Abstract
Purpose
Obesity is often associated with better clinical outcomes in heart failure (HF). This so-called obesity paradox remains controversial. The aim of present study was to investigate the prognostic value of obesity in patients hospitalized for systolic HF.
Materials and Methods
We performed a pooled analysis of data from two multicenter, observational HF studies. Patients hospitalized for systolic HF were eligible for the present study. We divided the subjects into two groups, a normal body mass index (BMI) group and a high BMI group. Study endpoints included all-cause mortality and any re-hospitalization within 1 year.
Results
We enrolled 3145 patients (male, 1824; female, 1321). The high BMI group was significantly associated with lower 1-year mortality rate [odds ratio (OR), 0.543; 95% confidence interval (CI), 0.355−0.832] after adjusting for age, hypertension, diabetes, ischemic HF, previous myocardial infarction, serum creatinine level, anemia, and ejection fraction in men. After adjustment for clinical characteristics, high BMI was not significantly associated with 1-year mortality (OR, 0.739; 95% CI, 0.450−1.216) or 1-year re-hospitalization (OR, 0.958; 95% CI, 0.696−1.319) in women.
Conclusion
In pooled analysis of data from two Korean HF registries, the high BMI group was independently associated with lower 1-year mortality rate from systolic HF, especially in men.
Keywords: Obesity, heart failure, systolic, sex difference
INTRODUCTION
Although obesity is an independent risk factor for cardiovascular disease, including heart failure (HF),1 an elevated body mass index (BMI) is paradoxically associated with improved clinical outcomes in the setting of established HF.2,3,4,5,6 This so-called obesity paradox seems to be more prominent in men according to several studies.2,3,4,5,6 This phenomenon is also apparent in Asians.7 Nevertheless, the existence of the obesity paradox remains controversial.
Although fat distribution varies by sex, the role of obesity according to sex in the outcomes of HF has not been well evaluated.6,7 Therefore, the primary aim of the present study was to confirm the existence of the obesity paradox in systolic HF and, if the obesity paradox exists, the existence of a sex-related difference therein.
MATERIALS AND METHODS
Study design
We merged data from two large registries, The Korean Heart Failure registry (KorHF), Survey of Guideline Adherence for Treatment of Systolic Heart Failure in Real World (SUGAR). KorHF is a nationwide, prospective, observational, multicenter, online registry for patients hospitalized for acute HF between June 2004 and April 2009, with 24 participating hospitals in Korea.8 The study population included all adult patients (age ≥18 years old) with a hospitalization for HF with a left ventricular ejection fraction (LVEF) of <45% at admission since January 2009. The SUGAR trial is a multicenter, retrospective observational study on subjects admitted for systolic HF [ejection fraction (EF) <45%] in 23 university hospitals since January 2008 (ClinicalTrials.gov Identifier: NCT01390935).
The study was approved by the Institutional Review Board of the Wonju Medical College of Yonsei University (No 311006).
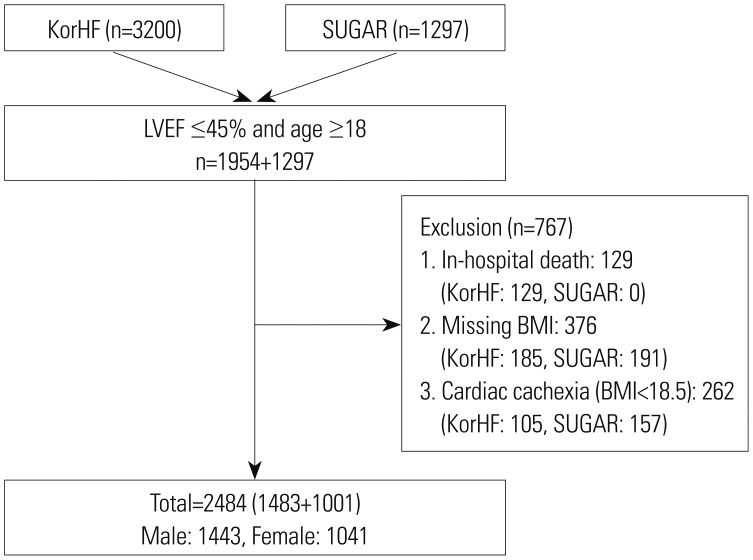
Patients aged ≥18 years with reduced LVEF ≤45% in three registries were eligible for the present study. The subjects without information on height and weight were excluded. Underweight HF patients may have “cardiac cachexia,” which is known to be associated with worse prognosis; thus, to adjust for the potential confounding effect of patients with cachexia or frailty, those classified as underweight (BMI <18.5 kg/m2) were excluded from the analysis.9 According to the World Health Organization criteria, overweight was defined as a BMI of ≥25 kg/m2.10 The selection of the study population is described in Fig. 1.
