Abstract
Crocetin is an ingredient of traditional Chinese medicine and has therapeutic potential in various diseases due to its pharmacological properties, such as neuroprotection, anti-oxidative stress, and anti-inflammation. These properties might benefit the treatment of spinal cord injury. In the present study, we tested the effect of crocetin on neurite growth and sensorimotor dysfunction in a rat model of spinal cord injury. We evaluated the viability of cultured hippocampal neurons with tetrazolium dye and lactate dehydrogenase assays, visualized neurites and axons with antibody staining, and monitored motor and sensorimotor functions in rats with spinal cord injury using the Basso, Beattie, and Bresnahan assay and the contact plantar placement test, respectively, and measured cytokine expression using enzyme-linked immuno-absorbent assays. We found that crocetin (1) did not alter the viability of cultured hippocampal neurons; (2) accelerated neurite growth with preference for the longest process in individual hippocampal neurons; (3) reversed the inhibition of neurite growth by chondroitin sulfate proteoglycan and NogoA; (4) facilitated the recovery of motor and sensorimotor functions after spinal cord injury; and (5) did not inhibit pro-inflammatory responses, but restored the innervation of the descending 5-HT system in injured spinal cord. Crocetin promotes neurite growth and facilitates the recovery of motor and sensorimotor functions after spinal cord injury, likely through repairing neuronal connections.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0157-7) contains supplementary material, which is available to authorized users.
Keywords: Crocetin, Spinal cord injury, Hippocampal neurons, Inflammation
Introduction
Crocetin is a natural apocarotenoid dicarboxylic acid found in the fruits of Gardenia jasminoides and in crocus flowers [1]. It forms the central core of crocin, the compound responsible for the color of saffron [2]. Both crocetin and crocin have long been used in traditional Chinese medicine, and have potential for treating diseases such as cerebral ischemia, memory impairment, and Parkinson’s disease [3–5]. An increasing number of in vitro and in vivo studies have also demonstrated that crocetin protects tissues from traumatic damage, including hepatocytes [6], cardiomyocytes [7, 8], hippocampal neurons [2], retina [9, 10], lung [11], and kidney [10]. Therefore, crocetin has therapeutic and interventional potential for a wide range of diseases. This is also supported by its diverse pharmacological properties of anti-apoptotic [12], anti-oxidative [8], and anti-inflammatory activity [2, 13].
Spinal cord injury (SCI) is a catastrophic event that drastically reduces the patient’s quality of life and imposes social and economic burdens. The annual incidence of SCI is estimated to be ~15–40 cases per million globally [14]. Great efforts have been made to alleviate the symptoms of SCI patients, to prevent the progress of injury, and to educate patients to cope with their inability to control the bowel and bladder [15]. In addition, animal research on SCI repair has been conducted with advanced strategies including transplantation of neural stem cells, biased polarization of macrophages, neurotrophic factors, anti-Nogo antibody, anti-inflammatory cytokines, and tissue engineering [15]. These strategies have shown considerable efficacy in improving locomotion and sensorimotor functions [15–20].
Considering that crocetin modulates several major targets that are known to be effective in the repair of SCI, we hypothesized that crocetin may facilitate neurite growth and improve functional recovery following SCI. In the present study, we tested this hypothesis in primary cultures of hippocampal neurons and in an SCI rat model.
Methods and Materials
Chemicals
Chondroitin sulfate proteoglycan (CSPG) and NogoA were from Sigma-Aldrich (St. Louis, MO). Crocetin (>95% purity) was from Haohua Industry (Jinan, China).
Animals
Rats were obtained from the Laboratory Animal Center of the The Second Hospital of Shandong University. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources 1996), and were approved by the Institutional Animal Care and Use Committee and by the Office of Laboratory Animal Resources at The Second Hospital of Shandong University. Animals were group-housed at ≤4 per cage in a room with a 12-h light/dark cycle, and food and water were provided ad libitum. All efforts were made to minimize the number of animals used and their suffering.
Primary Culture of Hippocampal Neurons
Primary cultures of hippocampal neurons were prepared as described previously [21]. Hippocampi were dissected from postnatal day 0 (P0) rat pups, immersed in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 10% Ham’s F-12 with glutamine (Gibco, Carlsbad, CA), and digested by trypsin in DMEM for 15 min at 37 °C. After trypsin was terminated by BSA, the tissue was washed and gently triturated. Then, the dissociated neurons were plated at 15,000 cells/mL–40,000 cells/mL in 35-mm Petri dishes (Costar, Cambridge, MA). The culture medium was replaced with DMEM containing Neurobasal medium and 5% B-27 supplement (Gibco) 24 h after plating, and the cultures were placed in an incubator at 37 °C, under 95% O2 and 5% CO2. The cultures were used at time points indicated in the results.
