Abstract
The symptoms of autism spectrum disorder (ASD) have been hypothesized to be caused by changes in brain connectivity. From the clinical perspective, the “disconnectivity” hypothesis has been used to explain characteristic impairments in “socio-emotional” function. Therefore, in this study we compared the facial emotional recognition (FER) feature and the integrity of social-emotional-related white-matter tracts between children and adolescents with high-functioning ASD (HFA) and their typically developing (TD) counterparts. The correlation between the two factors was explored to find out if impairment of the white-matter tracts is the neural basis of social-emotional disorders. Compared with the TD group, FER was significantly impaired and the fractional anisotropy value of the right cingulate fasciculus was increased in the HFA group (P < 0.01). In conclusion, the FER function of children and adolescents with HFA was impaired and the microstructure of the cingulate fasciculus had abnormalities.
Keywords: Autism spectrum disorder, Facial emotional recognition, Social-emotional related white matter fiber tracts, Diffusion tensor imaging, Tractography
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that has the core symptoms of impaired social communication as well as repetitive and stereotyped interests and behaviors [1]. In recent years, studies on brain connectivity in ASD patients have shown that impairment of the integrity of the large-scale neural network may be an important neurobiological basis of ASD. Studies have already confirmed that the integrity of the social-emotional-related structural network is associated with the impairment of social and emotional abilities in ASD patients [2]. Therefore, the long-range white-matter tract that connects this network may be an important causal factor for the core social communication disorder in ASD patients.
Studies on the neural correlation of complex socioemotional processing in normal participants have indicated that some long-range brain white matter tracts are the substrate of socioemotional function. The temporo-amygdala-orbitofrontal network is connected through the uncinate fasciculus. This fiber tract connects the temporal lobes with the insula and orbitofrontal cortex [3, 4], and the integrity of the uncinate fasciculus is associated with the recognition of complex human emotions [5]. The cingulate fasciculus is a long-range white-matter tract that connects the medial anterior and posterior cingulate cortex with the medial prefrontal, parietal, and temporal lobes (including the hippocampus) [6], and impairment of the cingulate fasciculus significantly influences extensive social motivation and psychological functions [3, 7, 8]. The superior longitudinal fasciculus, which connects the cortex surrounding the lateral sulcus of the frontal, parietal, and temporal lobes [6], provides the corresponding fiber connection for the mirror neuron system. Many studies have indicated that the mirror neuron system plays an important role in social cognition [9, 10]. The face processing system is composed of three parts: the superior temporal sulcus, the fusiform gyrus, and the amygdala [11, 12], which are connected through the inferior longitudinal fasciculus [13, 14]. Damage to the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus is associated with impaired facial emotional recognition (FER) [13]. Based on this functional study of white matter tracts associated with complex social emotional processing in normal participants, we selected the uncinate fasciculus, cingulate fasciculus, superior longitudinal fasciculus, inferior longitudinal fasciculus, and inferior fronto-occipital fasciculus as regions of interest (ROIs) to explore the structural integrity and related functional impairment in children/adolescents with ASD.
Previous studies on the neuropathological basis of ASD have reached inconsistent conclusions concerning the fasciculi listed above [15]. Some studies showed decreased fractional anisotropy (FA) values of these tracts compared to a control group [16–20], some showed increased FA values [21], and a very small number of studies noted that diffusion tensor imaging (DTI) values are associated with emotional cognitive functions in ASD. These results may be associated with the heterogeneity of samples and task settings of the emotional cognitive function. In patients with developmental disorders such as ASD, the DTI technique provides a unique opportunity to investigate the structural features of white-matter tracts and their related functions. FA and mean diffusivity (MD) are very sensitive to developmental changes and pathological differences in the density, size, myelin formation, and fibrous tissue continuity in voxels of axons; therefore, they are indicators for the structural integrity of white-matter tract tissues [22, 23].
Therefore, in this study we selected children and adolescents with HFA and their typically developing (TD) counterparts between 6–16 years of age to compare FER ability and the integrity of emotion-related white matter tracts, and at the same time we further explored the correlation between the integrity of emotion-related white matter tracts and the accuracy rates of FER as well as their clinical symptoms.
Participants and Methods
Ethics Statement
The present study was approved by the Medical Ethics Committee of the Brain Hospital Affiliated with Nanjing Medical University (KY043). The parents or legal guardians of the participants were informed of the purposes and detailed procedures of the investigation and all gave informed consent before initiation of the experimental procedures.
