Abstract
In the past decade, numerous genes associated with autism spectrum disorders (ASDs) have been identified. These genes encode key regulators of synaptogenesis, synaptic function, and synaptic plasticity. Drosophila is a prominent model system for ASD studies to define novel genes linked to ASDs and decipher their molecular roles in synaptogenesis, synaptic function, synaptic plasticity, and neural circuit assembly and consolidation. Here, we review Drosophila studies on ASD genes that regulate synaptogenesis, synaptic function, and synaptic plasticity through modulating chromatin remodeling, transcription, protein synthesis and degradation, cytoskeleton dynamics, and synaptic scaffolding.
Keywords: Autism spectrum disorders, Drosophila, Chromatin remodeling, Synaptic scaffolding, Synaptic transmission
Introduction
Autism spectrum disorders (ASDs) are complex developmental disabilities, whose prevalence is estimated to be 1 in 68 children under 8 years of age in the USA, and they differ substantially between boys (1 in 42) and girls (1 in 189) [1]. The core diagnostic features are impaired social interaction, and repetitive and restrictive behaviors [2]. In addition, children with ASDs frequently present with a host of associated behavioral issues, such as motor deficits (hypotonia, apraxia, or motor delay), sleep abnormalities, gastrointestinal disturbances, and epilepsy [3–7]. In the past decade, numerous mutations in ASD-associated genes have been identified and various mouse models of monogenic forms of ASDs have been generated and characterized [8]. So far, a variety of standard assessments for ASD-related behavioral phenotypes have been established in mouse models [9]. Meanwhile, ASD models in other animals such as nonhuman primates and Drosophila are necessary complements in ASD studies, and are valuable in translating genetic findings and deciphering the shared molecular pathways and phenotypes in ASDs [8, 10].
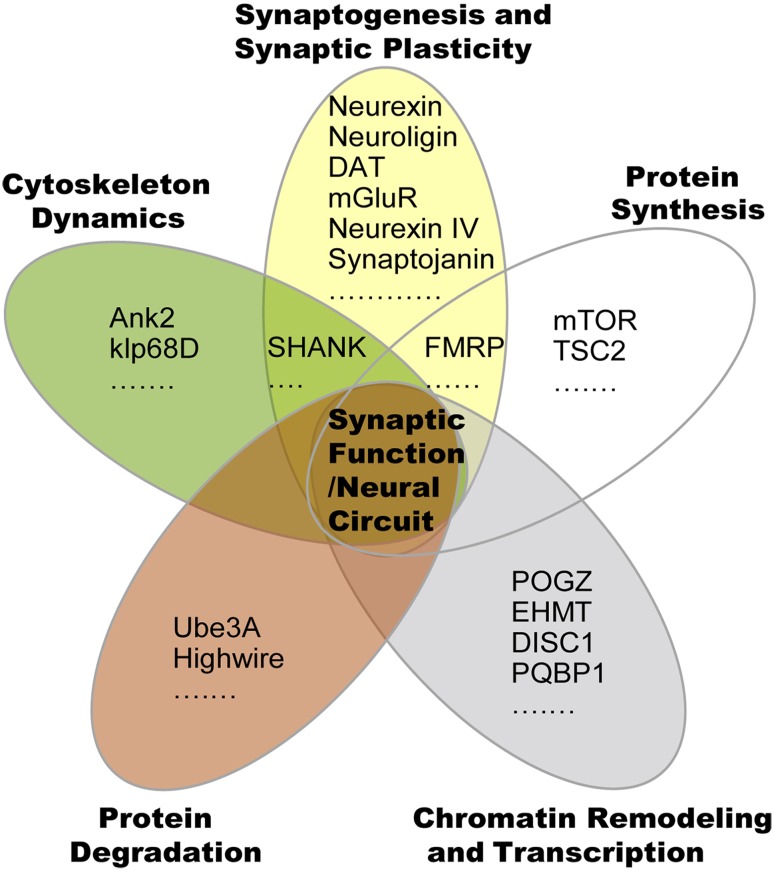
The fruit fly Drosophila melanogaster is a prominent model system in neuroscience. As a model system, it has a wide range of practical and genetic advantages, such as a short generation time (~10 days at room temperature) and a large number of offspring for rapid large-scale analysis (females can lay up to 100 eggs per day). In addition, Drosophila has some unique aspects for genetic studies, including the lack of meiotic recombination in males and the use of balancer chromosomes that carry visible genetic markers to facilitate the maintenance of mutant lines [10]. Drosophila is also useful for defining gene interaction networks and identifying novel regulatory connections. It offers efficient and high-throughput genetic manipulation, and greatly facilitates the discovery of single gene