Tetrazolium Dye (MTT) Assay
The viability of hippocampal neurons in the cultures was evaluated using the MTT assay [22] with a commercial kit from Abnova (Walnut, CA). The neurons (80 µL) were seeded in 96-well plates at 5000–10000 neurons per well. After the neurons attached to the plates, they were treated with 5 µmol/L, 10 µmol/L, or 20 µmol/L crocetin for 24 h, and then 15 µL MTT solution (5 mg/mL) was added into each well and incubated at 37 °C for 4 h to fully form formazan crystals. One hundred microliters of solubilizer (containing SDS, the concentration of which was not provided by Abnova) was added into each well, and the incubation continued for 4 h–6 h at 37 °C to dissolve the crystals. After these procedures, the OD values were measured at 570 nm on an absorbance plate reader (iMark™, Bio-Rad, Hercules, CA). The assays were performed in triplicate in each single experiment, and 3 individual experiments were performed for one set of data.
Lactate Dehydrogenase (LDH) Test
The death of hippocampal neurons in cultures was assessed using an LDH test kit from Roche (Penzberg, Upper Bavaria, Germany). The tests were performed according to the product manual. Briefly, 50 µL of cell suspension was seeded into the wells of a 96-well plate. Crocetin (50 µL) was added to wells to final concentrations of 5 µmol/L, 10 µmol/L, and 20 µmol/L, and kept in an incubator at 37 °C, 90% humidity, and supplied with 5% CO2, for 24 h. Culture medium only was used as a background control, and lysis buffer was added into wells containing neurons to set a positive control. Each sample or control were triplicates. For quantification of LDH activity, 100 µL freshly-prepared reaction mixture was added into each well and incubated for up to 30 min at room temperature in the dark. The reaction was ended by addition of 50 µL stop solution. The 96-well plate was placed on a spectrometer and the absorbance read at 490 nm.
Immunofluorescence Assay
Cultures in Petri dishes were fixed in 4% paraformaldehyde for 10 min, permeabilized with 0.01% Triton X-100 in phosphate-buffered saline (PBS) for 5 min, and blocked with 10% donkey serum in PBS for 30 min. After these processes, the cultures were incubated with rabbit anti-β-tubulin antibody for 1 h at room temperature (Millipore, Billerica, MA). The residual primary antibodies were washed out 3 times with PBS (5 min each), then the samples were incubated with fluorescence-conjugated secondary antibodies at room temperature for 1 h. After 3 washes, the samples were dried and immersed in Vectorshield mounting medium (Vector Laboratories, Cambridgeshire, UK) and coverslipped. Samples processed for immunofluorescence assay were imaged under a confocal microscope (Zeiss, Oberkochen, Germany). ImageJ (National Institutes of Health, Bethesda, MD) was used for image processing. All data were obtained from three Petri dishes in each experiment and from at least three different cell preparations. At least 100 neurites from 20 fields in each group were analyzed.
SCI Rat Model
The SCI rat model was produced according to a previous report with minor modifications [23]. Rats were anesthetized with 3% sevoflurane through a conical-shaped canine anesthesia mask. Under aseptic conditions, spinal segments T9 and T10 were exposed, and a transection was made between T9 and T10 with a sharp blade. During recovery, food and water were provided ad libitum and placed close to the rats for easier access. All rats were allowed to recover at room temperature (24 °C ± 1 °C). The rats were randomly divided into 2 groups: crocetin (40 mg/kg, intraperitoneal (i.p.) injection) treatment group and a vehicle group. The locomotion and sensorimotor behaviors were monitored every week for up to 6 weeks post-injury.
Locomotor Behavior Test
To assess locomotor behavior, we used the 21-point open field locomotion score, developed by Basso, Beattie, and Bresnahan (BBB) [24]. Based on the scores, recovery can be classified into early (score 0–7), intermediate (8–13), and late phases (14–21) [24]. An increased BBB score indicates functional recovery.
Sensorimotor Evaluation
The contact plantar placement (CPP) test was used to evaluate sensorimotor integration in SCI rats [25]. We performed the test once a week, starting 1 week post-injury. The dorsal surface of each hindpaw was lightly touched against the edge of a standard laboratory bench, and the ability of the rat to make plantar contact with the bench was scored by the number of times this response was elicited during three trials for each hindlimb (maximum score, 6).
Enzyme-Linked Immunoabsorbent Assay (ELISA)
Rats with SCI or sham operation were euthanized with CO2, and the T5 and L1 segments were removed and immediately homogenized in ice-cold PBS. The homogenate was centrifuged and the supernatant retained for measurement of the cytokines tumor necrosis factor alpha (TNF-α), interleukin 1-beta (IL-1β), IL-6, and IL-8 with sandwich ELISA [26]. Briefly, antibodies to these cytokines were non-covalently adsorbed onto 96-well plastic plates for 2 h. After washing out the free antibodies, the supernatant was applied to the plate, and incubated overnight at 4 °C. The solution was exchanged 3 times with PBS with Tween 20 (5 min each). Then, biotin-conjugated anti-cytokine antibodies were added to bind each cytokine, followed by adding horseradish peroxidase (HRP)-labeled avidin or streptavidin. An ABC HRP kit (MultiSciences, Bellingham, WA) was used to generate color, which was measured with a spectrophotometer (PerkinElmer, Waltham, MA).