Participants
High-functioning ASD (HFA) group: children and adolescents with HFA diagnosed at the Brain Hospital Affiliated with Nanjing Medical University between September 2013 and September 2015 were enrolled. On the basis of the completion of a clinical psychological evaluation, two senior pediatric psychiatrists made the diagnosis based on the relevant diagnostic criteria in the diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). If the diagnostic results were inconsistent, the patients were excluded. The inclusion criteria were: (1) meeting the ASD diagnostic criteria in the DSM-5; (2) being 6–16 years of age; (3) having an intelligence quotient (IQ) ≥ 70 points [24]; (4) being right-handed; and (5) obtaining consent from the legal guardians to participate in the study. The exclusion criteria were: (1) having a clear history of craniocerebral trauma; (2) presenting with a history of nervous system diseases and severe somatic diseases; and (3) having a definite mental illness such as attention deficit hyperactivity disorder and learning disabilities. After removing 5 participants whose images were of low quality, a total of 34 individuals were enrolled, including 30 males and 4 females. The mean age was 9.3 ± 2.2 years.
Control group: typically developing age-, gender-, and IQ-matched children and adolescents were selected over the same time period. The inclusion criteria were: (1) having normal physical development and intelligence; (2) being right-handed; and (3) obtaining consent from the legal guardians to participate in the study. The exclusion criteria were as follows: (1) presenting with a definite history of craniocerebral trauma; (2) having a history of nervous system diseases and severe somatic diseases; and (3) having any type of definite mental illness. A total of 39 individuals were recruited, 29 males and 10 females. The mean age was 10.0 ± 3.2 years.
Assessment of Autistic Symptoms
General condition survey: a self-compiled scale was used to survey the general demographic data, birth history, past history, and family history of all participants.
Clinical assessment: (1) the Autism Diagnostic Interview-Revised (ADI-R) [25] was the clinical diagnostic evaluation scale. The ADI-R is an extensively applied, standardized, and a structured diagnostic tool for parent interviews and is composed of three dimensions, Reciprocal Social Interaction, Abnormalities in Communication, and Restricted, Repetitive, and Stereotyped Patterns of Behavior, all of which reached the diagnostic criteria. When the three dimensions were all above the cut-off scores by >2 points, autism was diagnosed. (2) The Wechsler Intelligence Scale for Children-China Revised (WICS-CR): this instrument is an IQ determination scale that includes a total of 12 subtests. The verbal scale is composed of six subtests: information, similarities, arithmetic, vocabulary, comprehension, and digit span. The performance scale is composed of six subtests: picture completion, picture arrangement, block design, object assembly, coding, and mazes. The digit span in the verbal scale and the mazes in the performance scale were reserved tests. Each subtest was individually scored. The scoring was performed according to the manual during the tests.
FER accuracy rate measurements: as the presentation materials in the FER tasks, we chose 50 pictures in the Chinese facial affective picture system with relatively high recognition levels. There were 10 pictures each for five types of emotional faces, i.e., happy, sad, neutral, fearful, and angry. The pictures were presented on a computer using E-prim2.0 software. The children and adolescents were instructed to complete the recognition of 50 affective pictures. The task process was divided into a practice part and the formal test. After the participants accurately understood the testing procedure, the formal tasks were performed. The participants performed recognition of the facial emotional pictures and pressed a button to respond. The software automatically recorded the accuracy rate. During this process, the examiner did not provide any hints about facial emotion recognition.
Diffusion Tensor Imaging Acquisition
A 3.0 T superconducting magnetic resonance imaging system (Siemens, Germany) and a birdcage head quadrature coil were used. The head was immobilized using sponge mats, and cranial scanning was performed in a supine position. Cross-sectional T2W1 scanning was first performed in each case for anatomical positioning and the exclusion of patients with organic diseases in the nervous system visible by the naked eye. The planar echo sequence was used for DTI scanning at the axial position of the head. The scanning parameters were: diffusion sensitivity coefficient b value = 1000 s/mm2, 30 diffusion sensitivity gradient directions, collection matrix = 128 × 128, scanning field = 230 mm × 230 mm, slice thickness = 2.5 mm, repetitive time = 9000 ms, echo time = 104 ms, scanning time = 5 min 8 s, and 60 slices for each scan.
