-2407-11-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–649. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 12.Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, et al. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093–1108. doi: 10.1089/scd.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YD, Cai N, Wu XL, Cao HZ, Xie LL, Zheng PS. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 2013;4:e760. doi: 10.1038/cddis.2013.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang YA, Chen CH, Sun HS, Cheng CP, Tseng VS, Hsu HS, et al. Global Oct4 target gene analysis reveals novel downstream PTEN and TNC genes required for drug-resistance and metastasis in lung cancer. Nucleic Acids Res. 2015;43:1593–1608. doi: 10.1093/nar/gkv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Liu S, Xin H, Jiang J, Younglai E, Sun S, et al. Up-regulation of microRNA-145 promotes differentiation by repressing OCT4 in human endometrial adenocarcinoma cells. Cancer. 2011;117:3989–3998. doi: 10.1002/cncr.25944. [DOI] [PubMed] [Google Scholar]
- 16.Yu B, Cai H, Xu Z, Xu T, Zou Q, Gu M. Expressions of stem cell transcription factors Nanog and Oct4 in renal cell carcinoma tissues and clinical significance. Artif Cells Nanomed Biotechnol. 2016;44:1818–1823. doi: 10.3109/21691401.2015.1105238. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Wang J, Xu Z, Ahmad A, Li E, Wang Y, et al. Expression of Sox2 and Oct4 and their clinical significance in human non-small-cell lung cancer. Int J Mol Sci. 2012;13:7663–7675. doi: 10.3390/ijms13067663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Yan Y, Ji W, Bao L, Qian H, Chen L, et al. OCT4 positively regulates Survivin expression to promote cancer cell proliferation and leads to poor prognosis in esophageal squamous cell carcinoma. PLoS One. 2012;7:e49693. doi: 10.1371/journal.pone.0049693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka J, Yashiro M, Sakurai K, Kubo N, Tanaka H, Muguruma K, et al. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Surg Res. 2012;174:130–135. doi: 10.1016/j.jss.2010.11.903. [DOI] [PubMed] [Google Scholar]
- 20.Atlasi Y, Mowla SJ, Ziaee SA, Bahrami AR. OCT-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int J Cancer. 2007;120:1598–1602. doi: 10.1002/ijc.22508. [DOI] [PubMed] [Google Scholar]
- 21.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, et al. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 22.Shen L, Huang X, Xie X, Su J, Yuan J, Chen X. High expression of SOX2 and OCT4 indicates radiation resistance and an independent negative prognosis in cervical squamous cell carcinoma. J Histochem Cytochem. 2014;62:499–509. doi: 10.1369/0022155414532654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linn DE, Yang X, Sun F, Xie Y, Chen H, Jiang R, et al. A role for OCT4 in tumor initiation of drug-resistant prostate cancer cells. Genes Cancer. 2010;1:908–916. doi: 10.1177/1947601910388271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Yao Z, Wan Y, Lin D. Overexpression of OCT4 is associated with gefitinib resistance in non-small cell lung cancer. Oncotarget. 2016;7:77342–77347. doi: 10.18632/oncotarget.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan JP, Minna JD, Shay JW. Evidence for self-renewing lung cancer stem cells and their implications in tumor initiation, progression, and targeted therapy. Cancer Metastasis Rev. 2010;29:61–72. doi: 10.1007/s10555-010-9216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Zhou Y, Zhang X, Yang Y, Dan S, Su T, et al. Dual inhibiting OCT4 and AKT potently suppresses the propagation of human cancer cells. Sci Rep. 2017;7:46246. doi: 10.1038/srep46246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emhemmed F, Ali Azouaou S, Thuaud F, Schini-Kerth V, Désaubry L, Muller CD, et al. Selective anticancer effects of a synthetic flavagline on human Oct4-expressing cancer stem-like cells via a p38 MAPK-dependent caspase-3-dependent pathway. Biochem Pharmacol. 2014;89:185–196. doi: 10.1016/j.bcp.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, et al. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016;374:292–303. doi: 10.1016/j.canlet.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Teng HF, Li PN, Hou DR, Liu SW, Lin CT, Loo MR, et al. Valproic acid enhances Oct4 promoter activity through PI3K/Akt/mTOR pathway activated nuclear receptors. Mol Cell Endocrinol. 2014;383:147–158. doi: 10.1016/j.mce.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 30.PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute; 2002. Rectal Cancer Treatment (PDQ(R)): Health Professional Version. [Google Scholar]
- 31.Al-Marzoqee FY, Khoder G, Al-Awadhi H, John R, Beg A, Vincze A, et al. Upregulation and inhibition of the nuclear translocation of Oct4 during multistep gastric carcinogenesis. Int J Oncol. 2012;41:1733–1743. doi: 10.3892/ijo.2012.1608. [DOI] [PubMed] [Google Scholar]
- 32.Wen Y, Hou Y, Huang Z, Cai J, Wang Z. SOX2 is required to maintain cancer stem cells in ovarian cancer. Cancer Sci. 2017;108:719–731. doi: 10.1111/cas.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao L, Song Y, Huang W, Yang S, Fu J, Feng X, et al. Expression of SOX2, NANOG and OCT4 in a mouse model of lipopolysaccharide-induced acute uterine injury and intrauterine adhesions. Reprod Biol Endocrinol. 2017;15:14. doi: 10.1186/s12958-017-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piva M, Domenici G, Iriondo O, Rábano M, Simöes BM, Comaills V, et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol Med. 2014;6:66–79. doi: 10.1002/emmm.201303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sodja E, Rijavec M, Koren A, Sadikov A, KoroŠec P, Cufer T. The prognostic value of whole blood SOX2, NANOG and OCT4 mRNA expression in advanced small-cell lung cancer. Radiol Oncol. 2016;50:188–196. doi: 10.1515/raon-2015-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamawaki K, Ishiguro T, Mori Y, Yoshihara K, Suda K, Tamura R, et al. Sox2-dependent inhibition of p21 is associated with poor prognosis of endometrial cancer. Cancer Sci. 2017;108:632–640. doi: 10.1111/cas.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velcheti V, Schalper K, Yao X, Cheng H, Kocoglu M, Dhodapkar K, et al. High SOX2 levels predict better outcome in non-small cell lung carcinomas. PLoS One. 2013;8:e61427. doi: 10.1371/journal.pone.0061427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sholl LM, Barletta JA, Yeap BY, Chirieac LR, Hornick JL. Sox2 protein expression is an independent poor prognostic indicator in stage I lung adenocarcinoma. Am J Surg Pathol. 2010;34:1193–1198. doi: 10.1097/PAS.0b013e3181e5e024. [DOI] [PMC free article] [PubMed] [Google Scholar]