Statistical Analysis
SigmaPlot software was used for statistical analysis. Data are shown as the mean ± SD. The effects of crocetin on neurite growth were analyzed with one-way ANOVA. A repeated-measures ANOVA was used to analyze the time-course of locomotor and sensorimotor recovery after SCI. The significance of crocetin effects was tested with the unpaired Student’s t test or one-way ANOVA. A P value <0.05 was considered significant.
Results
Crocetin Does Not Affect the Viability of Primary Hippocampal Neurons
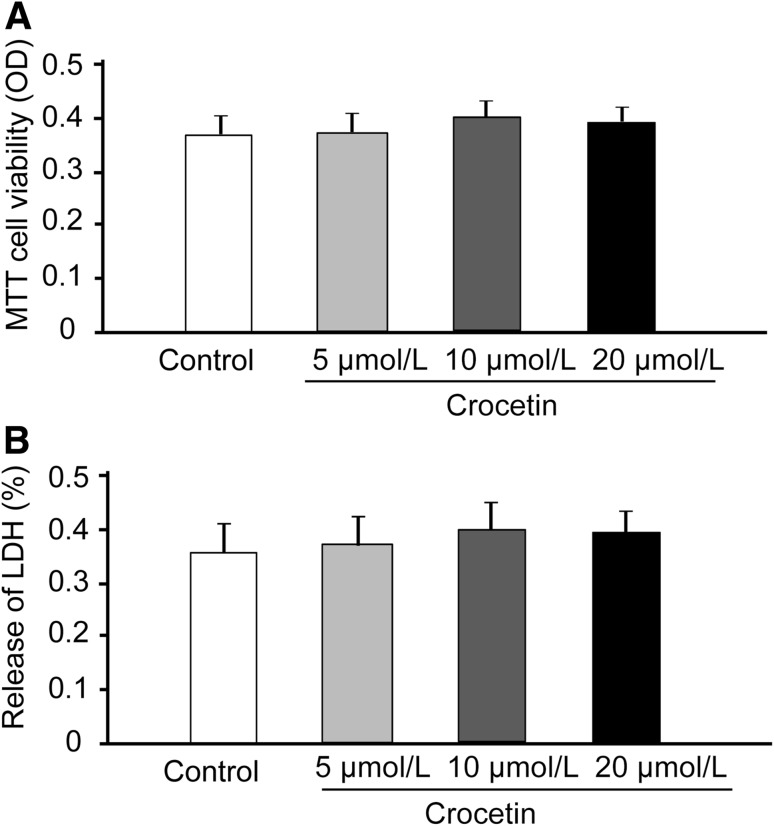
To assess the potential toxicity of crocetin on hippocampal neurons, we incubated hippocampal cultures with increasing concentrations of crocetin (5 µmol/L–20 µmol/L, Fig. 1A and B), as reported in other studies [2, 9], for up to 24 h. We then used MTT assays to determine the viability of hippocampal neurons, and LDH assays to evaluate the numbers of dying hippocampal neurons. We found that neither the viability nor the death of hippocampal neurons was altered by these concentrations of crocetin (Fig. 1).
Fig. 1.
Effects of crocetin on the viability of primary neurons in vitro. Cell viability was measured using the MTT assay and cell death was assessed by the LDH test. Primary neurons were treated with 5 μmol/L, 10 μmol/L, or 20 μmol/L crocetin for 24 h. A Effects of crocetin on the viability of primary cultures of hippocampal neurons. B Effects of crocetin on the death of hippocampal neurons in culture. Data are presented as mean ± SD.
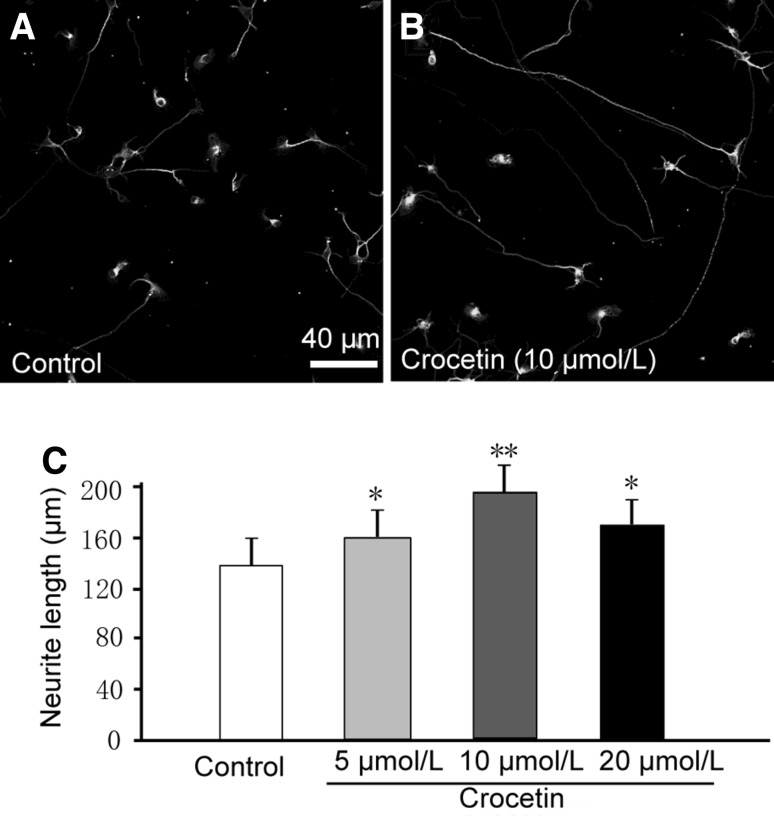
Crocetin Accelerates Growth of the Longest Neurite in Hippocampal Neurons
We labeled neurons with an antibody against β-tubulin (Fig. 2A and B), a marker in the somata, dendrites, axons, and axonal terminals of immature neurons [27] and analyzed the length of neuronal processes. We found that crocetin dramatically increased the length of the longest, but not other processes in hippocampal neurons after 2 days in culture (Fig. 2C). Hippocampal neurons, especially those in early cultures without spatial restriction, exhibit differential growth in processes with the fastest growth in the axon [28]. Therefore, the longest processes we observed here were likely to be axons. We found a bell-shaped dose-response relationship of crocetin effects on the growth of the longest process. The optimal concentration of crocetin was 10 µmol/L. These results indicated that crocetin facilitates neurite growth.
