Abstract
Purpose
We investigated associations between family history of diabetes (FHD) and hemoglobin A1c (HbA1c) level, among people with and without diabetes.
Materials and Methods
In total, 7031 people without diabetes and 1918 people with diabetes who participated in the Dong-gu Study were included. Data on FHD in first-degree relatives (father, mother, and siblings) were obtained. Elevated HbA1c levels in people without diabetes and high HbA1c levels in people with diabetes were defined as the highest quintiles of HbA1c ≥5.9% and ≥7.9%, respectively.
Results
In people without diabetes, the odds of elevated HbA1c levels [odds ratio (OR) 1.34, 95% confidence interval (CI) 1.13−1.59] were significantly greater in people with any FHD than in those without. Specifically, the odds of elevated HbA1c levels in people without diabetes with an FHD involving siblings were greater than in those without an FHD involving siblings. Additionally, in people with diabetes, the odds of high HbA1c levels (OR 1.33, 95% CI 1.02−1.72) were greater in people with any FHD than in those without such history. Moreover, people with diabetes with maternal FHD had increased odds of high HbA1c levels.
Conclusion
FHD was associated not only with high HbA1c levels in people with diabetes, but also with elevated HbA1c levels in people without diabetes.
Keywords: Family history, diabetes mellitus, HbA1c, blood glucose
INTRODUCTION
Diabetes is a major public health issue worldwide, and the prevention of diabetes improves quality of life and reduces financial burden on patients, their families, and society.1,2 Thus, the early detection of people without diabetes at high risk for diabetes is important. People who are identified as being at high risk of diabetes should undergo regular screenings.3 Additionally, it is important to identify people with diabetes who should be more concerned about glycemic control and to manage them appropriately, thereby reducing the risks of diabetes complications and mortality.4,5
Family history of diabetes (FHD), which reflects both genetic susceptibility and environmental influences shared by families,6 is a well-known risk factor for diabetes. FHD is independently associated with the prevalence and incidence of diabetes in the general population, although the degree of association varies by race and country.7,8,9,10,11 FHD is also associated with earlier onset of diabetes,12 often resulting in worse glycemic control.13,14 Therefore, assessing FHD is a useful screening tool for diabetes due to its low cost and accuracy.6,11
Many epidemiological studies have reported an association between FHD and an increased prevalence or incidence of diabetes; however,6,7,8,10,15,16 evidence of an association between FHD and blood glucose concentration in people with diabetes, or in the general population without diabetes, is insufficient. Moreover, there have been few studies on the association of FHD and blood glucose concentration in Asian populations, especially Koreans. The impact of FHD on blood glucose management would be expected to vary between races and countries. Therefore, in a large community-dwelling population in Korea, we assessed whether FHD is associated with elevated hemoglobin A1c (HbA1c) levels in both people with and without diabetes. We further investigated the association between an FHD in individual first-degree relatives (fathers, mothers, and siblings) and HbA1c in people with and without diabetes.
MATERIALS AND METHODS
Study population
The study population consisted of community-dwelling adults aged 50 years and older who participated in the baseline survey of the Dong-gu Study from 2007 to 2010.17 Of the 9260 people enrolled, 41 whose records lacked information on HbA1c and 270 whose records lacked information on anthropometric or laboratory measures were excluded. The remaining 8949 people were categorized as those without diabetes (n=7031) or those with diabetes (n=1918). Diabetes was defined as fasting plasm glucose (FPG) ≥126 mg/dL or HbA1c ≥6.5% or having been prescribed an anti-diabetic medication. All participants provided informed consent, and the study was conducted in accordance with the guidelines of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of Chonnam National University Hospital (IRB no. I-2008-05-056).
Family history of diabetes
Data on the FHD of the participants were collected by trained staff using standardized questionnaires. The presence of diabetes in first-degree relatives, including fathers, mothers, and siblings, was identified. If any first-degree relatives had diabetes, the subject was considered FHD-positive; if none had diabetes, the subject was considered FHD-negative. The relatives affected were also recorded and assessed separately, as history of diabetes in father (fHD), history of diabetes in mother (mHD), and history of diabetes in siblings (sHD). Participants were additionally categorized based on the combination of fHD, mHD, and sHD, as follows: none, father only, mother only, siblings only, father and mother, father and siblings, mother and siblings, and father, mother, and siblings.
Venous blood samples were extracted from all participants after at least a 12-hour overnight fast. Serum was separated on-site within 30 min using a high-speed cold centrifuge, and the remaining samples were cryopreserved at -70℃ until analysis. HbA1c levels were analyzed by high-performance liquid chromatography using a VARIANT II system (Bio-Rad; Hercules, CA, USA). FPG was measured using an enzymatic technique with an automated analyzer (Hitachi-7600, Hitachi Ltd., Tokyo, Japan). Elevated HbA1c levels in people without diabetes and high HbA1c levels in people with diabetes were defined as the highest quintiles of HbA1c ≥5.9% and ≥7.9%, respectively.
Covariates
Data on demographic characteristics, smoking habits, alcohol consumption, and medications were collected from each subject by well-trained interviewers using standardized questionnaires. Education level was classified as elementary school or lower, middle or high school, or college or higher. Smoking status was classified as never smoking, former smoking, or current smoking. Drinking amount was classified as none, ≤1 drink/day, >1 and ≤2 drinks/day, >2 and ≤4 drinks/day, or ≥4 drinks/day. Prescriptions for medication to treat hypertension and dyslipidemia were noted for all patients. Prescriptions for medication to treat diabetes and diabetes duration were additionally noted for people with diabetes.