Image Analysis
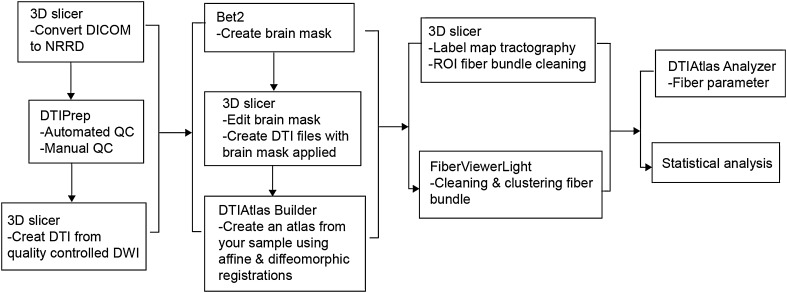
The images and data processing used the Atlas-based local analysis method. The software 3D-Slicer (http://www.slicer.org/), DTIPrep (http://www.nitrc.org/projects/dtiprep/), DTIAtlasBuilder (http://www.nitrc. org/projects/dtiatlasbuilder/), FIberViewerLight (http://www.nitrc.org/projects/ fvlight/), and DTIAtlasFiberAnalyzer (http://www.nitrc.org/projects/dti_tract_stat) were used for DTI image processing. The data processing framework was a template surrounding the DTI. The template was constructed using a deformation map and was used as an unbiased average atlas. On the basis of this compressed template, the bilateral uncinate fasciculus, cingulate fasciculus, superior longitudinal fasciculus, inferior longitudinal fasciculus, and inferior fronto-occipital fasciculus were used as the regions of interest (ROIs) to perform interactive fiber tractography to construct the fiber tracts. The fiber tractography was based on the ROI selection method of fiber tracts by Catani [26]. The DTI parameter files of fiber tracts were automatically extracted through the template information. The major steps were: (1) using 3D Slicer software for format conversion; (2) automatic diffusion weighted imaging (DWI) quality control using a tool named DTIPrep, which includes a variety of quality checks as well as eddy current and motion correction [27]. Visual quality control was then performed to eliminate DWI suffering from artifacts that were not picked up in the automatic step. Finally, the overall quality of the resulting DTI data was assessed in 3D Slicer. (3) DTIAtlasBuilder software was used to establish the template to compress the images of all participants into an unbiased atlas. Atlases were created iteratively starting from affine atlases (step 1) over deformable diffeomorphic atlases (step 2 and 3), and the process was fully automatic. All atlas registrations were performed via FA image intensity normalized to the atlas of the current step. (4) The 3D Slicer software platform was used to select the five fasciculi as ROIs to perform interactive fiber tractography. (5) DTIAtlasFiberAnalyzer software was used to obtain the FA and MD values of these fiber tracts. All the steps were made according to the corresponding software manuals. All of the tools referenced in the description of our workflow can be used as stand-alone command lines for scripting and grid computing, or interactively as part of 3D Slicer as external modules. The tool workflow and fiber reconstruction results are shown in Figs. 1 and 2.
Fig. 1.
Pipeline for the analysis of DTI images.
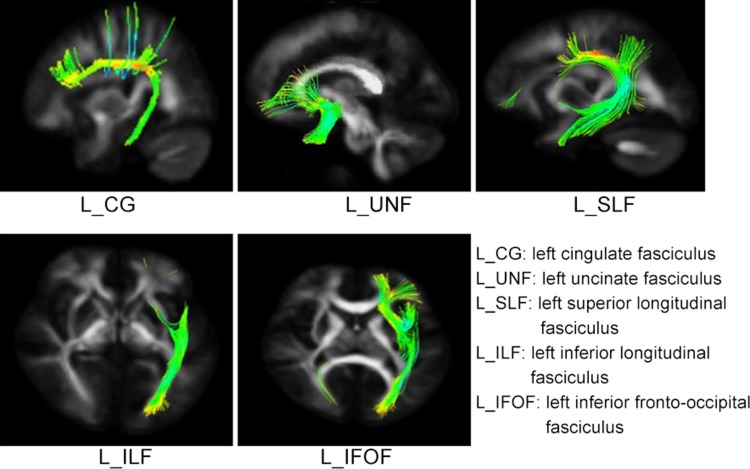
Fig. 2.
Example of fiber reconstruction results (L left, R right, UF uncinate fasciculus, CG cingulate fasciculus, SLF superior longitudinal fasciculus, ILF inferior longitudinal fasciculus, IFOF inferior fronto-occipital fasciculus).