Fig. 2.
Crocetin increases neurite growth in cultured hippocampal neurons. A, B Representative images of cultured hippocampal neurons two days after plating without (Control) and with 10 μmol/L crocetin. Cells were immunostained with β-tubulin antibody. Crocetin (5 μmol/L–20 μmol/L) promoted growth of the longest neurite (C) in individual neurons. At least 100 neurites were analyzed in 20 fields from each group. Data are presented as mean ± SD; *P < 0.05, **P < 0.01 versus control.
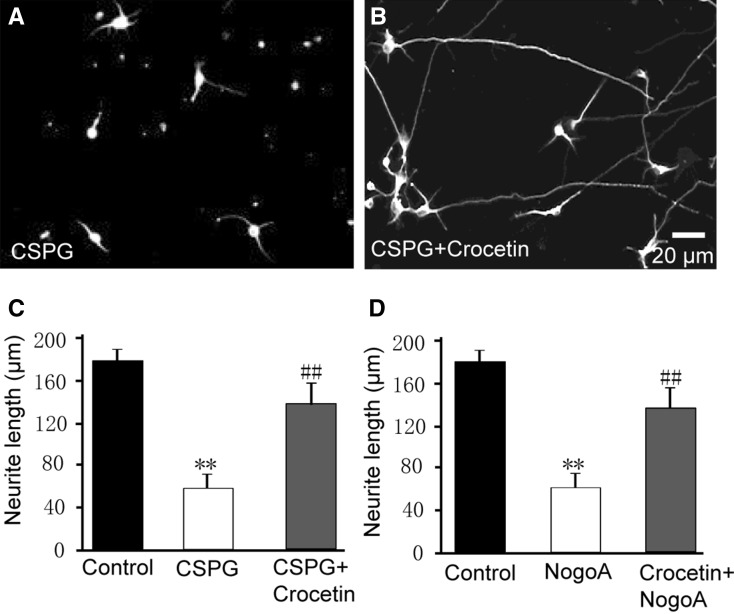
Crocetin Reverses the Inhibition of Neurite Growth by Chondroitin Sulfate Proteoglycan (CSPG) and NogoA
As noted above, crocetin accelerated neurite growth in immature neurons, which possess an intrinsic drive for growth. This experiment mimicked physiological development of neurons. Neurite growth is usually negatively or positively regulated by a variety of factors, including CSPG [29] and NogoA [30]. We next determined whether crocetin is able to reverse the inhibition of neurite growth by these factors. Addition of 1 µg/mL CSPG to the culture medium slowed neurite growth, (neurite length, 59 µm ± 16 µm in CSPG versus 176 µm ± 14 µm in Control; P < 0.01; Fig. 3A–C). Interestingly, CSPG + crocetin resulted in ~2-fold longer neurites than CSPG alone, and the length of neurites almost reached control levels (Fig. 3C). Similarly, 2 µg/mL NogoA inhibited neurite growth in hippocampal cultures, and crocetin reversed this inhibition (Fig. 3D). These results indicated that crocetin counteracts the inhibition of neurite growth by CSPT and NogoA.
Fig. 3.
Crocetin reverses the inhibition of neurite growth in cultured hippocampal neurons by CSPG and NogoA. A Typical image showing that CSPG inhibited the neurite growth in cultured hippocampal neurons. B Typical image showing that crocetin treatment reversed the inhibition of neurite growth by CSPG. C Summary of the length of the longest neurite in cultured hippocampal neurons in control (Control) and in the presence of CSPG (1 μg/mL) and CSPG + crocetin (10 µmol/L). D Summary of the length of the longest neurite in cultured hippocampal neurons in the presence of NogoA (2 μg/mL) and NogoA + crocetin. At least 100 neurites from 20 fields in each group were analyzed. Data are mean ± SD; **P < 0.01 compared with Control; ## P < 0.01 compared with CSPG or NogoA.