functions, neurogenetic events, and advanced behaviors [10, 11]. Despite the low anatomical conservation, the biological processes are highly conserved between Drosophila and humans at the molecular, cellular, and synaptic levels. About 75% of human disease genes have identifiable homologs in Drosophila, 44% of which are sufficiently conserved for functional study [12, 13]. Here, we review the studies in Drosophila that characterize the genetic and molecular pathology of ASDs. These studies involve many ASD-associated genes that influence the structure and the turnover of synapses at different levels, including chromatin remodeling, transcription, protein synthesis and degradation, actin cytoskeleton dynamics, and synaptic transmission (Fig. 1).
Fig. 1.
ASD-associated genes regulate synaptic function and neural circuits through various cellular events.
Chromatin Remodeling and Transcription
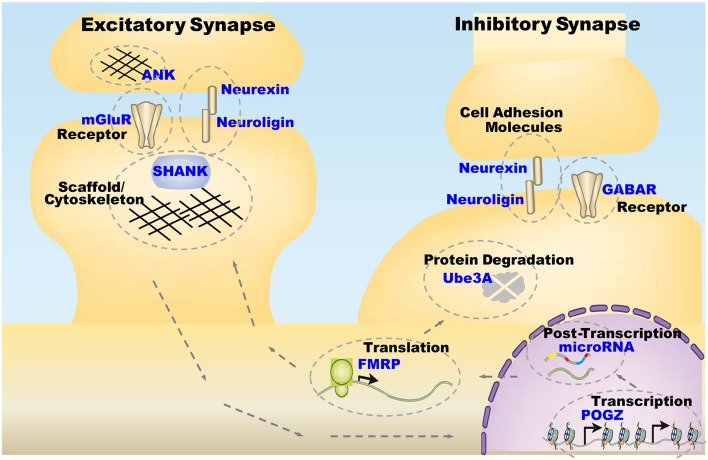
Some important regulators of chromatin remodeling and transcription are promising genetic factors for ASDs. However, how changes in these genes affect neuronal morphology and activity is unclear. Several studies in Drosophila have revealed the underlying molecular mechanisms of chromatin remodeling and transcription regulators in neural development and ASD-related behaviors (Figs. 1, 2).
Fig. 2.
Functions of ASD-associated genes in different cellular processes.
Mutations in POGZ have been reported in individuals with ASDs, intellectual disability, and schizophrenia [14–16]. POGZ encodes a heterochromatin protein 1 α-binding protein and is hypothesized to function as a transcriptional regulator in molecular networks crucial for neuronal function [17]. Downregulation of row (Drosophila ortholog of POGZ) in neurons leads to deficits in habituation, a form of non-associative learning that is relevant to both intellectual disability and ASDs [18]. Euchromatin histone methyltransferase (EHMT) is another ASD risk gene, which encodes a member of the evolutionarily-conserved protein family methylates histone 3 at lysine 9 [19–21]. In Drosophila, loss of Ehmt results in a significant decrease of dendrite end number, higher-order branching, and dendritic field complexity, as well as learning and memory deficits [22].
Disrupted-in-schizophrenia-1 (DISC1) is associated with a wide range of mental illnesses, including ASDs [23]. DISC1 interacts with and activates transcription factor 4 (ATF4)/CREB2 in the nucleus [24]. A fly model expressing human DISC1 has shown that accumulation of exogenous human DISC1 in the nucleus disturbs sleep homeostasis, implying a deficit in neuronal activity. This function is modulated by interaction with ATF4/CREB2 and recruitment of a co-repressor, N-CoR, to the CRE-mediated transcriptional machinery [25].