Fig. 1. Selection of study population. KorHF, The Korean Heart Failure registry; SUGAR, Survey of Guideline Adherence for Treatment of Systolic Heart Failure in Real World; LVEF, left ventricular ejection fraction; BMI, body mass index.
Endpoints
The study endpoints included 1-year all-cause mortality and 1-year re-hospitalization. Given the nature of systolic HF, which is frequently aggravated by trivial triggers and require hospitalization, any re-hospitalization was selected as an endpoint. Owing to ambiguity in distinguishing cardiac death from non-cardiac death in systolic HF, we also chose all-cause mortality as a study endpoint, instead of cardiac death.
Statistical analysis
Statistical analyses were conducted using SPSS version 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean±standard deviation. Categorical variables were expressed as absolute numbers and percentages. All analyses were performed separately according to sex. To adjust for significant covariates, multivariable models were developed for all-cause mortality and re-hospitalization. We used binary logistic regression models to estimate unadjusted and adjusted relationships between each variable and patient outcomes. The adjusted model was controlled for baseline demographic and clinical characteristics. These included age, hypertension, diabetes mellitus (DM), ischemic HF, previous myocardial infarction (MI), EF, serum creatinine (Cr) level, and anemia [hemoglobin (Hb) <10 g/dL]. Kaplan-Meier analyses were used to compare between endpoints. The log-rank test was used to test for differences in unadjusted survival curves. A two-sided p-value of <0.05 was considered significant.
RESULTS
Demographics
Finally, 2484 patients (men, 1443; women, 1041) were included in the present study (Fig. 1). For the analysis, we considered two BMI groups, a normal BMI group (BMI, 18.5−24.9 kg/m2) and a high BMI group (BMI ≥25.0 kg/m2). The baseline characteristics of the patients are presented in Table 1 (men) and Table 2 (women). Among the men, subjects in the high BMI group were younger (58.13 years vs. 66.41 years, p<0.001) and had a higher blood pressure (133/83 mm Hg vs. 126/77 mm Hg, p<0.001). The subjects with high BMI more frequently presented with prior hypertension (52.9% vs. 47.9%), but less frequently with ischemic HF (35.0% vs. 44.1%, p<0.001), left bundle branch block (3.8% vs. 7.9%), and chronic kidney disease (9.7% vs. 13.3%, p=0.032). Among women, subjects in the high BMI group were younger (67.17 years vs. 70.51 years, p<0.001) and more frequently presented with prior hypertension (61.4% vs 54.1%, p=0.016), diabetes (45.2% vs. 35.2%, p<0.001), dyslipidemia (31.0% vs. 20.7%), and chronic kidney disease (12.9% vs. 8.1%, p=0.008).
Table 1. Baseline Characteristics of the Male Population According to BMI.