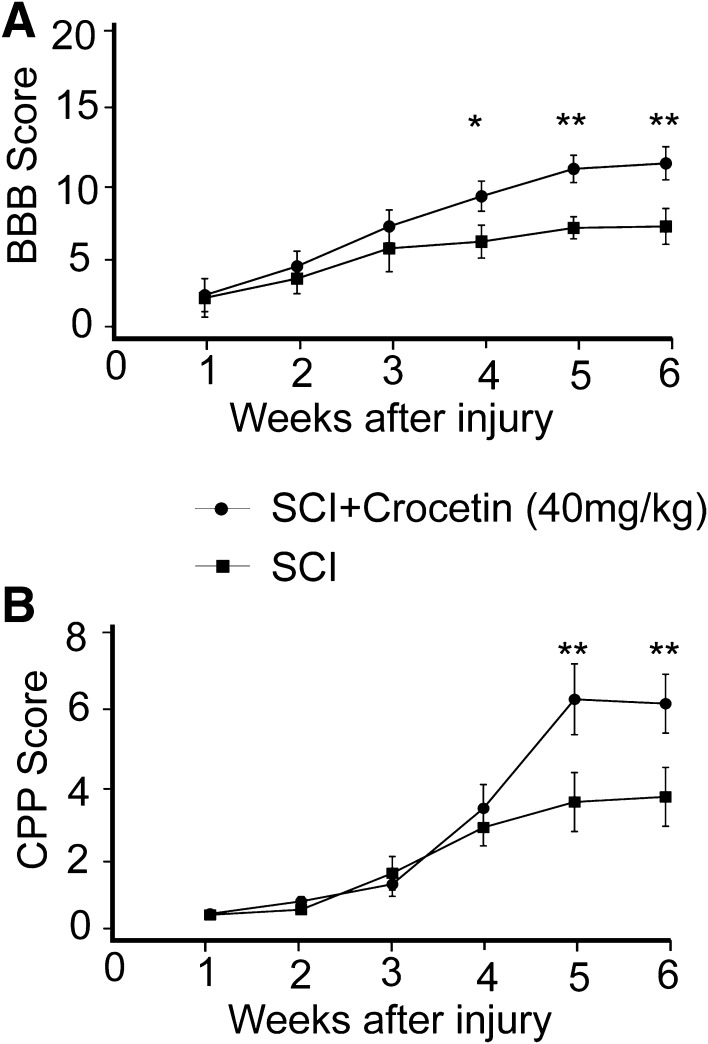
Crocetin Improves Locomotion and Sensorimotor Functions After SCI
To test whether crocetin has potential to treat neuronal injury, we established a rat model of SCI, and monitored the recovery of locomotion with the BBB assay, and examined sensorimotor integration with the CPP test. We performed these tests once a week for up to 6 weeks. In both tests, rats exhibited gradual recovery of locomotor (Fig. 4A) and sensorimotor functions (Fig. 4B), and crocetin accelerated these processes after 4 weeks (Fig. 4A, B). Particularly, crocetin promoted locomotor recovery from the early phase (BBB score 0–7) to the intermediate phase (BBB score 8–15) (Fig. 4A). These results suggested that crocetin has potential for SCI treatment. It is worth noting that crocetin treatment did not affect the cell number in the lesion site at 7 days post-injury (supplementary Fig. S1).
Fig. 4.
Crocetin facilitates the recovery of motor and sensorimotor functions in a rat model of spinal cord injury. A Time-course of BBB scores showing the recovery of motor function after spinal cord injury with and without crocetin. B Time-course of contact plantar placement (CPP) scores showing the recovery of sensorimotor function after spinal cord injury with and without crocetin (40 mg/kg). Data are presented as mean ± SD; n = 6/group; *P < 0.05, **P < 0.01.