All participants received a standardized physical examination performed by well-trained research staff members. Height and weight were measured while lightly dressed without shoes. Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Blood pressure was measured using a customized cuff and a mercury sphygmomanometer in the right arm after individuals rested for 5 min in a sitting position. The readings obtained from three consecutive measurements of systolic blood pressure (SBP) and diastolic blood pressure were recorded at 1-min intervals, and the mean value was used for the analysis. Lipid profiles, including total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides, were assessed using enzymatic techniques and an automated analyzer.
Statistical analysis
All analyses were performed separately for the non-diabetes and diabetes groups. The general characteristics of the study population according to presence of an FHD were compared using Student's t-test for continuous variables and the χ2 test for categorical variables. Differences in the proportions of people without diabetes with elevated HbA1c ≥5.9% and the proportions of people with diabetes with high HbA1c ≥7.9% ac cording to the presence of any FHD or individual history of diabetes were compared using the χ2 test.
Multiple logistic regression analysis was used to evaluate the associations of FHD with elevated HbA1c levels (≥5.9%) in people without diabetes and high HbA1c levels (≥7.9%) in people with diabetes. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were estimated by FHD status. All analyses were performed in sequential order in three models: model 1 was unadjusted; model 2 was adjusted for age, gender, and education; model 3 was further adjusted for BMI, waist circumference, smoking status, alcohol consumption, hypertension medication, dyslipidemia medication, SBP, total cholesterol, HDL cholesterol, and triglycerides; and model 4, for people with diabetes only, also included diabetes duration and diabetes medication. Fully adjusted means (95% CI) of HbA1c levels according to presence of any FHD or individual FHD were calculated and compared using a general linear model. All statistical analyses were conducted with SPSS ver. 22.0 software (IBM Corp., Armonk, NY, USA). A p value <0.05 was considered statistically significant.
RESULTS
Characteristics of the study population according to FHD
The characteristics of people with and without diabetes according to the presence of an FHD are shown in Table 1. Among the 7031 people without diabetes, 985 (14.0%) reported an FHD. Additionally, people without diabetes with an FHD tended to be female, younger, and non-smokers and to have a higher BMI, lower SBP, higher total cholesterol, and a higher level of education. Among the 1918 people with diabetes, 552 (28.8%) reported an FHD. People with diabetes with an FHD tended to be female and younger and to have smaller waist circumferences, higher levels of education, and longer diabetes duration; they were also more likely to have been prescribed dyslipidemia or diabetes medication.
Table 1. Characteristics of Those with and without Diabetes According to FHD.
| Variables | Non-diabetes (n=7031) | p value | Diabetes (n=1918) | p value | ||
|---|---|---|---|---|---|---|
| FHD-negative (n=6046) | FHD-positive (n=985) | FHD-negative (n=1366) | FHD-positive (n=552) | |||
| Gender | <0.001 | 0.013 | ||||
| Male | 2333 (38.6) | 303 (30.8) | 687 (50.3) | 243 (44.0) | ||
| Female | 3713 (61.4) | 682 (69.2) | 679 (49.7) | 309 (56.0) | ||
| Age (yr) | 65.1±8.3 | 62.0±7.4 | <0.001 | 67.6±7.6 | 65.0±7.5 | <0.001 |
| Body mass index (kg/m2) | 24.2±2.9 | 24.4±2.8 | 0.043 | 25.0±3.0 | 24.8±3.0 | 0.429 |
| Waist circumference (cm) | 87.4±8.6 | 87.2±8.3 | 0.425 | 90.5±8.2 | 89.2±8.2 | 0.001 |
| Systolic blood pressure (mm Hg) | 122.7±17.1 | 120.9±15.5 | 0.002 | 127.1±16.5 | 125.8±16.2 | 0.104 |
| Diastolic blood pressure (mm Hg) | 74.5±10.2 | 74.4±9.9 | 0.750 | 74.0±10.4 | 73.4±10.1 | 0.282 |
| Total cholesterol (mg/dL) | 202.5±38.4 | 205.3±38.5 | 0.030 | 195.8±43.4 | 195.2±46.7 | 0.776 |
| HDL cholesterol (mg/dL) | 52.1±12.0 | 52.5±12.0 | 0.307 | 49.6±12.0 | 49.0±10.7 | 0.317 |