MicroRNA (miRNA) is another way to post-transcriptionally regulate gene expression. The autism susceptibility gene A2bp1 has been identified in Drosophila as an mRNA target of miR-980 [26]. MiR-980 inhibition enhances olfactory learning and memory stability, while its over-expression in the mushroom bodies impairs 3-h memory. Overexpression of its target A2bp1 in the mushroom bodies enhances memory. These defects may be attributed to the role of miR-980 in inhibiting excitability, as projection neurons overexpressing miR-980 exhibit a strong trend for a lower mean firing frequency with an injected current at 40–50 pA[26].
Protein Synthesis and Degradation
Neuronal activity and function are partially determined by synaptic protein levels, which are strictly regulated by protein synthesis and degradation. On the other hand, the levels of synaptic proteins are also influenced by neuronal activity [27]. Mutations of the genes involved in such homeostatic regulation have been found in ASD patients [28]. Numerous studies in Drosophila have illustrated that dysfunction of ASD-related genes affects protein synthesis and degradation, and subsequently results in deficits in synaptogenesis and synaptic function, as well as synaptic plasticity (Fig. 2).
The fragile X mental retardation 1 gene (FMR1) encodes a pan-neuronal RNA-binding protein, FMR1, that associates with specific mRNAs to repress their translation [29–33]. So far, >800 distinct mRNA targets of FMR1 have been found, and it has been implicated in many aspects of brain development and function [34]. Fragile X Syndrome (FXS), the leading monogenetic cause of autism, is caused by transcriptional silencing of the FMR1 gene due to a trinucleotide repeat expansion in its 5’-UTR [35, 36]. Since the generation of the first Fmr1-knockout mouse model to study FXS, other animal models including the fly FXS model have been further developed and studied, providing more cellular and molecular clues to explain this complex syndrome. There is only one FMR1 homolog, named dfmr1 in Drosophila, which encodes the dFmr1 protein that shares high identity with mammalian FMR1 in the functional domains [37]. The Drosophila neuromuscular junction (NMJ) is a glutamatergic synapse characterized by stereotypic innervation patterns of motor neurons into well-defined target body-wall muscles, making it easier to study synaptogenesis, synaptic transmission, and plasticity [38]. Drosophila dfmr1 loss-of-function mutants show synapse overelaboration (overgrowth, over-branching, and excess synaptic boutons) in peripheral NMJs [39] as well as in the mushroom body (MB) of the central nervous system [40], accompanied by altered neurotransmission. The hypermorph mutants of dfmr1 show opposite defects. A further rescue study indicated a pre-synaptic requirement of dFMR1 for synapse structuring, along with both a pre- and post-synaptic requirement for functional neurotransmission [41]. Furthermore, dfmr1 loss-of-function mutants exhibit more dendritic branching in dendritic arborization neurons and its role in dendrite development is partially mediated by Rac1 as well as microRNA-124a [42, 43]. In addition, deficits in axonal targeting have been extensively reported in dfmr1mutants [40, 44–47].
Loss of Fmr1 up-regulates the translation of its target mRNAs [39, 42, 48, 49]. In the fly NMJ, Adar acts downstream of Fmr1 for proper NMJ architecture [50]. The other two dFmr1 targets, the synaptic heparan sulfate proteoglycans glycosylphosphatidyl inositol-anchored Dally-like protein (Dlp) and transmembrane Syndecan (Sdc), play important roles in modulating synaptic structure and function [51]. The expression of these two proteins is markedly elevated in dfmr1-null NMJs [52]. Bone morphogenetic protein type II receptor (BMPR2) is also one of the targets of FMR1. The structural defects at the NMJ in loss-of-function dfmr1 mutants can be rescued by reducing Wit, the Drosophila ortholog of BMPR2 [53]. The role of FMR1 in mRNA translation is regulated by polyglutamine-binding protein 1 (PQBP1), whose mutations cause Renpenning syndrome [54]. Drosophila Pqbp1 interacts with Fmr1 and facilitates target mRNA assembly into ribosomes [54]. Two of the common mutations found in Renpenning syndrome, PQBP1 c.459_462delAGAG and c.463_464dupAG, encode a distinct C-terminal epitope that preferentially binds non-phosphorylated FMRP and promotes its ubiquitin-mediated degradation [55]. Therefore, PQBP1 c.463_464dupAG transgenic flies show remarkable defects of synaptic over-growth similar to dfmr1 mutants, which can be rescued by exogenously-expressed dFmr1 [55].