Crocetin Differentially Affects Cytokine Expression After SCI
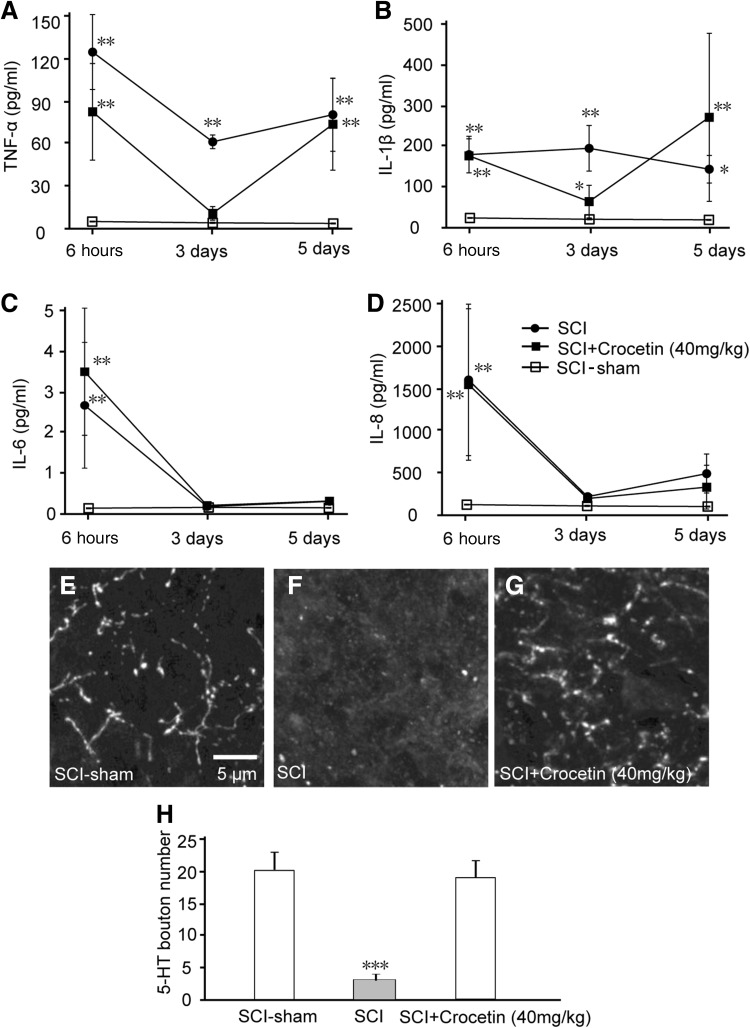
An inflammatory response is one of the major events occurring in the secondary phase of SCI [31] and may in turn lead to dysfunction in signal transduction in the spinal cord and impair behavior. To determine whether crocetin has an anti-inflammatory action in the injured spinal cord, we measured cytokine levels in the injured spinal cord segments of the SCI rat model and the corresponding segments in sham rats. We found that TNF-α, IL-1β, IL-6, and IL-8 showed different patterns of change in the first week post-injury (Fig. 5A–D). Specifically, TNF-α and IL-1β dramatically decreased at day 3 post-injury, but at day 5 returned to the initial levels (at 6 h post-injury) (Fig. 5A, B). On the other hand, IL-6 and IL-8 diminished after 3 days post-injury (Fig. 5C, D). These results suggested that the levels of pro-inflammatory cytokines fluctuated within 5 days post-injury. Crocetin attenuated the decrease in TNF-α and IL-1β at day 3, smoothing the fluctuations in these two cytokines within the first week (Fig. 5A, B), but did not change the trend of either IL-6 or IL-8 (Fig. 5C, D). These data suggested that crocetin may not alter the inflammatory responses to the injury process in the SCI rat model.
Fig. 5.
Effects of crocetin on pro-inflammatory cytokines and 5-HT boutons in rats with spinal cord injury (SCI). The levels of TNF-α (A), IL-1β (B), IL-6 (C), and IL-8 (D) were monitored within 5 days post-injury in the SCI rat model. The SCI rats were treated with i.p. injection of 40 mg/kg crocetin (Crocetin), vehicle (SCI), or sham surgery. 5-HT antibody staining was used to visualize 5-HT bouton and fibers in sections from T5-L1 spinal segments of sham (E), SCI (F), and SCI + crocetin (G) rats. Mean ± SD, n = 6/group; **P < 0.01, ***P < 0.001.
Crocetin Restored 5-HT Boutons After SCI
5-HT in the spinal cord is implicated in both sensory and motor circuits [32]. To investigate whether loss of 5-HT-positive terminals occurred after SCI, and whether crocetin induced regeneration of these terminals, we stained spinal sections close to the injury site with anti-5-HT antibody (Fig. 5E–G). We found that spinal sections close to the injury site had fewer 5-HT-positive terminals. In contrast, crocetin administration dramatically increased the 5-HT-positive terminals in spinal sections (Fig. 5H).
Discussion
Cumulative evidence has demonstrated the neuroprotective property of crocetin in various situations. For instance, crocetin protects dopaminergic neurons in the substantia nigra pars compacta from lesion by 6-hydroxydopamine [3]. It improves the viability of cultured hippocampal neurons during lipopolysaccharide (LPS) exposure [2]. In retinal ganglia cell cultures, crocetin reduces the damage caused by tunicamycin, a stressor of the endoplasmic reticulum, and hydrogen peroxide [9]. In the present study, we incubated hippocampal cultures with crocetin at concentrations used in other studies [2, 9], but did not expose the cultures to chemical insults. We found that this neither increased cell viability (Fig. 1A), nor exacerbated cell death (Fig. 1B). Taken together with previous studies, our results support the hypothesis that crocetin protects neurons only when the neurons are subject to damage from stressors or chemical insults.
Neurite growth plays important roles in promoting neural plasticity and neuronal communication, and facilitating neurogenesis for the repair of injury in the central nervous system. We showed that crocetin differentially enhanced neurite growth in cultured hippocampal neurons with preference for the longest neurite in an individual neuron (Fig. 2A–D). These results support the hypothesis that crocetin targets positive regulators of neurite growth, and the neurite containing the highest level of these regulators and showing the fastest growth may be the preferential target of crocetin. Hippocampal neurons, especially those in early cultures without spatial restriction, exhibit differential growth which is fastest in the axon [28]. Therefore, the longest processes we observed here were likely to be axons.