| Triglycerides (mg/dL) | 114.0 (82.0–165.0) | 116.0 (83.0–165.0) | 0.529 | 134.0 (92.8–196.0) | 135.0 (91.0–194.8) | 0.926 |
| Medication for hypertension, n (%) | 0.155 | 0.462 | ||||
| No | 4129 (68.3) | 695 (70.6) | 675 (49.4) | 283 (51.3) | ||
| Yes | 1917 (31.7) | 290 (29.4) | 691 (50.6) | 269 (48.7) | ||
| Medication for dyslipidemia, n (%) | 0.118 | 0.009 | ||||
| No | 5632 (93.2) | 904 (91.8) | 1198 (87.7) | 459 (83.2) | ||
| Yes | 414 (6.8) | 81 (8.2) | 168 (12.3) | 93 (16.8) | ||
| Smoking status, n (%) | 0.001 | 0.163 | ||||
| Never | 4193 (69.4) | 741 (75.2) | 817 (59.8) | 356 (64.5) | ||
| Former | 1225 (20.3) | 154 (15.6) | 360 (26.4) | 128 (23.2) | ||
| Current | 628 (10.4) | 90 (9.1) | 189 (13.8) | 68 (12.3) | ||
| Alcohol consumption, n (%) | 0.628 | 0.712 | ||||
| None | 3333 (55.1) | 538 (54.6) | 746 (54.6) | 298 (54.0) | ||
| ≤1 drink/day | 1748 (28.9) | 283 (28.7) | 354 (25.9) | 152 (27.5) | ||
| >1 and ≤2 drinks/day | 348 (5.8) | 65 (6.6) | 86 (6.3) | 35 (6.3) | ||
| >2 and ≤4 drinks/day | 333 (5.5) | 60 (6.1) | 84 (6.1) | 37 (6.7) | ||
| >4 drinks/day | 284 (4.7) | 39 (4.0) | 96 (7.0) | 30 (5.4) | ||
| Educational level, n (%) | <0.001 | 0.001 | ||||
| Elementary school or lower | 2581 (42.7) | 332 (33.7) | 655 (48.0) | 211 (38.2) | ||
| Middle or high school | 2626 (43.4) | 490 (49.7) | 507 (37.1) | 242 (43.8) | ||
| College or higher | 839 (13.9) | 163 (16.5) | 204 (14.9) | 99 (17.9) | ||
| Diabetes duration (yr) | <0.001 | |||||
| 0 | 608 (44.5) | 160 (29.0) | ||||
| 0.1–4 | 294 (21.5) | 104 (18.8) | ||||
| 5–9 | 157 (11.5) | 84 (15.2) | ||||
| 10–19 | 190 (13.9) | 127 (23.0) | ||||
| ≥20 | 117 (8.6) | 77 (13.9) | ||||
| Medication for diabetes, n (%) | <0.001 | |||||
| No | 616 (45.1) | 157 (28.4) | ||||
| Yes | 750 (54.9) | 395 (71.6) | ||||
FHD, family history of diabetes; HDL, high-density lipoprotein.
Data are presented as means±standard deviations or medians (interquartile ranges) or numbers (percentages).
Prevalence of an FHD
The frequencies and proportions of people with an FHD among people with and without diabetes are shown in Table 2. Among those without diabetes, 6046 (86.0%) had no FHD, 903 (12.8%) had one family member with diabetes, and 82 (1.2%) had two or more family members with diabetes. The proportions of those without diabetes with an fHD, mHD, and sHD were 1.9, 3.5, and 9.8%, respectively. Among those with diabetes, 1366 (71.2%) had no FHD, 467 (24.4%) had one family member with diabetes, and 85 (4.4%) had two or more family members with diabetes. The proportions of those with diabetes with an fHD, mHD, and sHD were 4.0, 7.9, and 21.8%, respectively.
Table 2. HbA1c Levels According to FHD.
| FHD | Non-diabetes | Diabetes | ||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | HbA1c* | p (p-trend) | n (%) | HbA1c* | p (p-trend) | |||
| <5.9% | ≥5.9% | <7.9% | ≥7.9% | |||||
| Total | 7031 (100.0) | 81.4 | 18.6 | 1918 (100.0) | 79.3 | 20.7 | ||
| Presence of any FHD | 0.047 | <0.001 | ||||||
| Negative | 6046 (86.0) | 81.7 | 18.3 | 1366 (71.2) | 82.1 | 17.9 | ||
| Positive | 985 (14.0) | 79.1 | 20.9 | 552 (28.8) | 72.3 | 27.7 | ||
| Frequency of FHD | 0.132 (0.068) | <0.001 (<0.001) | ||||||
| None | 6046 (86.0) | 81.7 | 18.3 | 1366 (71.2) | 82.1 | 17.9 | ||
| One | 903 (12.8) | 79.0 | 21.0 | 467 (24.4) | 73.2 | 26.8 | ||
| ≥Two | 82 (1.2) | 80.5 | 19.5 | 85 (4.4) | 67.1 | 32.9 | ||
| Presence of individual FHD | ||||||||
| fHD | 0.797 | 0.379 | ||||||
| Absence | 6896 (98.1) | 81.4 | 18.6 | 1841 (96.0) | 79.5 | 20.5 | ||
| Presence | 135 (1.9) | 82.2 | 17.8 | 77 (4.0) | 75.3 | 24.7 | ||
| mHD | 0.889 | <0.001 | ||||||
| Absence | 6785 (96.5) | 81.4 | 18.6 | 1766 (92.1) | 80.5 | 19.5 | ||
| Presence | 246 (3.5) | 81.7 | 18.3 | 152 (7.9) | 65.1 | 34.9 | ||
| sHD | 0.012 | 0.001 | ||||||
| Absence | 6341 (90.2) | 81.8 | 18.2 | 1500 (78.2) | 81.0 | 19.0 | ||
| Presence | 690 (9.8) | 77.8 | 22.2 | 418 (21.8) | 73.2 | 26.8 | ||
HbA1c, hemoglobin A1c; FHD, family history of diabetes; fHD, history of diabetes in father; mHD, history of diabetes in mother; sHD, history of diabetes in siblings.
*Row percentile.