| Variable | 18.5≤BMI<25 n=978 |
25≤BMI<30 n=396 |
30≤BMI n=69 |
p for trend |
|---|---|---|---|---|
| BMI (kg/m2) | 22.1±1.7 | 26.8±1.3 | 33.6±3.5 | <0.001 |
| Age (yr) | 65.8±13.4 | 59.6±14.1 | 48.1±15.0 | <0.001 |
| SBP (mm Hg) | 126.8±27.0 | 132.0±30.1 | 140.3±29.6 | <0.001 |
| Heart rate (BPM) | 90.4±24.2 | 91.8±26.3 | 93.3±21.4 | 0.367 |
| Ischemic HF | 424 (43.8) | 155 (39.7) | 20 (29.0) | 0.013 |
| De novo HF | 594 (64.9) | 257 (69.8) | 42 (68.9) | 0.117 |
| Ejection fraction | 29.0±8.6 | 29.2±9.4 | 27.2±9.4 | 0.104 |
| LVEDD | 61.6±11.0 | 62.2±11.9 | 65.8±7.2 | 0.009 |
| LVESD | 50.9±13.3 | 51.9±13.3 | 55.4±8.2 | 0.014 |
| E/E' | 19.7±10.4 | 18.7±8.8 | 20.5±10.0 | 0.295 |
| LBBB | 71 (8.0) | 15 (4.3) | 1 (1.7) | 0.005 |
| Atrial fibrillation | 222 (24.3) | 117 (31.8) | 10 (16.4) | 0.299 |
| NYHA class 3 or 4 | 627 (70.9) | 252 (68.5) | 48 (76.2) | 0.980 |
| Past history | ||||
| HTN | 449 (45.9) | 199 (50.3) | 42 (60.9) | 0.011 |
| DM | 324 (33.2) | 127 (32.1) | 21 (30.4) | 0.571 |
| Stroke | 108 (15.3) | 42 (14.9) | 4 (7.5) | 0.265 |
| Previous MI | 190 (19.4) | 69 (17.4) | 10 (14.5) | 0.211 |
| COPD | 63 (6.9) | 15 (4.1) | 1 (1.6) | 0.016 |
| CKD | 127 (13.0) | 36 (9.1) | 10 (14.5) | 0.255 |
| Laboratory finding | ||||
| Hemoglobin (g/dL) | 13.0±2.2 | 14.1±2.1 | 14.8±2.7 | <0.001 |
| Na (mmol/L) | 138.3±4.8 | 139.1±4.0 | 139.1±4.1 | 0.194 |
| Glucose (mg/dL) | 156.5±80.4 | 149.9±63.9 | 126.1±44.1 | 0.001 |
| BUN (mg/dL) | 26.0±16.8 | 22.5±12.4 | 21.0±15.6 | 0.010 |
| Cr (mg/dL) | 1.6±1.5 | 1.4±0.6 | 1.5±0.9 | 0.365 |
| NT-proBNP (pg/mL) | 8832.3±9671.9 | 5249.3±6317.7 | 4260.6±3746.9 | 0.001 |
| Discharge medication | ||||
| ACEi or ARB | 773 (79.3) | 319 (80.6) | 61 (88.4) | 0.123 |
| Beta blocker | 521 (53.4) | 212 (53.5) | 45 (65.2) | 0.214 |
| MRA | 508 (52.0) | 226 (57.1) | 38 (55.1) | 0.138 |
BMI, body mass index; SBP, systolic blood pressure; BPM, beats per minute; HF, heart failure; LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LBBB, left bundle branch block; NYHA, New York Heart Association; HTN, hypertension; DM, diabetes mellitus; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; BUN, blood urea nitrogen; Cr, creatinine; BNP, brain natriuretic peptides; NT-proBNP, N terminal pro brain natriuretic peptides; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist.
Values are expressed as mean±SD or n (%).
Table 2. Baseline Characteristics of the Female Population According to BMI.
| Variable | 18.5≤BMI<25 n=723 |
25≤BMI<30 n=261 |
30≤BMI n=57 |
p for trend |
|---|---|---|---|---|
| BMI (kg/m2) | 21.9±1.7 | 26.6±1.3 | 32.8±2.7 | <0.001 |
| Age (yr) | 70.4±12.9 | 67.3±12.9 | 64.8±14.4 | 0.002 |
| SBP (mm Hg) | 128.8±27.6 | 130.7±27.6 | 128.4±25.7 | 0.918 |
| Heart rate (BPM) | 91.3±24.5 | 92.1±27.1 | 89.1±19.8 | 0.540 |
| Ischemic HF | 272 (37.9) | 105 (41.0) | 25 (43.9) | 0.247 |
| De novo HF | 430 (63.2) | 150 (60.7) | 34 (64.2) | 0.720 |