The mTOR pathway controls global mRNA translation and has been shown to play roles in many fundamental cellular processes including autophagy, transcription, and cytoskeletal dynamics [56]. It is a key regulator of neuronal differentiation [57, 58], and hyper-activation of this pathway increases the risk of ASDs [59]. In Drosophila, the gene unkempt (unk) has been identified as a novel negative regulator of photoreceptor differentiation acting downstream of mTORC1. Unk together with its binding partner headcase (Hdc) negatively regulates the InR/mTOR pathway and controls the timing of neurogenesis [60, 61]. Tuberous sclerosis complex (TSC) is an autosomal dominant disorder that shows clinical features of epilepsy and autism. It is caused by mutations in the TSC1 or TSC2 genes. TSC protein regulates synaptic growth via the TORC2-Akt pathway, and the mTOR pathway is upregulated by TSC2 protein[59]. In the Drosophila NMJ, Tsc2 mutants show increased synaptic growth [62].
Duplications of the genomic region encompassing UBE3A (15q11–q13) are the second most common genetic lesions found in autism (3%–5% of cases) [63]. UBE3A is a maternally-expressed gene and its product, the ubiquitin protein ligase E3A, is present in many regions of the brain, with highest expression in the hippocampus and cerebellum [64]. In Drosophila, loss of Ube3a significantly increases the number of both total and satellite boutons in conjunction with compromised endocytosis in the NMJs [65]. Ube3a specifically ubiquitinates the type I BMP receptor Tkv, and promotes its proteasomal degradation. Therefore Ube3a has a critical role in regulating NMJ development by repressing BMP signaling [65]. Over-expression of Ube3a in Drosophila results in a decreased number of active zones per bouton and vesicle area, and 40%–50% of larvae intermittently fail to evoke junction potentials at rapid stimulation rates (15 Hz) [66]. Both loss-of-function and over-expression of Ube3a decrease dendritic branching, suggesting that the proper level of Ube3a is critically important for normal dendritic patterning [67]. Moreover, improper expression of Ube3a appears to be detrimental to learning, thereby recapitulating the learning deficits in autism [68]. Over-expression of human Ube3a in Drosophila that mimics the gene duplication in autism patients has been used to screen UBE3A substrates [69]. A key regulator of monoamine synthesis, the gene Punch or GCH1 encodes the enzyme GTP cyclohydrolase I [70]. It is interesting that the mRNA and protein levels of the Drosophila vesicular monoamine transporter dVMAT are also elevated in the absence of dFmr1 [71]. The altered monoamine (dopamine/serotonin) synthesis pathway found both in dUBE3A and dFMR1 mutants may provide a potential explanation for the repetitive behaviors and hyperactivity associated with autism and also explain why some individuals with ASDs respond better to selective serotonin reuptake inhibitors than others.
Highwire is another highly-conserved E3 ligase associated with ASDs, and it functions presynaptically to negatively regulate synaptic growth at the Drosophila NMJ [72]. Mutations of wallenda, which encodes an MAP kinase kinase kinase homologous to the vertebrate dual leucine zipper-bearing kinases DLK and LZK, completely suppress the synaptic overgrowth phenotype in highwire mutants [73]. In addition, Rae1 has been identified as a Highwire cofactor to prevent the autophagy-mediated degradation of Highwire protein in post-mitotic neurons [74].