Neurite growth is controlled by a balance between positive and negative molecules. CSPGs are complex macromolecules that negatively regulate neurite growth both in vivo and in vitro [29]. Consistent with previous studies, the application of CSPGs in cultured hippocampal neurons dramatically shortened the length of neurites (Fig. 3A, C). In contrast, crocetin restored neurite growth to levels similar to hippocampal neurons not subjected to CSPG treatment (Fig. 3B, C). Meanwhile, we also tested the inhibitory effects of another negative factor of neurite growth, NogoA [30], and found that it strongly reduced neurite growth in hippocampal neurons, whereas crocetin reversed the inhibition (Fig. 3D).
Therefore, we have provided evidence showing that crocetin facilitates neurite growth by favorably potentiating positive regulators, and counteracts negative modulators of neurite growth. CSPG acts on its receptor, PTP-sigma [33], a transmembrane protein tyrosine phosphatase, while NogoA binds to the Nogo receptor [34], and both connect to downstream Rho-associated kinase pathways [34, 35]. Further investigation is required to elucidate whether Rho-associated pathways are involved in the promotion of axon growth by crocetin.
In the SCI rat model, we assessed the gradual recovery of motor and sensorimotor functions, which were accelerated by crocetin (Fig. 4A, B). The effects of crocetin became significant after 4 weeks of treatment. This time-course suggests that crocetin affects processes involved in the secondary phase of SCI (minutes to weeks after injury). This secondary phase consists of several events [31]: vascular changes, free radical formation and lipid peroxidation, disruption of ionic balance, glutamate excitotoxicity, apoptosis, and inflammatory responses. Crocetin has been reported to attenuate the responses of rat brain microglial cell cultures to LPS, reducing the LPS-induced upregulation of TNF-α and IL-1β [2]. However, in our study, crocetin increased the levels of these two cytokines 3 days post-injury, but they were not altered either 6 h or 5 days post-injury. In addition, crocetin did not change the time-courses of another two pro-inflammatory factors, IL-6 and IL-8, after 6 h and up to 5 days post-injury (Fig. 5A–D). As a matter of fact, the levels of TNF-α and IL-1β in the injured spinal cord of our rat model were higher than those in sham rats, but the levels were much lower than those in microglial cell cultures exposed to LPS [2], suggesting that in our study, the injury triggered a much weaker inflammatory response, which may explain why we did not find inhibitory effects of crocetin on the cytokines tested.
The serotoninergic (5-HT) system, originating from the dorsal raphe nucleus in the brainstem, coordinates complex sensory and motor patterns during different behavioral states by regulating the neuronal excitability in diverse regions of the brain and spinal cord [32]. We showed that in the SCI rat model, 5-HT-positive axons and terminals significantly diminished in the injured spinal cord, and 5-HT levels were much lower than in sham rats as well. Crocetin restored the 5-HT-positive structures and returned 5-HT levels to normal levels (Fig. 5E–G). These results provide evidence to support the idea that crocetin facilitates the repair of neuronal connections after injury. As injury is followed by the formation of a glial scar, which contains CSPG [35], inhibition or blockade of the signaling of CSPG and NogoA promotes axon growth [34, 35]. Therefore, we propose that crocetin improves 5-HT fiber growth through its action on CSPG and NogoA signaling.
However, our data did not exclude the possibility that other ascending and descending pathways regulated the motor and sensorimotor functions, and we cannot conclude that repair of the descending 5-HT system to the spinal cord contributes to the improvement of motor and sensorimotor functions following crocetin administration. Further pharmacological investigations are needed to clarify these issues.
In summary, in primary cultures of hippocampal neurons, we revealed that crocetin promotes neurite growth with preference for the longest neurite (i.e. axon) in individual neurons. In the SCI rat model, administration of crocetin facilitated the recovery of motor and sensorimotor functions. This effect may not be mediated by anti-inflammatory activity of crocetin, but could be related to the repair of injury-caused damage in neuronal connections through inhibiting the CSPG and NogoA signaling pathways. Therefore, crocetin could be a potential drug to treat SCI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Conflict of interest
We declare no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0157-7) contains supplementary material, which is available to authorized users.