| Ejection fraction | 30.8±8.7 | 31.4±8.1 | 32.1±9.1 | 0.272 |
| LVEDD | 57.6±10.6 | 57.7±13.2 | 59.4±13.7 | 0.531 |
| LVESD | 47.4±11.5 | 47.0±12.2 | 18.8±14.0 | 0.585 |
| E/E' | 23.4±12.7 | 23.2±15.8 | 17.6±5.8 | 0.095 |
| LBBB | 72 (11.0) | 17 (7.1) | 6 (11.5) | 0.311 |
| Atrial fibrillation | 161 (23.8) | 55 (22.3) | 15 (28.3) | 0.843 |
| NYHA class 3 or 4 | 477 (72.1) | 171 (69.5) | 44 (80.0) | 0.699 |
| Past history | ||||
| HTN | 385 (53.3) | 152 (58.2) | 36 (63.2) | 0.060 |
| DM | 247 (34.2) | 117 (44.8) | 27 (47.4) | 0.001 |
| Stroke | 53 (10.6) | 27 (14.1) | 1 (2.5) | 0.823 |
| Previous MI | 108 (14.9) | 46 (17.6) | 8 (14.0) | 0.593 |
| COPD | 20 (2.9) | 5 (2.0) | 1 (1.9) | 0.419 |
| CKD | 54 (7.5) | 36 (13.8) | 8 (14.0) | 0.002 |
| Laboratory finding | ||||
| Hemoglobin (g/dL) | 11.7±2.0 | 12.0±2.0 | 12.4±2.0 | 0.011 |
| Na (mmol/L) | 138.2±5.3 | 139.0±4.7 | 137.9±4.7 | 0.704 |
| Glucose (mg/dL) | 166.8±92.6 | 173.3±95.8 | 175.6±80.5 | 0.503 |
| BUN (mg/dL) | 23.8±14.3 | 25.1±15.1 | 15.2±14.1 | 0.489 |
| Cr (mg/dL) | 1.3±1.2 | 1.5±1.4 | 1.5±1.3 | 0.398 |
| NT-proBNP (pg/mL) | 10317.6±10426.8 | 7956.2±8981.9 | 6192.7±8103.5 | 0.018 |
| Discharge medication | ||||
| ACEi or ARB | 563 (77.9) | 206 (78.9) | 48 (84.2) | 0.320 |
| Beta blocker | 393 (54.4) | 156 (59.8) | 31 (54.4) | 0.332 |
| MRA | 371 (51.3) | 140 (53.6) | 27 (47.4) | 0.983 |
BMI, body mass index; SBP, systolic blood pressure; BPM, beats per minute; HF, heart failure; LBBB, left bundle branch block; NYHA, New York Heart Association; HTN, hypertension; DM, diabetes mellitus; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; BUN, blood urea nitrogen; Cr, creatinine; BNP, brain natriuretic peptides; NT-proBNP, N terminal pro brain natriuretic peptides; ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist.
Values are expressed as mean±SD or n (%).
Clinical outcomes
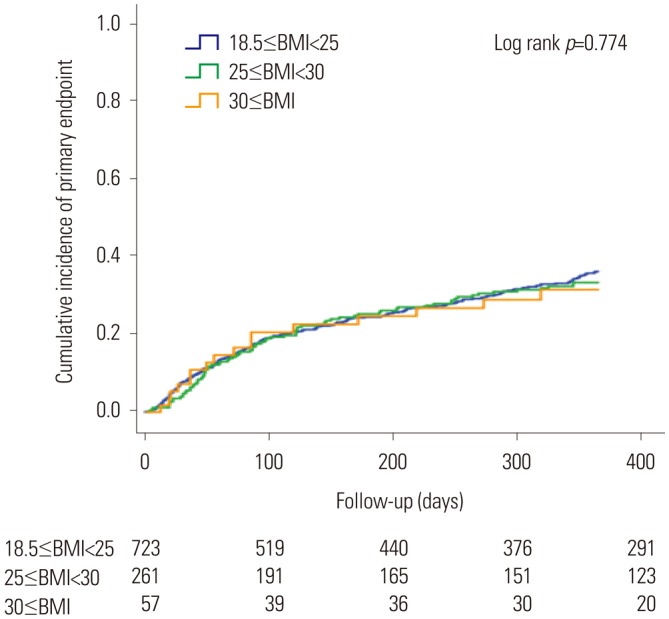
Among the 3251 subjects, 2484 (76.4%; men, 1483; women, 1041) had available information on the study endpoints (Table 3). In men, a larger proportion of patients in the normal BMI group than in the high BMI group (40.3% vs. 29.4%, p<0.001) reached the study endpoints. One-year all-cause mortality (17.2% vs. 6.9%, p<0.001) and re-hospitalization rates (31.0% vs. 25.4%, p=0.024) were significantly higher in the normal BMI group than in the high BMI group. Meanwhile, no significant differences in study endpoints were found between women in the normal BMI group and those in the high BMI group (37.7% vs. 35.4%, p=0.544). No significant differences in all-cause mortality and re-hospitalization rates were found between the two groups. The Kaplan-Meier event free curves of each study endpoints are depicted in Fig. 2. Among men, the high BMI group showed improved clinical outcomes in comparison with the normal BMI group. However, no significant differences in the study endpoints were observed between the two groups of women (Fig. 3).