Prevalence of higher HbA1c levels according to FHD
Among people without diabetes, those with an FHD were significantly more likely than those without such history to have elevated HbA1c levels (20.9% vs. 18.3%). Among people without diabetes with an FHD, elevated HbA1c levels were significantly more prevalent only among those with an sHD, compared with those without an sHD. Among those with diabetes, those with an FHD were significantly more likely than those without to have high HbA1c levels (27.7% vs. 17.9%). Among people with diabetes with an FHD, high HbA1c levels were significantly more prevalent in those with an mHD and sHD than in those without an mHD and sHD (Table 2).
Associations of FHD with HbA1c levels in people without diabetes
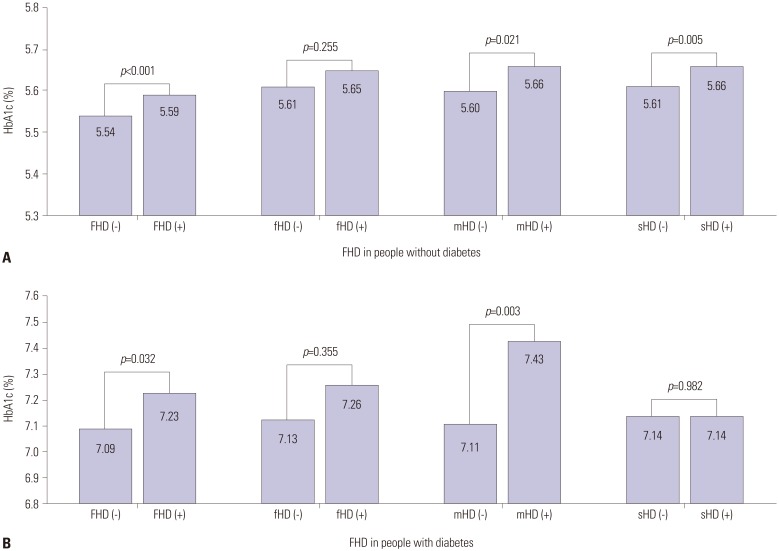
People with an FHD had significantly higher adjusted HbA1c levels (5.59% vs. 5.54%) than people without (p<0.001). Adjusted HbA1c levels were significantly higher among people without diabetes with an sHD than among people without (p=0.005). Additionally, the adjusted HbA1c level was significantly higher for people without diabetes with an mHD than for those without (p=0.021) (Fig. 1A).
Fig. 1. Fully adjusted means (95% confidence interval) of HbA1c levels according to the presence of any FHD or individual FHD in people without (A) and with (B) diabetes. HbA1c, hemoglobin A1c; FHD, family history of diabetes; fHD, history of diabetes in father; mHD, history of diabetes in mother; sHD, history of diabetes in siblings.
For people without diabetes, the ORs (95% CI) for elevated HbA1c by FHD status are shown in Table 3. In models 1, 2, and 3, the odds of elevated HbA1c were significantly higher in people with an FHD (OR 1.34, 95% CI 1.13−1.59 in model 3) than in those without. In analyses of individual FHD, the odds of elevated HbA1c were significantly higher only for people with an sHD (OR 1.34, 95% CI 1.11−1.63 in model 3). In the analysis by FHD classification presented in Table 4, the odds of elevated HbA1c were significantly higher (OR 1.35, 95% CI 1.10−1.66) only in the comparison between people with a diabetes history of siblings only and FHD-negative people.
Table 3. Odds Ratios for Elevated HbA1c (≥5.9%) in People without Diabetes according to FHD Using Logistic Regression Analysis.
| FHD | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Presence of any FHD | |||
| Negative | 1.00 | 1.00 | 1.00 |
| Positive | 1.18 (1.00–1.40) | 1.34 (1.13–1.59) | 1.34 (1.13–1.59) |
| Presence of individual FHD | |||
| fHD | |||
| Absence | 1.00 | 1.00 | 1.00 |
| Presence | 0.94 (0.60–1.47) | 1.19 (0.76–1.86) | 1.17 (0.74–1.85) |
| mHD | |||
| Absence | 1.00 | 1.00 | 1.00 |
| Presence | 0.98 (0.70–1.36) | 1.24 (0.88–1.73) | 1.26 (0.90–1.77) |
| sHD | |||
| Absence | 1.00 | 1.00 | 1.00 |
| Presence | 1.28 (1.06–1.55) | 1.35 (1.11–1.64) | 1.34 (1.11–1.63) |
HbA1c, hemoglobin A1c; FHD, family history of diabetes; fHD, history of diabetes in father; mHD, history of diabetes in mother; sHD, history of diabetes in siblings. Model 1 was unadjusted. Model 2 was adjusted for age, gender, and education. Model 3 was further adjusted for body mass index, waist circumference, smoking status, alcohol consumption, medication to treat hypertension, medication to treat dyslipidemia, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, and triglycerides plus model 2.
Data are presented as odds ratio (95% confidence interval).