Statistical Analysis
The statistical analyses were performed using SPSS 20.0 software. The differences of accuracy rates of FER and FA, and MD values of the emotion-related fiber tracts between the HFA and control groups were analyzed using the two independent samples t test. The correlation of FA and MD values of abnormal fiber tracts with accuracy rates of emotion recognition and ADI-R scores were analyzed using Pearson correlation. To reduce error variance and increase statistical power, age and IQ were used as covariates to perform Pearson partial correlation analysis to further assess the correlation between the FA and MD values of abnormal fiber tracts and the emotion recognition accuracy rate and clinical symptoms.
Results
Demographics
The comparative analysis of gender (x 2 = 2.26, P = 0.13), age (t = −1.19, P = 0.24), and IQ (t = −0.69, P = 0.49) of the children and adolescents between the HFA and TD groups did not show any significant difference. The mean scores of the three ADI-R dimensions in the HFA group all reached the diagnostic criteria (Table 1).
Table 1.
Participant demographics (mean ± SD).
| Items | HFA group (n = 34) | TD group (n = 39) | t/x 2 value | P value |
|---|---|---|---|---|
| Gender (male:female) | 30:4 | 29:10 | 2.26 | 0.13 |
| IQ | 109.94 ± 20.82 | 113.03 ± 16.83 | −0.69 | 0.49 |
| Age (years) | 9.27 ± 2.23 | 10.05 ± 3.20 | −1.19 | 0.24 |
| ADI-R: reciprocal social deficits score | 14.76 ± 7.38 | – | – | – |
| ADI-R: abnormalities in communication score | 8.88 ± 4.55 | – | – | – |
| ADI-R: restricted, repetitive behavior score | 4.44 ± 3.14 | – | – | – |
ADI-R Autism Diagnostic Interview-Revised, HFA high-functioning autism spectrum disorder, TD typically developing.
Between-Group Differences in FER Accuracy Rates
The comparative analysis of the HFA and TD groups showed that the FER accuracy rates for the recognition of the facial emotions of fear and happiness were not significantly different; however, the rates for the recognition of facial emotions of sadness, anger, and neutral had significant differences (Table 2). In addition, the total accuracy rate for the recognition of facial emotions was also significantly different between the two groups (t = −4.53, P < 0.001) (Table 2).
Table 2.
Between-group differences in recognition accuracy rates of the five basic types and total facial emotion results (percentage, mean ± SD).
| HFA (n = 34) | TD (n = 39) | t | P | |
|---|---|---|---|---|
| Sad | 0.50 ± 0.24 | 0.77 ± 0.19 | −5.39 | 0.00 |
| Angry | 0.47 ± 0.24 | 0.66 ± 0.21 | −3.67 | 0.00 |
| Fear | 0.34 ± 0.22 | 0.47 ± 0.30 | −1.98 | 0.05 |
| Neutral | 0.64 ± 0.37 | 0.89 ± 0.21 | −3.62 | 0.00 |
| Happy | 0.81 ± 0.20 | 0.90 ± 0.19 | −1.82 | 0.07 |
| Total accuracy rate | 0.55 ± 0.19 | 0.73 ± 0.14 | −4.53 | 0.00 |
Statistically significant differences are shown in bold, P < 0.01
HFA high-functioning autism spectrum disorder, TD typically developed.
Comparison of FA and MD Values of Social-Emotional-Related White Matter Tracts Between the Two Groups
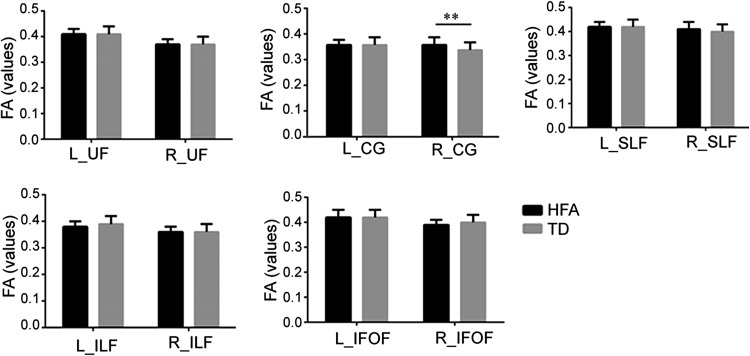
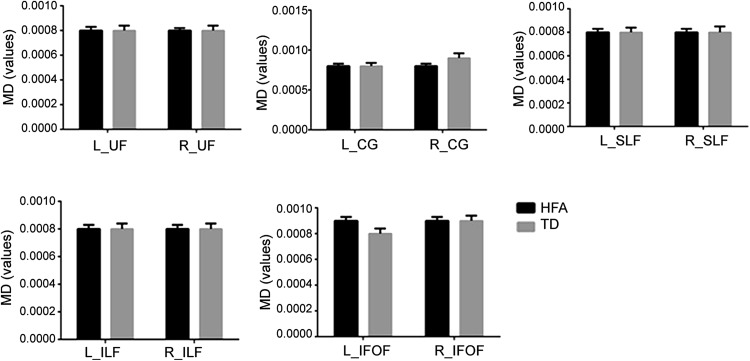
The FA and MD values of the bilateral social-emotional-related fiber tracts were compared between HFA and TD groups. Compared to the TD group, the HFA group had an increased FA value for the right cingulate fasciculus (R_CG) (t = 2.74, P < 0.01), while the FA and MD values of the left (L)_CG and the MD value of the R_CG were not significantly different. The FA and MD values of the bilateral uncinate fasciculus, bilateral superior longitudinal fasciculus, bilateral inferior longitudinal fasciculus, and bilateral inferior fronto-occipital fasciculus between the HFA and the TD groups did not show significant differences (Figs. 3, 4).