Table 3. Incidence of the Primary Endpoint According to BMI Category.
| Variable | 18.5≤BMI<25 | 25≤BMI<30 | 30≤BMI | p for trend |
|---|---|---|---|---|
| Male, n (%) | ||||
| Primary endpoint | 329 (33.6) | 106 (26.8) | 41 (20.3) | 0.001 |
| All cause death | 84 (8.6) | 15 (3.8) | 2 (2.9) | 0.001 |
| Re-hospitalization | 293 (30.0) | 101 (25.5) | 14 (20.3) | 0.025 |
| Female, n (%) | ||||
| Primary endpoint | 228 (31.6) | 79 (30.3) | 16 (28.1) | 0.522 |
| All cause death | 50 (6.9) | 11 (4.2) | 2 (3.5) | 0.085 |
| Re-hospitalization | 207 (28.6) | 72 (27.6) | 16 (28.1) | 0.789 |
BMI, body mass index
Fig. 2. Kaplan-Meier curve for incidence of the primary endpoint in the male population. BMI, body mass index.
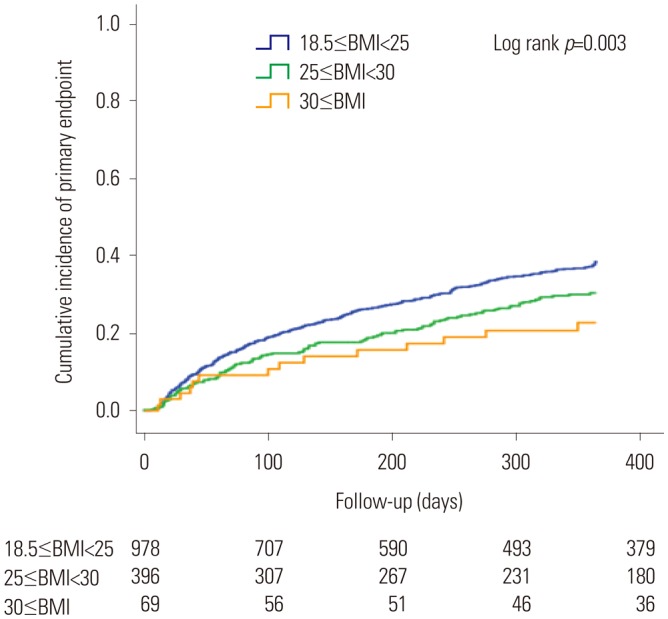
Fig. 3. Kaplan-Meier curve for incidence of the primary endpoint in the female population. BMI, body mass index.
After adjustment for age, hypertension, DM, ischemic HF, previous MI, serum Cr level, hemoglobin, and EF, the odds ratios for the study endpoints were 0.786 [95% confidence interval (CI), 0.620−0.998] and 0.545 (95% CI, 0.302−0.985), respectively, in males. However, among women, those with high BMI were not associated with either of the study endpoints, even after adjustment (Table 4).
Table 4. Adjusted Hazard Ratios for the Primary Endpoint According to BMI Category.
| Categorical | Continuous | ||||
|---|---|---|---|---|---|
| 18.5≤BMI<25 | 25≤BMI<30 | 30≤BMI | p for trend | 1-SD increase in BMI | |
| Male | 1 | 0.786 (0.620−0.998)* | 0.545 (0.302−0.985)* | 0.008 | 0.889 (0.795−0.995)* |
| Female | 1 | 0.965 (0.737−1.263) | 0.945 (0.563−1.584) | 0.755 | 1.022 (0.909−1.149) |
BMI, body mass index; CI, confidence interval.