References
- 1.Umigai N, Murakami K, Ulit MV, Antonio LS, Shirotori M, Morikawa H, et al. The pharmacokinetic profile of crocetin in healthy adult human volunteers after a single oral administration. Phytomedicine. 2011;18:575–578. doi: 10.1016/j.phymed.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN, et al. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav. 2005;81:805–813. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Shoyama Y, Sugiura M, Saito H. Effects of Crocus sativus L. on the ethanol-induced impairment of passive avoidance performances in mice. Biol Pharm Bull. 1994;17:217–221. doi: 10.1248/bpb.17.217. [DOI] [PubMed] [Google Scholar]
- 5.Zheng YQ, Liu JX, Wang JN, Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 6.Wang CJ, Shiow SJ, Lin JK. Effects of crocetin on the hepatotoxicity and hepatic DNA binding of aflatoxin B1 in rats. Carcinogenesis. 1991;12:459–462. doi: 10.1093/carcin/12.3.459. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Sun J, Liu C, Fang C. Protective effects of crocetin pretreatment on myocardial injury in an ischemia/reperfusion rat model. Eur J Pharmacol. 2014;741:290–296. doi: 10.1016/j.ejphar.2014.07.052. [DOI] [PubMed] [Google Scholar]
- 8.Yan J, Qian Z, Sheng L, Zhao B, Yang L, Ji H, et al. Effect of crocetin on blood pressure restoration and synthesis of inflammatory mediators in heart after hemorrhagic shock in anesthetized rats. Shock. 2010;33:83–87. doi: 10.1097/SHK.0b013e3181a98f55. [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi M, Tsuruma K, Imai S, Nakanishi T, Umigai N, Shimazawa M, et al. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur J Pharmacol. 2011;650:110–119. doi: 10.1016/j.ejphar.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Yan J, Xi L, Qian Z, Wang Z, Yang L. Protective effect of crocetin on hemorrhagic shock-induced acute renal failure in rats. Shock. 2012;38:63–67. doi: 10.1097/SHK.0b013e3182596ec4. [DOI] [PubMed] [Google Scholar]
- 11.Yang R, Yang L, Shen X, Cheng W, Zhao B, Ali KH, et al. Suppression of NF-kappaB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2012;674:391–396. doi: 10.1016/j.ejphar.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Xiang M, Yang M, Zhou C, Liu J, Li W, Qian Z. Crocetin prevents AGEs-induced vascular endothelial cell apoptosis. Pharmacol Res. 2006;54:268–274. doi: 10.1016/j.phrs.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001, 26: S2–12. [DOI] [PubMed]
- 15.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Ahuja CS, Fehlings M. Concise Review: Bridging the Gap: Novel neuroregenerative and neuroprotective strategies in spinal cord injury. Stem Cells Transl Med. 2016;5:914–924. doi: 10.5966/sctm.2015-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflamm. 2016;2016:9476020. doi: 10.1155/2016/9476020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech. 2016;9:1125–1137. doi: 10.1242/dmm.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong X, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J Cell Mol Med. 2017;21:941–954. doi: 10.1111/jcmm.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Gorp S, Leerink M, Kakinohana O, Platoshyn O, Santucci C, Galik J, et al. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res Ther. 2013;4:57. doi: 10.1186/scrt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang M, Wang M, Xing T, Zeng J, Wang H, Ruan DY. Mechanisms of unmodified CdSe quantum dot-induced elevation of cytoplasmic calcium levels in primary cultures of rat hippocampal neurons. Biomaterials. 2008;29:4383–4391. doi: 10.1016/j.biomaterials.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Leung DY, Nordeen SK, Goleva E. Estrogen inhibits glucocorticoid action via protein phosphatase 5 (PP5)-mediated glucocorticoid receptor dephosphorylation. J Biol Chem. 2009;284:24542–24552. doi: 10.1074/jbc.M109.021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato A, Ohtaki H, Tsumuraya T, Song D, Ohara K, Asano M, et al. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J Neuroinflammation. 2012;9:65. doi: 10.1186/1742-2094-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 25.Abrams MB, Nilsson I, Lewandowski SA, Kjell J, Codeluppi S, Olson L, et al. Imatinib enhances functional outcome after spinal cord injury. PLoS One. 2012;7:e38760. doi: 10.1371/journal.pone.0038760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods. 2001;255:149–157. doi: 10.1016/S0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 27.Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto H, Demura T, Morita M, Banker GA, Tanii T, Nakamura S. Differential neurite outgrowth is required for axon specification by cultured hippocampal neurons. J Neurochem. 2012;123:904–910. doi: 10.1111/jnc.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snow DM, Smith JD, Cunningham AT, McFarlin J, Goshorn EC. Neurite elongation on chondroitin sulfate proteoglycans is characterized by axonal fasciculation. Exp Neurol. 2003;182:310–321. doi: 10.1016/S0014-4886(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 30.Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Yang Q, Gao L, Tao M, Chen Z, Yang X, Cao Y. Transcriptomics analysis of candida albicans treated with huanglian jiedu decoction using RNA-seq. Evid Based Complement Alternat Med. 2016;2016:3198249. doi: 10.1155/2016/3198249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aghajanian GK, Sanders-Bush E. Serotonin. Neuropsychopharmacology: The Fifth Generation of Progress 2002: 15–34.
- 33.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montani L, Gerrits B, Gehrig P, Kempf A, Dimou L, Wollscheid B, et al. Neuronal Nogo-A modulates growth cone motility via Rho-GTP/LIMK1/cofilin in the unlesioned adult nervous system. J Biol Chem. 2009;284:10793–10807. doi: 10.1074/jbc.M808297200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/S1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.