Cell Adhesion Molecules
ASDs also involve many proteins mediating neuronal connectivity and synaptic transmission, such as the synaptic adhesion molecules neurexin (NRX) and neuroligin (NLG) in synaptogenesis, various neurotransmitters and proteins associated with synaptic vesicle recycling in synaptic transmission, contactin-associated proteinlike 2 (CNTNAP2) in neuronal conduction, and Ca2+ channels in ion permeability (Fig. 1). Given that the Drosophila genome is relatively less redundant than the human genome, a single mutation in the homolog of an ASD-related gene is more likely to avoid compensatory effects and yield a measurable phenotype.
The well-known autism candidate genes NRX and NLG are classical trans-synaptic partners that play critical roles in synaptogenesis and synaptic transmission (Fig. 2). Loss of Nlgs and Nrx results in reduced bouton numbers, aberrant presynaptic and postsynaptic development at NMJs, and impaired synaptic transmission [75–79]. It has been found that Drosophila neuroligin 1 (dNlg1) and dNlg3 act predominantly in pre-synaptic terminals, while dNlg2 functions both pre- and post-synaptically [75–78]. Nrx and Nlgs also play critical roles in synaptic transmission. dNrx has been shown to functionally couple with Ca2+ channels to regulate synaptic transmission. And dNlg4 modulates GABA transmission in large ventral lateral neurons through recruiting GABAA receptors resistance to dieldrin [80]. Interestingly, both dnrx mutants and dnlg4 mutants exhibit reduced night-time sleep, even though they function in different brain regions [80, 81]. In addition, impaired dNrx and dNlgs result in neuronal plasticity defects [80, 82]. dNrx has been shown to interact with N-ethylmaleimide-sensitive factor (NSF), an enzyme that mediates disassembly of the soluble NSF attachment protein receptor (SNARE) complex, and plays an important role in synaptic vesicle release [83]. Besides, dNrx is expressed beginning from an early neurodevelopmental stage prior to synaptogenesis. dNrx plays an essential role in columnar restriction during L4 axon branching in the Drosophila visual system through clustering of one of the classical axon guidance molecules, Ephrin, which implies a novel role of dNrx in early neural development [84].
Neurexin IV (NrxIV) is an ortholog of the autism gene CNTNAP2 [85]. NrxIV homozygous-null mutants display reduced bouton numbers, while heterozygous-nulls do not observably differ from the wild-type [86]. The polygenic causes of ASDs have also been investigated in Drosophila models using orthologs to human ASD genes with different copy number variants (CNVs) [87]. Two ASD candidate genes encoding adherens junction proteins, NOTCH1 and p120ctn (orthologs of human NOTCH1 and catenin delta 2 (CTNND2)) that show gain or loss of CNVs, respectively, were tested pairwise and have shown synergistic effects on NMJ bouton number [87]. These studies provide evidence for synergistic interactions between CNV candidate gene sets, supporting shared and distinct genetic etiologies of ASDs.
Synaptic Receptors and Ion Channels
The Drosophila NMJ is an asymmetric glutamatergic synapse formed between motor neurons and muscle cells. Since it displays some advantageous features, including structural accessibility, stereotypic features, and amenability to genetic manipulations as well as electrophysiological and microscopic analyses, it is considered to be a convenient and useful model for elucidating the mechanisms underlying synapse formation, synaptic transmission and plasticity, and synaptic degeneration. Many mutants of genes encoding the components in synaptic transmission are also associated with ASDs, including synaptic receptors, components in synaptic vesicle cycling, and ion channels (Figs. 1, 2).
Mutations in the Drosophila group II metabolic glutamate receptor gene (DmGluRA) increase neuronal excitability by preventing PI3 kinase activation and consequently hyper-activating the transcription factor Foxo [88]. Loss of dFmr1 also results in excessive activity of metabotropic glutamate receptors (mGluRs) and learning and memory deficits, which are related to the inhibition of cAMP signaling reported in patients and animal models [36]. These deficits can be rescued by pharmacological inhibition of mGluRs, which provides further support for the agonistic effects of dFmr1 and mGluR signaling [89].