Table 4. Odds Ratios for Elevated HbA1c (≥5.9%) in People without Diabetes and High HbA1c (≥7.9%) in People with Diabetes according to FHD Using Logistic Regression Analysis.
| Classification of FHD | Non-diabetes (n=7031) | Diabetes (n=1918) | ||
|---|---|---|---|---|
| n (%) | HbA1c≥5.9%* | n (%) | HbA1c≥7.9%† | |
| Negative (none) | 6046 (86.0) | 1.00 | 1366 (71.2) | 1.00 |
| Father only | 103 (1.5) | 1.22 (0.72–2.06) | 37 (1.9) | 0.78 (0.32–1.88) |
| Mother only | 183 (2.6) | 1.34 (0.91–1.98) | 88 (4.6) | 2.23 (1.35–3.70) |
| Siblings only | 617 (8.8) | 1.35 (1.10–1.66) | 342 (17.8) | 1.22 (0.90–1.65) |
| Father and mother | 9 (0.1) | 0.81 (0.10–6.64) | 9 (0.5) | 1.28 (0.27–6.00) |
| Father and siblings | 19 (0.3) | 1.84 (0.64–5.29) | 21 (1.1) | 1.02 (0.36–2.89) |
| Mother and siblings | 50 (0.7) | 1.44 (0.70–2.93) | 45 (2.3) | 1.45 (0.73–2.88) |
| Father, mother, and siblings | 4 (0.1) | - | 10 (0.5) | 1.45 (0.33–6.42) |
HbA1c, hemoglobin A1c; FHD, family history of diabetes; SBP, systolic blood pressure; HDL, high-density lipoprotein.
Data are presented as odds ratio (95% confidence interval).
*Adjusted for age, gender, education, body mass index, waist circumference, smoking status, alcohol consumption, medication to treat hypertension, medication to treat dyslipidemia, SBP, total cholesterol, HDL cholesterol, and triglycerides, †Adjusted for age, gender, education, body mass index, waist circumference, smoking status, alcohol consumption, medication to treat hypertension, medication to treat dyslipidemia, SBP, total cholesterol, HDL cholesterol, triglycerides, diabetes duration, and medication to treat diabetes.
Associations of FHD with HbA1c levels in people with diabetes
People with diabetes with an FHD had significantly higher adjusted HbA1c levels (7.23% vs. 7.09%) than did people without (p=0.032). Adjusted HbA1c levels were significantly higher in people with an mHD than in people without (p=0.003) (Fig. 1B).
The ORs (95% CI) for high HbA1c in people with diabetes are shown in Table 5. In models 1, 2, 3, and 4, the odds of high HbA1c were significantly higher in FHD-positive than in FHD-negative people. In a fully adjusted model (model 4), the odds for high HbA1c (OR 1.33, 95% CI 1.02−1.72) in people with an FHD were still higher than in those without, although the magnitude of the OR decreased slightly, compared with the ORs in models 1 to 3. In analyses of individual FHD, the odds of high HbA1c (OR 1.77, 95% CI 1.19−2.62 in model 4) were significantly higher in people with an mHD than in people without an mHD, in all models. The odds of high HbA1c were significantly higher in people with an sHD than in people without an sHD in models 1–3, although these associations were not statistically significant after further controlling for diabetes duration and diabetes medication (model 4). In the analysis performed according to FHD classification, as presented in Table 4, the odds of high HbA1c levels (OR 2.23, 95% CI 1.35−3.70) were significantly higher only for people with a history of diabetes in their mother only, compared with those without an FHD.
Table 5. Odds Ratios for High HbA1c (≥7.9%) in People with Diabetes according to FHD Using Logistic Regression Analysis.
| FHD | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Presence of any FHD | ||||
| Negative | 1.00 | 1.00 | 1.00 | 1.00 |
| Positive | 1.76 (1.40–2.22) | 1.72 (1.36–2.18) | 1.80 (1.41–2.29) | 1.33 (1.02–1.72) |
| Presence of individual FHD | ||||
| fHD | ||||
| Absence | 1.00 | 1.00 | 1.00 | 1.00 |
| Presence | 1.27 (0.75–2.16) | 1.25 (0.73–2.15) | 1.24 (0.71–2.14) | 0.85 (0.47–1.51) |
| mHD | ||||
| Absence | 1.00 | 1.00 | 1.00 | 1.00 |
| Presence | 2.21 (1.55–3.15) | 2.17 (1.51–3.12) | 2.27 (1.56–3.28) | 1.77 (1.19–2.62) |
| sHD | ||||
| Absence | 1.00 | 1.00 | 1.00 | 1.00 |
| Presence | 1.56 (1.21–2.01) | 1.50 (1.16–1.93) | 1.55 (1.19–2.01) | 1.16 (0.88–1.53) |
HbA1c, hemoglobin A1c; FHD, family history of diabetes; fHD, history of diabetes in father; mHD, history of diabetes in mother; sHD, history of diabetes in siblings. Model 1 was unadjusted. Model 2 was adjusted for age, gender, and education. Model 3 was further adjusted for body mass index, waist circumference, smoking status, alcohol consumption, medication to treat hypertension, medication to treat dyslipidemia, systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, and triglycerides plus model 2. Model 4 was further adjusted for diabetes duration and medication to treat diabetes plus model 3.
Data are presented as odds ratio (95% confidence interval).
DISCUSSION
This study of a large community-dwelling population in Korea investigated the association between an FHD and HbA1c. Our results show that any FHD was significantly associated with elevated HbA1c levels among people without diabetes and high HbA1c levels among people with diabetes, and these associations were independent of socioeconomic factors, health behaviors, and clinical indicators. In particular, an sHD among people without diabetes and an mHD among people with diabetes showed a stronger association with increased HbA1c levels.