Fig. 3.
FA values of social-emotional-related white matter tracts in the two groups. The high-functioning ASD group had an FA value of the right cingulate fasciculus higher than the TD group (t = 2.74, P = 0.008), but no significant differences were found in left cingulated fasciculus and other social-emotional-related white matter tracts. **P < 0.01; all values expressed as mean ± SEM (L left, R right; UF uncinate fasciculus, CG cingulate fasciculus, SLF superior longitudinal fasciculus, ILF inferior longitudinal fasciculus, IFOF inferior fronto-occipital fasciculus).
Fig. 4.
MD values of social-emotional-related white matter tracts in the two groups. No significant difference were found in the social-emotional-related white matter tracts; all values expressed as mean ± SEM (L left, R right, UF uncinate fasciculus, CG cingulated fasciculus, SLF superior longitudinal fasciculus, ILF inferior longitudinal fasciculus, IFOF inferior fronto-occipital fasciculus).
Analysis of Correlation Between FA Values for the Cingulate Fasciculus and Age, IQ, and Accuracy Rates of FER in the Two Groups
The FA values for the bilateral cingulate fasciculus and age showed a significantly positive correlation in the HFA group [L_CG (r = 0.42, P = 0.02) and R_CG (r = 0.38, P = 0.03), respectively].
The correlation between FA values and age, IQ, and accuracy rates of FER in the TD group showed a significantly positive correlation between age and FA value for the L_CG (r = 0.35, P = 0.03). For both groups, the IQ was not significantly correlated with the FA values for the bilateral cingulate fasciculus.
To reduce error variance and increase statistical power, we performed a bivariate correlation analysis between FER ability and FA values for the bilateral cingulate fasciculus. The results showed a positive correlation between L_CG FA and the accuracy rate of FER (r = 0.232 P = 0.048). The following partial analysis using age and IQ as covariates further excluded a correlation between L_CG FA and the accuracy rate of facial FER (Table 3).
Table 3.
Analysis of the correlation between FA value for the cingulate fasciculus and accuracy rate of facial emotion recognition in the HFA group.
| Accuracy rate of facial emotion recognition | ||||||
|---|---|---|---|---|---|---|
| r | P | r a | P a | r b | P b | |
| L_CG FA | 0.23 | <0.05 | 0.11 | 0.36 | 0.22 | 0.06 |
| R_CG FA | 0.02 | 0.84 | −0.07 | 0.57 | 0.01 | 0.92 |
FA fractional anisotropy, L_CG left_ cingulate fasciculus, R_CG right_ cingulate fasciculus. r a partial correlation between FA and accuracy rate of facial emotion recognition using age as covariate; r b partial correlation between FA and accuracy rate of facial emotion recognition using IQ as covariate. Statistically significant differences are shown in bold.
Analysis of the Correlation Between the FA Values for the CG and Clinical Symptoms in the HFA Group
Considering impact of age and IQ on the results, age and IQ were used as covariates at the same time to perform the Pearson partial correlation analysis to further assess the correlation between the FA values of abnormal fiber tracts and the clinical symptoms. No significant relationships were found between DTI measures and any dimension of the ADI-R scores in the HFA group (Table 4).
Table 4.