Data were presented as hazard ratio (95% CI). Adjusted for age, hypertension, diabetes, chronic kidney disease, ischemic etiology, New York Heart Association class, previous myocardial infarction, left ventricle ejection fraction, N terminal pro brain natriuretic peptides, left ventricle end diastole dimension, hemoglobin, discharge medication (angiotensin converting enzyme inhibitor or angiotensin receptor blocker and Beta blocker).
*p value<0.05.
DISCUSSION
The present study demonstrated the existence of the obesity paradox and a sex-related difference in the obesity paradox in systolic HF. To date, many studies have demonstrated the obesity paradox in systolic HF. Unfortunately, the mechanism of the obesity paradox remains unclear. Multiple explanations have been proposed for the obesity paradox in HF. Cardiac cachexia is one of the representative explanations for the obesity paradox. Systolic HF is well known to be a catabolic status.11 Cardiac cachexia was previously shown to be associated with neurohormonal imbalance, inflammation, and poor prognosis.12,13 First, obesity patients may have better metabolic reserve in systolic HF against catabolism.14 Second, obesity patients may present an earlier stage of HF because of the in-creased symptoms and functional impairment caused by excess body weight;4,5 thus, the patients could receive treatments at an earlier stage. In addition, the cardioprotective role of obesity has also been suggested, such as decreased catecholamine response and high cholesterol level, which has an anti-inflammatory property to neutralize circulating lipopolysaccharides.15,16 Adipose tissue is known to produce soluble tumor necrosis factor (TNF)-alpha receptors, which could have a protective effect in obese patients with HF by neutralizing the adverse effect of TNF.17
The present study demonstrated a clear sex-related difference in the obesity paradox in a large number of patients with systolic HF. However, the sex-related difference in the obesity paradox has not been clearly understood yet. Recently, a neurohormonal sex-related difference in HF was reported.18 The levels of biomarkers related to inflammation and extracellular matrix remodeling were found to be significantly lower in women than in men. Considering the anti-inflammatory effect of obesity, the protective effect of obesity might be more prominent in men than in women. The sex-related difference in systolic HF was demonstrated in a previous study.2 In the study, high BMI and waist circumference did not predict improved survival in women. However, the number of female subjects were so small (n=94) that it had limited power to detect any difference. Some studies suggest differences of gender in obesity and its effects, awareness of symptoms, and medical treatment in patients with cardiac diseases, including HF. There was a gender difference between obesity and associates of HF symptoms.19 In a study of the effects of obesity on myocardial and vascular stiffness, myocardial hypertrophy in males was different from that in females.20 Male patients were better at interpreting their HF symptoms, compared to female patients.21 Thus, the relationship between obesity and HF may differ in male and female patients. In 2014, Shah, et al.22 performed a 1-year prospective global registry to explore the obesity paradox in HF, and revealed that the inverse association was stronger in subjects with older age, nondiabetes, or systolic HF. In addition, the study showed a racial difference wherein the inverse BMI association with mortality was stronger in Asian patients than in European and American patients. Previous study of the obesity paradox in patients with HF showed contradictory results with our study.23 While it is difficult to explain the reason, the overweight group in the study, not the obese group, showed survival advantage in the study. We suspect that various factors may have affected the results, such as selection bias or race, etc.
BMI is the most commonly used epidemiologic measure of obesity. Most studies regarding the obesity paradox used BMI to measure adiposity. However, BMI also reflects lean body mass. We excluded underweight from analysis to adjust for potent confounding factors.22,24 To better measure pure adiposity, waist circumference has been used in several previous studies to understand the obesity paradox in HF. However, waist circumference has a limitation in that it cannot be used to distinguish between visceral and subcutaneous fats, which have different properties in the human body. To know which kind of adiposity is more related to the obesity paradox, further investigations using imaging studies are needed. In the present study, additional analysis using waist circumference could not be performed because of the lack of data.
Out study has several limitations. All the subjects were hospitalized for acute decompensated HF at study enrollment. Thus, body weight could be measured in terms of fluid retention status. We merged two registries designed for different purposes. However, we believe a large number of subjects could be enough to cover this limitation. We did not have information on the use of inotropics or cholesterol and cytokine levels, which would be helpful to understand the pathophysiology of the obesity paradox. Lastly, this study was conducted as a retrospective analysis, and pre-hospital course or detailed symptoms were not specified. The distribution of many of the variables between the groups was largely uneven; however, this is a natural characteristic of registry studies.