Recent studies have shown that people with an FHD have a negative lifestyle, including greater prevalences of smoking, drinking, obesity, and metabolic abnormalities, including increased BMI, blood pressure, and lipid levels.10 Therefore, in this study, we investigated whether FHD was independently associated with HbA1c levels after adjustment for health behaviors and metabolic parameters. A population-based study of 2059 young healthy Koreans showed that people with FHD had higher FPG than people without.11 In line with previous studies, our study revealed that an FHD is significantly associated with increased HbA1c levels, suggesting a higher likelihood of progression to diabetes. Inadequate management of glycemic control in people with diabetes leads to macrovascular and microvascular complications,4,18,19 thereby increasing the public health burden. Although many epidemiological studies have shown the association between an FHD and an increased prevalence of diabetes in the general population, until now, only a few studies explored whether an FHD is associated with glycemic levels among adults with diabetes. In line with previous research,20,21,22 our study showed that people with diabetes with an FHD had higher odds of high HbA1c (OR 1.36) than those without.
There is conflicting data regarding the impact of gender on an FHD and glucose homeostasis. Our study showed a higher prevalence of diabetes in males (26.1%) than in females (18.4%). However, the proportion with an mHD was higher than that with an fHD among both those with and those without diabetes (1.9% with an fHD and 3.5% with an mHD among those without diabetes vs. 4.0% with an fHD and 7.9% with an mHD among those with diabetes). One of the reasons for the family history of maternal diabetes is reported more often than family history of paternal diabetes can be a fact that females live longer than males. Although many epidemiological studies have observed excess maternal transmission of diabetes to off-spring,23,24,25,26,27 a few studies have not reported these findings.28,29 The Framingham Offspring Study showed that maternal and paternal diabetes equally affect the risk for offspring diabetes; however, those with an mHD were slightly more likely to have increased FPG and abnormal glucose tolerance than those with an fHD.16 A previous Korean study suggested that both fHD and mHD were independent risk factors for offspring di-abetes, whereas no predominance of an mHD was found.29 In our study, an mHD, but not an fHD, was an independent risk factor for high HbA1c levels in the people with diabetes. Our findings are similar to the results of previous studies showing the potent effects of an mHD on offspring, although we did not find any influence of an fHD. However, there are several explanations for the greater importance of maternal diabetes. First, fetal exposure to maternal hyperglycemia during pregnancy may explain the excess maternal transmission of diabetes.30 The mother's glucose tolerance during pregnancy may additionally influence the future development of diabetes in offspring.31,32 Second, maternally inherited mutations in mitochondrial DNA may play a role in the development of diabetes.33,34 Third, because the mother usually spends more time raising children than the father, children's lifestyle factors, including eating habits, food preferences, and other lifestyle-related behaviors, are more strongly influenced by the mother.8
Until now, studies that assessed the association between sHD and glycemic levels in people without diabetes were very rare. This study identified the independent and combined influences of an sHD on elevated HbA1c in the general population without diabetes. Thus far, there has been only one related study, which showed a significant association between sHD and diabetes risk.35 The mechanisms underlying this association are poorly understood, but possible explanations are as follows. First, siblings have a more similar environment persisting into adulthood than do parent−offspring pairs,36 and childhood socioeconomic circumstances contribute to a variety of causes of disease and factors relating to death as adults.37,38 Second, sHD information may be better known than parental FHD information, as one is more likely to be aware of a sibling's diagnosis than a parent's. Third, one may have several siblings and, even if only one has a history of diabetes, it was counted as an sHD for this study. The prevalence of an sHD was significantly higher than the prevalence of fHD or mHD.
Recall and misclassification biases may have affected the results of the present study, because the FHD data were obtained from participants by interviewers using questionnaires. In addition, information on FHD was not confirmed by biochemical or prescription data. Recall bias is a major threat to the internal validity of studies that use self-reported data.39 Differential recall can lead to differential misclassification of the study population with regard to the exposure variable, and consequently, the estimated measure of effect size may deviate away from or toward the null value.40 In our study, a person with diabetes was more likely to be cognizant of and remember his/her FHD. This may lead to overestimation of the risk estimate. However, our study did not directly compare the exposure or outcome variable between those with and those without diabetes. We assessed whether the presence of an FHD was associated with higher glycemic levels among people without diabetes. In addition, we investigated whether the presence of an FHD was associated with poor glycemic control among people with diabetes. Therefore, non-differential rather than differential misclassification was potentially present, and thus the risk estimate may have been underestimated in our study.
This study has several limitations. First, its cross-sectional design does not allow us to infer a causal relationship between an FHD and HbA1c levels. Second, although people with diabetes use different types of medications, number of classes, and medication doses, we assumed that everyone who is being treated for diabetes is receiving appropriate treatment regardless of the type or dose of treatment. Since there is no detailed information on diabetes treatment in our study, it is possible that there was an error in the analysis process in diabetic patients. Third, because postprandial 2-hour glucose levels were not measured, any undiagnosed patients with diabetes could not be detected and therefore would have been misclassified as not having diabetes. Fourth, the dietary and exercise variables that potentially affect glycemic levels were not adjusted for in the analysis. Fifth, when interpreting the results regarding the effect of the diabetes status of the parents on HbA1c levels, the possibility of a difference in the reported rates of diabetes between the father and mother should be considered. Despite these limitations, to the best of our knowledge, this is the first study to investigate the associations between FHD and HbA1c levels in people without diabetes in Korea. The effects of FHD in people with diabetes have been studied extensively; however, we also confirmed that FHD is significantly associated with HbA1c even in people without diabetes. This study is also the first large community-based study of an Asian population on this topic, and we further identified and ana-lyzed an FHD with regard to particular relatives. Moreover, compared to previous studies that primarily examined the influence of a parental history of diabetes, this study observed the effects of an FHD on HbA1c levels with regard to multiple first-degree relatives, both individually and in combination.