Correlation between the FA values for the cingulate fasciculus and the scores of all dimensions of the ADI-R in the HFA group.
| ADI-R_social | ADI-R_communication | ADI-R_behavior | ||||
|---|---|---|---|---|---|---|
| r c | P c | r c | P c | r c | P c | |
| L_CG (FA) | −0.15 | 0.42 | −0.08 | 0.65 | −0.24 | 0.17 |
| R_CG(FA) | −0.13 | 0.49 | −0.09 | 0.61 | −0.28 | 0.11 |
L_CG left_ cingulate fasciculus, R_CG right_cingulate fasciculus. r c partial correlation between FA and all dimension of the ADI-R scores in HFA group using age and IQ as covariate at the same time.
Discussion
The selection of stimulation materials is the most important step in studying FER; it directly influences the test results. Previous studies on school-age children and adolescents have chosen cartoon pictures [28] or facial emotion pictures of real people [29–31] as the stimulation materials for FER. Because the emotion information delivered by real people is the most real and enriched, the use of the facial emotions of real people as the stimulation materials is most common. In addition, the recognition of the facial emotion of other races is more difficult than that of one’s own [32]. Therefore, after combination with the above factors, the present study selected the emotional faces of real Chinese people as the stimulation materials to reduce the effect of unrelated interfering factors in this experiment. The major findings of the present study in the behavioral experiment of the FER ability of HFA children was that the accuracy rates for the recognition of sad, angry, and neutral emotional faces in the HFA group were significantly lower than those of the children and adolescents in the TD group.
A 2014 meta-analysis on FER in ASD by Fink et al. [33] showed that school-age ASD children had a significantly lower recognition ability on four types of basic facial expression (happy, sad, angry, and fearful) than the control group. In the present study, the accuracy rates for the recognition of sad, angry, and neutral faces in the HFA group were significantly lower than those of the TD group, which is basically consistent with the current international research results.
For social-emotional-related white-matter tracts, the present study used the template-based local analysis method in DTI data processing to define target fibers in the uncinate, cingulate, arcuate, inferior longitudinal, and inferior fronto-occipital fasciculi as the ROIs to perform fiber tractography uniformly on the compressed template. This method reduced the errors caused by manual delineation of the ROIs during fiber tractography of paerticipants one by one, increased the precision of the fiber tractography, and simultaneously measured the FA and MD values of the ROIs. The major factors affecting the FA value are the integrity of the axonal cell membrane and the level of myelination [34]. When the integrity of the cell membrane is higher and myelination is more mature, the FA value is higher; otherwise, the FA value is lower.
The results of the comparison between the social-emotional related fiber tracts of the two groups showed a significant difference in the right cingulate fasciculus. The cingulate fasciculus is a major long-range tract that represents connectivity in the brain, providing the connection among the gray matter areas that regulate psychological, abstract, and emotional responses. These are the core deficiencies in ASD patients [35]. In the present study, compared to that in the TD group, the FA value for the R_CG in the HFA group significantly increased, and the L_CG did not present a significant difference. This result is not completely consistent with previous research results on ASD. There are many previous studies on the FA values for the cingulate fasciculus in ASD; however, the results are not consistent [36, 37]. In a previous study, the FA value in ASD patients increased when they were <6 years old; these results support previous claims of abnormal brain overgrowth in young children with autism, and the excessive cerebral growth not only in gray but also in white matter [38]. By contrast, the FA values of ASD patients at adolescence or early adulthood decreased or were close to those in a control group [39]. The study by Billeci et al. [39] showed that, compared to a control group, there was an intersection point during the increase in the FA values in ASD patients. This intersection point was at 7–8 years of age, indicating that the FA value of ASD patients was higher than that of the control group before 7–8 years of age. The study by Lee et al. [40] also showed that there was an intersection point during the increase in the FA values of the white-matter fiber tract with age; however, the intersection point was at 12 years. The combination of the results in the previous literature and the present study shows that the FA value of the right cingulate fasciculus in HFA groups is significantly higher than that in control groups, possibly due to the presence of an intersection point during the increase in the FA value of white matter tracts with age in ASD patients. The inconsistent results may also be associated with the homogeneity of participants. The selection of participants in previous studies has been broader and included various subtypes of ASD, but subjects in our study were all HFA, so the results of the present study are cleaner, ruling out the confounding factors of comorbidities. Next, the present study selected HFA patients between 6 and 16 years of age. The age range was larger, and the ages in the HFA group were mainly distributed in the low age period, which may be a factor that has a larger influence on the differences in FA values.