Several genes involved in the dopamine (DA) network are also associated with ASDs, including the plasma membrane protein syntaxin 1 (STX1) [90] and the DA transporter (DAT) [91]. A novel de novo missense mutation in the human DAT (hDAT) gene results in a Thr-to-Met substitution at site 356 (hDAT-T356M). Expression of hDAT-T356M in DA neurons with the Drosophila DAT-null allele leads to hyper-locomotion, indicating that alterations in DA homeostasis may confer risks for ASDs and related neuropsychiatric conditions [91]. Another two missense variants, SLC6A3 R/W and STX1A R/Q [17, 92, 93], disrupt the reverse transport of DA, resulting in DA dysfunction and associated locomotor behavioral abnormalities [94].
Acetylcholine is the major excitatory neurotransmitter in the central nervous system of insects [95]. The α7 subunit of the nicotinic acetylcholine receptor is one of the most prevalent receptors that are involved in neurological pathologies including autism [96]. In Drosophila, Dα7 protein is enriched at the dendrites of the giant fiber that integrates sensory input, activates flight motor neurons, and mediates synaptic transmission [97].
To date, three GABA receptor subunit classes have been cloned in Drosophila. They are Rdl (resistant to dieldrin), Grd (GABA and glycine-like receptor of Drosophila) and Lcch3 (ligand-gated Cl– channel homolog 3) [98]. A significant reduction has been found in all three subunits and glutamic acid decarboxylase in dfmr1 mutants [99, 100].
Normal synaptic vesicle recycling contributes to functional neurotransmission. Synaptojanin (Synj) is a phosphoinositide phosphatase known to play an important role in synaptic vesicle recycling [101]. Dyrk1A, also known as Minibrain (Mnb), is a serine/threonine kinase implicated in ASDs [15, 102]. The protein encoded by the Drosophila Mnb gene has been shown to interact with the INI1 ortholog Snr1, which is a chromatin-remodeling factor involved in the morphogenesis of dendritic arbors in Drosophila sensory neurons [103, 104]. Phosphorylation of Synj by Mnb kinase enhances Synj activity and is required for reliable synaptic vesicle recycling [105]. Hence, it is not surprising that Synj and Mnb mutations have been linked to autism [15, 102].
The α2δ gene family plays roles in Ca2+ channel trafficking and membrane stabilization-dependent synaptic morphogenesis [106], and is associated with a wide range of neurological diseases, including ASDs [17]. Presynaptic homeostatic potentiation is disturbed when α2δ-3 is lost, due to a failure to potentiate presynaptic Ca2+ influx and the Rab-3 interacting molecule-dependent readily-releasable vesicle pool [107].
Scaffolding Proteins and the Actin Cytoskeleton
The correct positioning of cell-adhesion molecules, receptors, and channels at the synapse requires the complex assembly of scaffolding proteins and the actin cytoskeleton. Many mutations in these genes are also found in ASD patients (Figs. 1, 2).
The SHANK family gene SHANK3 is considered to be one of the most prevalent causes of ASDs [108, 109]. Prosap/Shank family proteins have multi-domains including ankyrin repeats, SH3, PDZ, proline-rich, and SAM domains, and are the key organizers of the postsynaptic density (PSD) [110]. One possible molecular pathogenesis is an imbalance between excitatory and inhibitory receptors linked with the Nlgs-PSD-95-SHANK complex via PDZ binding. In mammals, the Shank family binds Nlgs and functions to coordinate pre/postsynaptic signaling through Neurexin-Neuroligin signaling complexes [111, 112]. There are three Shank family genes in mammals but only a single homolog of Shank in Drosophila, which makes it easier to implement in vivo null-mutant studies in Drosophila [113]. Both loss and over-expression of Shank decrease synaptic bouton number and maturity, and result in defects in the organization of the sub-synaptic reticulum, a complex system of folding the postsynaptic membrane at the NMJ. Furthermore, Shank regulates a non-canonical Wnt signaling pathway in postsynaptic cells by modulating the internalization of the Wnt receptor Fz2 [114]. These findings imply that Shank dosage is critical for synaptic development and establish a novel connection between Shank and synaptic Wnt signaling. The Neurobeachin (NBEA) gene has been shown to be disrupted in patients with idiopathic autism [115]. NBEA encodes a neuron-specific multi-domain signal scaffold protein that is predominantly expressed in the brain during development [116]. Loss of Rugose (rg), the Drosophila homolog of human NBEA, results in abnormal synaptic architecture and physiology [117].