In conclusion, an FHD was associated not only with high HbA1c levels in people with diabetes but also with elevated HbA1c levels in people without diabetes. Although an FHD is an irreversible risk factor, assessment of FHD is essential for the prevention and management of diabetes in the community, regardless of the current diabetes status of community-dwelling adults.
ACKNOWLEDGEMENTS
This study was supported by Wonkwang University in 2017.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Schunk M, Reitmeir P, Rückert-Eheberg IM, Tamayo T, Schipf S, Meisinger C, et al. Longitudinal change in health-related quality of life in people with prevalent and incident type 2 diabetes compared to diabetes-free controls. PLoS One. 2017;12:e0176895. doi: 10.1371/journal.pone.0176895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, et al. The global economic burden of diabetes in adults aged 20-79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017;5:423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- 3.Dall TM, Narayan KM, Gillespie KB, Gallo PD, Blanchard TD, Solcan M, et al. Detecting type 2 diabetes and prediabetes among asymptomatic adults in the United States: modeling American Diabetes Association versus US Preventive Services Task Force diabetes screening guidelines. Popul Health Metr. 2014;12:12. doi: 10.1186/1478-7954-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2012;7:e42551. doi: 10.1371/journal.pone.0042551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buehler AM, Cavalcanti AB, Berwanger O, Figueiro M, Laranjeira LN, Zazula AD, et al. Effect of tight blood glucose control versus conventional control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Cardiovasc Ther. 2013;31:147–160. doi: 10.1111/j.1755-5922.2011.00308.x. [DOI] [PubMed] [Google Scholar]
- 6.Harrison TA, Hindorff LA, Kim H, Wines RC, Bowen DJ, McGrath BB, et al. Family history of diabetes as a potential public health tool. Am J Prev Med. 2003;24:152–159. doi: 10.1016/s0749-3797(02)00588-3. [DOI] [PubMed] [Google Scholar]
- 7.Valdez R, Yoon PW, Liu T, Khoury MJ. Family history and prevalence of diabetes in the U.S. population: the 6-year results from the National Health and Nutrition Examination Survey (1999-2004) Diabetes Care. 2007;30:2517–2522. doi: 10.2337/dc07-0720. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Sasaki S, et al. Family history of diabetes, lifestyle factors, and the 7-year incident risk of type 2 diabetes mellitus in middle-aged Japanese men and women. J Diabetes Investig. 2013;4:261–268. doi: 10.1111/jdi.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelis MC, Zaitlen N, Hu FB, Kraft P, Price AL. Genetic and environmental components of family history in type 2 diabetes. Hum Genet. 2015;134:259–267. doi: 10.1007/s00439-014-1519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Yang Z, Xiao J, Xing X, Lu J, Weng J, et al. Association between family history risk categories and prevalence of diabetes in Chinese population. PLoS One. 2015;10:e0117044. doi: 10.1371/journal.pone.0117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon JH, Roh E, Oh TJ, Kim KM, Moon JH, Lim S, et al. Increased risk of metabolic disorders in healthy young adults with family history of diabetes: from the Korea National Health and Nutrition Survey. Diabetol Metab Syndr. 2017;9:16. doi: 10.1186/s13098-017-0210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molyneaux L, Constantino M, Yue D. Strong family history predicts a younger age of onset for subjects diagnosed with type 2 diabetes. Diabetes Obes Metab. 2004;6:187–194. doi: 10.1111/j.1462-8902.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- 13.Berkowitz SA, Meigs JB, Wexler DJ. Age at type 2 diabetes onset and glycaemic control: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2010. Diabetologia. 2013;56:2593–2600. doi: 10.1007/s00125-013-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamshirgaran SM, Mamaghanian A, Aliasgarzadeh A, Aiminisani N, Iranparvar-Alamdari M, Ataie J. Age differences in diabetes-related complications and glycemic control. BMC Endocr Disord. 2017;17:25. doi: 10.1186/s12902-017-0175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariri S, Yoon PW, Qureshi N, Valdez R, Scheuner MT, Khoury MJ. Family history of type 2 diabetes: a population-based screening tool for prevention? Genet Med. 2006;8:102–108. doi: 10.1097/01.gim.0000200949.52795.df. [DOI] [PubMed] [Google Scholar]
- 16.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49:2201–2207. doi: 10.2337/diabetes.49.12.2201. [DOI] [PubMed] [Google Scholar]
- 17.Kweon SS, Shin MH, Jeong SK, Nam HS, Lee YH, Park KS, et al. Cohort Profile: The Namwon Study and the Dong-gu Study. Int J Epidemiol. 2014;43:558–567. doi: 10.1093/ije/dys244. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein BE, Moss SE. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med. 1996;124(1 Pt 2):90–96. doi: 10.7326/0003-4819-124-1_part_2-199601011-00003. [DOI] [PubMed] [Google Scholar]
- 19.Gaster B, Hirsch IB. The effects of improved glycemic control on complications in type 2 diabetes. Arch Intern Med. 1998;158:134–140. doi: 10.1001/archinte.158.2.134. [DOI] [PubMed] [Google Scholar]