In the correlation analysis between the DTI eigenvalues and age and IQ in the HFA and TD groups, the results showed that the FA values of the bilateral cingulate fasciculus positively correlated with age. Then, we made a bivariate correlation analysis between the DTI eigenvalues and the FER ability. The results showed a positive correlation between L_CG (FA) and the accuracy rate of FER. Considering the influencing factor of age, we conducted a partial correlation analysis using age and IQ as covariates. The correlation between L_CG (FA) and the accuracy rate of FER disappeared. In the sample of children and adolescents with ASD, age and IQ are important factors that may influence the results. In further studies, we will increase the sample size, and refine the age group to control the impact of these two factors. These results may also have been influenced by the reliability of the indicators used for measuring the self-reported symptoms and the parents’ reports of ASD patients. Future studies should use cross-field clinical diagnostic tools to measure the severity of symptoms to better detect the association between the eigenvalues of white-matter tract diffusivity and the behaviors of ASD patients.
Studies have shown that the limbic system is one of the neural bases of social emotion. The cingulate fasciculus is a large long-range fiber tract in the limbic system; its impairment is associated with damage to FER and socialization in ASD [41]. The present study did not show a correlation between abnormal structure of the cingulate fasciculus and the accuracy rate of FER, and this may be associated with the presentation methods of the tasks. In the present study, the task presentation consisted of static emotional faces rather than a dynamic and situational presentation. Therefore, the response of the FER ability could only be reflected in the static facial emotion level and could not reflect the comprehensive ability of FER; therefore, the results have a certain limitation.
Although the FA values of the right cingulate fasciculus between the HFA group and the control group had a significant difference, the fiber tract characteristic values of other large fiber tracts tracked by the DTI method, the uncinate, arcuate, inferior longitudinal, and inferior fronto-occipital fasciculi, did not show significant differences between these two groups. Previous studies have reported a reduction in the FA values in ASD patients for the fiber tracts included in the present study. However, these reports are not consistent [42]. In addition, previous studies have covered very large differences in the age, IQ, and symptom severity of participants. Different fiber tracts have different development tracks, and the association between fiber tract diffusivity and the severity of ASD symptoms is also not consistent; therefore, the participants’ characteristics may be a factor that can strongly influence the results. Furthermore, the methods used for studying fiber tracts in previous studies are also not consistent. These factors may all have caused the differences in the results for large fiber tracts.
In summary, the present study mainly showed that children and adolescents with HFA had an impaired FER ability. There was a significant reduction in the accuracy rate of their FER, particularly the recognition of sad, angry, and neutral emotions. The integrity study of social-emotional-related white-matter tracts among children and adolescents with HFA showed that the cingulate fasciculus has been overgrowth; specifically, age is an important factor influencing the FER function and the integrity of the cingulate fasciculus in ASD patients. Children and adolescents with HFA had impaired FER ability and emotional recognition that was associated with the cingulate fasciculus, and the impairment levels were closely associated with age. Whether the impairment to FER ability can become a reliable indicator for the clinical phenotype of ASD still requires increasing the sample size and implementing a more detailed discussion in future studies. The deficiencies of this research are provided below. Due to the restriction of IQ and age, the recruitment of participants was difficult; thus, our sample size was relatively small. In addition, the number of females was limited; therefore, comparison between genders and a longitudinal comparison across ages could not be performed. The present study used only fiber tractography based on ROIs and did not combine these with other image-processing methods for white-matter tracts to validate and process data from the entire brain to local areas; therefore, the results have certain limitations. The aforementioned limitation will be improved in future studies.
Acknowledgements
This work was supported by The National Key Research and Development Program of China (2016YFC1306200), the National Natural Science Foundation of China (91132750), Major Projects of the National Social Science Foundation of China (14ZDB161), and the Key Research and Development Program of Jiangsu Province, China (BE2016616).