Cytoskeleton dysregulation is one of the major problems in Fmr1 mutant neurons. The microtubule (MT) network is apparently altered in both loss- and gain-of-function dfmr1 mutants [48, 118]. The number and transport of mitochondria in axons are also affected by dFmr1 [118]. Besides, live imaging shows that dFmr1-associated mRNA granules are less motile and show decreased directional movement in cultured dfmr1 mutant neurons, demonstrating that FMR1 indeed regulates the association between mRNA cargos and microtubules [119]. Drosophila Fmr1 targets like Futsch and Profilin regulate MT- or actin-dependent synaptic growth and function [39, 49]. Drosophila Ank2 is the closest homolog of human ANK2 and ANK3. It is expressed specifically in the nervous system and associates with the presynaptic membrane cytoskeleton, similar to the human autism gene ANK3 [120]. Ank2 functions downstream of Spectrin in the anchorage of synaptic microtubules. As a consequence, synaptic stability is severely disrupted in Ank2 mutants, resulting in a reduction in overall terminal size, withdrawal of synaptic boutons, and disassembly of presynaptic active zones [120, 121]. Knock-down of Ank2 in mushroom bodies shows normal learning but a significant reduction in short-term memory, suggesting a specific role of Ank2 in cognition [122]. CNVs at 16p11.2 have recently been implicated in the pathogenesis of ASDs [123], but the genes responsible for the increased risk of ASDs are currently unknown. A Kinesin-2-encoding gene klp68D closely related to human KIF22 at the 16p11.2 locus has been identified by genetic screening using the Drosophila NMJ system. Disruption of klp68D induces ectopic targeting of motor axons [124], suggesting that Kinesin proteins are important for synaptic connectivity.
Others
Children born to older parents are at a higher risk for disorders such as schizophrenia and autism. Studies in Drosophila models have provided evidence for parental age-related memory impairment [125]. Bisphenol A (BPA), a widely-used chemical in plastic containers, has been suggested to play a role in developmental disorders including autism [126]. Flies exposed to BPA show many autistic-like behaviors, which emphasizes the importance of environment etiology for neurodevelopmental disorders such as autism.
Conclusions and Perspective
In the past decade, ASDs have undergone considerable diagnostic evolution. To discover the novel biomarkers and molecular pathology underlying these disorders, several animal models have made contributions. While fruit-flies and humans have very different body plans, Drosophila has been a prominent model system in neuroscience since the 1960s for the remarkable similarity at the biological process level, such as a similar origin of the central nervous system [127] and several similar neurobiological processes including membrane excitability, neuronal signaling, and classes of neurotransmitters [128]. Several landmark studies have made discoveries ranging from single genes and neurogenetic events to advanced behaviors. Neuroscientists have taken advantage of Drosophila for its relatively simple nervous system, a powerful genetic toolkit, high throughput, and low cost. One of the best successes in Drosophila is the Fragile X story, reviewed above. In this case, the fly model can phenocopy FXS patients and FMR1-knockout mice, while pharmacological tests have been used to develop a potential treatment. Moreover, taking advantage of high-throughput screening, several Fmr1 targets have been screened in Drosophila, which help to explain the pathogenesis of FXS.
However, with the increasing thorough research on ASDs and the use of primates in ASD studies, what can be further contributed by research in Drosophila? Growing evidence supports a causal role for combinatorial contributions of multiple loci in ASD pathogenesis. Thus, it is necessary to investigate the roles of multiple gene interactions in ASD cases. As a good in vivo model with a powerful genetic toolkit, accompanied by the development of informatics-targeted screening, Drosophila will definitely show its power in identifying multiple candidate interactions, revealing distinct molecular etiologies underlying ASDs.