- 20.Gong L, Kao WH, Brancati FL, Batts-Turner M, Gary TL. Association between parental history of type 2 diabetes and glycemic control in urban African Americans. Diabetes Care. 2008;31:1773–1776. doi: 10.2337/dc08-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo CK, Lin LY, Yu YH, Chang CH, Kuo HK. A family history of diabetes mellitus is associated with poor glycemic control and increased metabolic risks among people with diabetes: data from the National Health and Nutrition Examination Survey 1999-2004. Intern Med. 2010;49:549–555. doi: 10.2169/internalmedicine.49.2880. [DOI] [PubMed] [Google Scholar]
- 22.Wu M, Wen J, Qin Y, Zhao H, Pan X, Su J, et al. Familial history of diabetes is associated with poor glycaemic control in type 2 diabetics: a cross-sectional study. Sci Rep. 2017;7:1432. doi: 10.1038/s41598-017-01527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Silva SN, Weerasuriya N, De Alwis NM, De Silva MW, Fernando DJ. Excess maternal transmission and familial aggregation of type 2 diabetes in Sri Lanka. Diabetes Res Clin Pract. 2002;58:173–177. doi: 10.1016/s0168-8227(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 24.Tan JT, Tan LS, Chia KS, Chew SK, Tai ES. A family history of type 2 diabetes is associated with glucose intolerance and obesity-related traits with evidence of excess maternal transmission for obesity-related traits in a South East Asian population. Diabetes Res Clin Pract. 2008;82:268–275. doi: 10.1016/j.diabres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Otabe S, Nakayama H, Fukutani T, Yuan X, Wada N, Hashinaga T, et al. Excessive maternal transmission of diabetes in Japanese families with young-onset type 2 diabetes and insulin secretion defect according to clinical features. Acta Diabetol. 2010;47(Suppl 1):133–138. doi: 10.1007/s00592-009-0152-1. [DOI] [PubMed] [Google Scholar]
- 26.Benrahma H, Arfa I, Charif M, Bounaceur S, Eloualid A, Boulouiz R, et al. Maternal effect and familial aggregation in a type 2 diabetic Moroccan population. J Community Health. 2011;36:943–948. doi: 10.1007/s10900-011-9393-3. [DOI] [PubMed] [Google Scholar]
- 27.Tam CH, Wang Y, Luan J, Lee HM, Luk AO, Tutino GE, et al. Maternal history of diabetes is associated with increased cardiometabolic risk in Chinese. Nutr Diabetes. 2014;4:e112. doi: 10.1038/nutd.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viswanathan M, McCarthy MI, Snehalatha C, Hitman GA, Ramachandran A. Familial aggregation of type 2 (non-insulin-dependent) diabetes mellitus in south India; absence of excess maternal transmission. Diabet Med. 1996;13:232–237. doi: 10.1002/(SICI)1096-9136(199603)13:3<232::AID-DIA27>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Kim DJ, Cho NH, Noh JH, Lee MS, Lee MK, Kim KW. Lack of excess maternal transmission of type 2 diabetes in a Korean population. Diabetes Res Clin Pract. 2004;65:117–124. doi: 10.1016/j.diabres.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Fetita LS, Sobngwi E, Serradas P, Calvo F, Gautier JF. Consequences of fetal exposure to maternal diabetes in offspring. J Clin Endocrinol Metab. 2006;91:3718–3724. doi: 10.1210/jc.2006-0624. [DOI] [PubMed] [Google Scholar]
- 31.Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women. Long-term effects on offspring. Diabetes. 1991;40(Suppl 2):126–130. doi: 10.2337/diab.40.2.s126. [DOI] [PubMed] [Google Scholar]
- 32.Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22:944–950. doi: 10.2337/diacare.22.6.944. [DOI] [PubMed] [Google Scholar]
- 33.Rigoli L, Salpietro DC, Caruso RA, Chiarenza A, Barberi I. Mitochondrial DNA mutation at np 3243 in a family with maternally inherited diabetes mellitus. Acta Diabetol. 1999;36:163–167. doi: 10.1007/s005920050161. [DOI] [PubMed] [Google Scholar]
- 34.Maassen JA, Janssen GM, Hart LM. Molecular mechanisms of mitochondrial diabetes (MIDD) Ann Med. 2005;37:213–221. doi: 10.1080/07853890510007188. [DOI] [PubMed] [Google Scholar]
- 35.Chien KL, Hsu HC, Su TC, Chang WT, Chen PC, Chen MF, et al. Sibling and parental history in type 2 diabetes risk among ethnic Chinese: the Chin-Shan Community Cardiovascular Cohort Study. Eur J Cardiovasc Prev Rehabil. 2008;15:657–662. doi: 10.1097/HJR.0b013e32830fe451. [DOI] [PubMed] [Google Scholar]
- 36.Harrap SB, Stebbing M, Hopper JL, Hoang HN, Giles GG. Familial patterns of covariation for cardiovascular risk factors in adults: The Victorian Family Heart Study. Am J Epidemiol. 2000;152:704–715. doi: 10.1093/aje/152.8.704. [DOI] [PubMed] [Google Scholar]
- 37.Galobardes B, Lynch JW, Davey Smith G. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 38.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 39.Basso O, Olsen J, Bisanti L, Karmaus W. The performance of several indicators in detecting recall bias. European Study Group on Infertility and Subfecundity. Epidemiology. 1997;8:269–274. doi: 10.1097/00001648-199705000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Hassan E. Recall bias can be a threat to retrospective and prospective research designs. Internet J Epidemiol. 2005;3(2) [Google Scholar]