References
- 1.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 4.Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
- 5.Fujie S, Namiki C, Nishi H, Yamada M, Miyata J, Sakata D, et al. The role of the uncinate fasciculus in memory and emotional recognition in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;26:432–439. doi: 10.1159/000165381. [DOI] [PubMed] [Google Scholar]
- 6.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 8.Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, Menon V. Development of functional and structural connectivity within the default mode network in young children. Neuroimage. 2010;52:290–301. doi: 10.1016/j.neuroimage.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- 10.Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cog Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabi Res Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 13.Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29:15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 15.Ameis SH, Catani M Altered white matter connectivity as a neural substrate for social impairment in autism spectrum disorder. Cortex 2015, 62: 158–181. [DOI] [PubMed]
- 16.Alexander AL, Lee JE, Lazar M, Boudos R, DuBray MB, Oakes TR, et al. Diffusion tensor imaging of the corpus callosum in autism. Neuroimage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 17.Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Bloemen OJ, Deeley Q, Sundram F, Daly EM, Barker GJ, Jones DK. White matter integrity in asperger syndrome: A preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res. 2010;3:203–213. doi: 10.1002/aur.146. [DOI] [PubMed] [Google Scholar]
- 19.Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. Neuroreport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- 20.Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 21.Roine U, Salmi J, Roine T, Wendt TN, Leppämäki S, Rintahaka P, et al. Constrained spherical deconvolution-based tractography and tract-based spatial statistics show abnormal microstructural organization in Asperger syndrome. Mol Autism. 2015;6:4. doi: 10.1186/2040-2392-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 23.Basser PJ, Pierpaoli C. Microstructural and Physiological Features of Tissues Elucidated by Quantitative-Diffusion-Tensor MRI. J Magn Reson. 2011;13:560–570. doi: 10.1016/j.jmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter M, Soorya L, Halpern D. Asperger’s syndrome and high-functioning autism. Pediatr Ann. 2009;38:30–35. doi: 10.3928/00904481-20090101-01. [DOI] [PubMed] [Google Scholar]
- 25.Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, et al. Diagnosing autism: analyses of data from the autism diagnostic interview. J Autism Dev Disord. 1997;27:501–517. doi: 10.1023/A:1025873925661. [DOI] [PubMed] [Google Scholar]
- 26.Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Wang Y, Gerig G, Gouttard S, Tao R, Fletcher T, Styner M. Quality Control of Diffusion Weighted Images. Proc SPIE Int Soc Opt Eng 2010, 7628. doi:10.1117/12.844748. [DOI] [PMC free article] [PubMed]
- 28.Teunisse JP, de Gelder B. Impaired categorical perception of facial expressions in high-functioning adolescents with autism. Child Neuropsychol. 2001;7:1–14. doi: 10.1076/chin.7.1.1.3150. [DOI] [PubMed] [Google Scholar]
- 29.Humphreys K, Minshew N, Leonard GL, Behrmann M. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia. 2007;45:685–695. doi: 10.1016/j.neuropsychologia.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Castelli F. Understanding emotions from standardized facial expressions in autism and normal development. Autism. 2005;9:428–449. doi: 10.1177/1362361305056082. [DOI] [PubMed] [Google Scholar]
- 31.Herba CM, Landau S, Russell T, Ecker C, Phillips ML. The development of emotion-processing in children: effects of age, emotion, and intensity. J Child Psychol Psychiatry. 2006;47:1098–1106. doi: 10.1111/j.1469-7610.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- 32.Malpass RS, Kravitz J. Recognition for faces of own and other race. J Pers Soc Psychol. 1969;13:330–334. doi: 10.1037/h0028434. [DOI] [PubMed] [Google Scholar]
- 33.Fink E, de Rosnay M, Wierda M, Koot HM, Begeer S. Brief report: accuracy and response time for the recognition of facial emotions in a large sample of children with autism spectrum disorders. J Autism Dev Disord. 2014;44:2363–2368. doi: 10.1007/s10803-014-2084-z. [DOI] [PubMed] [Google Scholar]
- 34.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 35.Dell’Acqua F, Simmons A, Williams SC, Catani M. Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp. 2013;34:2464–2483. doi: 10.1002/hbm.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinstein M, Ben-Sira L, Levy Y, Zachor DA, Ben Itzhak E, Artzi M, et al. Abnormal white matter integrity in young children with autism. Hum Brain Mapp. 2011;32:534–543. doi: 10.1002/hbm.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jou RJ, Mateljevic N, Kaiser MD, Sugrue DR, Volkmar FR, Pelphrey KA. Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. AJNR Am J Neuroradiol. 2011;32:1607–1613. doi: 10.3174/ajnr.A2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 39.Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurol. 2012;12:148. doi: 10.1186/1471-2377-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JE, Bigler ED, Alexander AL, Lazar M, DuBray MB, Chung MK, et al. Diffusion tensor imaging of white matter in the superior temporal gyrus and temporal stem in autism. Neurosci Lett. 2007;424:127–132. doi: 10.1016/j.neulet.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 41.Hall GB, Szechtman H, Nahmias C. Enhanced salience and emotion recognition in Autism: a PET study. Am J Psychiatry. 2003;160:1439–1441. doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- 42.Travers BG, Adluru N, Ennis C, do Tromp PM, Destiche D, Doran S, et al. Diffusion tensor imaging in autism spectrum disorder: a review. Autism Res. 2012;5:289–313. doi: 10.1002/aur.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]