In conclusion, in the pooled analysis of data from two Korean HF registries, obesity (high BMI) was independently associated with lower 1-year mortality rate in systolic HF in men, but not in women. A sex-related difference in the obesity paradox in systolic HF was confirmed. To understand the pathophysiology of the obesity paradox, further investigation is needed.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 2.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17:374–380. doi: 10.1016/j.cardfail.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 5.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Clark AL, Chyu J, Horwich TB. The obesity paradox in men versus women with systolic heart failure. Am J Cardiol. 2012;110:77–82. doi: 10.1016/j.amjcard.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Komukai K, Minai K, Arase S, Ogawa T, Nakane T, Nagoshi T, et al. Impact of body mass index on clinical outcome in patients hospitalized with congestive heart failure. Circ J. 2012;76:145–151. doi: 10.1253/circj.cj-11-0727. [DOI] [PubMed] [Google Scholar]
- 8.Choi DJ, Han S, Jeon ES, Cho MC, Kim JJ, Yoo BS, et al. Characteristics, outcomes and predictors of long-term mortality for patients hospitalized for acute heart failure: a report from the korean heart failure registry. Korean Circ J. 2011;41:363–371. doi: 10.4070/kcj.2011.41.7.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 10.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 11.Berry C, Clark AL. Catabolism in chronic heart failure. Eur Heart J. 2000;21:521–532. doi: 10.1053/euhj.1999.1882. [DOI] [PubMed] [Google Scholar]
- 12.Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115:836–847. doi: 10.1378/chest.115.3.836. [DOI] [PubMed] [Google Scholar]
- 13.Mustafa I, Leverve X. Metabolic and nutritional disorders in cardiac cachexia. Nutrition. 2001;17:756–760. doi: 10.1016/s0899-9007(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 14.Imbeault P, Tremblay A, Simoneau JA, Joanisse DR. Weight loss-induced rise in plasma pollutant is associated with reduced skeletal muscle oxidative capacity. Am J Physiol Endocrinol Metab. 2002;282:E574–E579. doi: 10.1152/ajpendo.00394.2001. [DOI] [PubMed] [Google Scholar]
- 15.Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356:930–933. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 16.Lavie CJ, Mehra MR, Milani RV. Obesity and heart failure prognosis: paradox or reverse epidemiology? Eur Heart J. 2005;26:5–7. doi: 10.1093/eurheartj/ehi055. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277:E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 18.Meyer S, van der Meer P, van Deursen VM, Jaarsma T, van Veldhuisen DJ, van der Wal MH, et al. Neurohormonal and clinical sex differences in heart failure. Eur Heart J. 2013;34:2538–2547. doi: 10.1093/eurheartj/eht152. [DOI] [PubMed] [Google Scholar]
- 19.Heo S, Moser DK, Pressler SJ, Dunbar SB, Lee KS, Kim J, et al. Association between obesity and heart failure symptoms in male and female patients. Clin Obes. 2017;7:77–85. doi: 10.1111/cob.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regitz-Zagrosek V, Brokat S, Tschope C. Role of gender in heart failure with normal left ventricular ejection fraction. Prog Cardiovasc Dis. 2007;49:241–251. doi: 10.1016/j.pcad.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Riegel B, Dickson VV, Kuhn L, Page K, Worrall-Carter L. Gender-specific barriers and facilitators to heart failure self-care: a mixed methods study. Int J Nurs Stud. 2010;47:888–895. doi: 10.1016/j.ijnurstu.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Shah R, Gayat E, Januzzi JL, Jr, Sato N, Cohen-Solal A, diSomma S, et al. Body mass index and mortality in acutely decompensated heart failure across the world: a global obesity paradox. J Am Coll Cardiol. 2014;63:778–785. doi: 10.1016/j.jacc.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 23.Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The heart failure overweight/obesity survival paradox: the missing sex link. JACC Heart Fail. 2015;3:917–926. doi: 10.1016/j.jchf.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Parissis J, Farmakis D, Kadoglou N, Ikonomidis I, Fountoulaki E, Hatziagelaki E, et al. Body mass index in acute heart failure: association with clinical profile, therapeutic management and in-hospital outcome. Eur J Heart Fail. 2016;18:298–305. doi: 10.1002/ejhf.489. [DOI] [PubMed] [Google Scholar]