Acknowledgements
We apologize to those whose works have contributed greatly to our knowledge but were not sufficiently reviewed or were not cited owing to space limitations. We thank members of the Han laboratory for proof-reading. This review was supported by the National Natural Science Foundation of China (31471031, 31400927, and 31671045), and the Natural Science Foundation of Jiangsu Province, China (BK20140623).
References
- 1.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ 2014, 63: 1–21. [PubMed]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, Viginia: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Schreck KA, Mulick JA. Parental report of sleep problems in children with autism. J Autism Dev Disord. 2000;30:127–135. doi: 10.1023/A:1005407622050. [DOI] [PubMed] [Google Scholar]
- 4.Honomichl RD, Goodlin-Jones BL, Burnham M, Gaylor E, Anders TF. Sleep patterns of children with pervasive developmental disorders. J Autism Dev Disord. 2002;32:553–561. doi: 10.1023/A:1021254914276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders: a multidimensional approach. Sleep. 2006;29:1563–1571. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- 6.Souders MC, Mason TB, Valladares O, Bucan M, Levy SE, Mandell DS, et al. Sleep behaviors and sleep quality in children with autism spectrum disorders. Sleep. 2009;32:1566–1578. doi: 10.1093/sleep/32.12.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geschwind DH. Advances in autism. Ann Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Torre-Ubieta L, Won H, Stein JL, Geschwind DH. Advancing the understanding of autism disease mechanisms through genetics. Nat Med. 2016;22:345–361. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCammon JM, Sive H. Addressing the genetics of human mental health disorders in model organisms. Ann Rev Genomics Hum Genet. 2015;16:173–197. doi: 10.1146/annurev-genom-090314-050048. [DOI] [PubMed] [Google Scholar]
- 11.Konopka RJ, Benzer S. Clock mutants of drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiter LT, Potocki L, Chien S, Gribskov M, Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 14.Gilissen C, Hehir-Kwa JY, Thung DT, van de Vorst M, van Bon BW, Willemsen MH, et al. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 15.Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2011;506:179–184. doi: 10.1038/nature12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silvia DR, Xin H, Goldberg AP, Poultney CS, Kaitlin S, Erucment Cicek A, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stessman HAF, Willemsen MH, Fenckova M, Penn O, Hoischen A, Xiong B, et al. Disruption of POGZ is associated with intellectual disability and autism spectrum disorders. Am J Hum Genet. 2016;98:541–552. doi: 10.1016/j.ajhg.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa H, Ishiguro K, Gaubatz S, Livingston DM, Nakatani Y. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science. 2002;296:1132–1136. doi: 10.1126/science.1069861. [DOI] [PubMed] [Google Scholar]
- 20.Mis J, Ner SS, Grigliatti TA. Identification of three histone methyltransferases in Drosophila: dG9a is a suppressor of PEV and is required for gene silencing. Mol Genet Genomics. 2006;275:513–526. doi: 10.1007/s00438-006-0116-x. [DOI] [PubMed] [Google Scholar]
- 21.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer JM, Kochinke K, Oortveld MA, Marks H, Kramer D, de Jong EK, et al. Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol. 2011;9:e1000569. doi: 10.1371/journal.pbio.1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilpinen H, Ylisaukko-Oja T, Hennah W, Palo OM, Varilo T, Vanhala R, et al. Association of DISC1 with autism and Asperger syndrome. Mol Psychiatr. 2008;13:187–196. doi: 10.1038/sj.mp.4002031. [DOI] [PubMed] [Google Scholar]
- 24.Morris JA, Kandpal G, Ma L, Austin CP. DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- 25.Sawamura N, Ando T, Maruyama Y, Fujimuro M, Mochizuki H, Honjo K, et al. Nuclear DISC1 regulates CRE-mediated gene transcription and sleep homeostasis in the fruit fly. Mol Psychiatr. 2008;13:1138–1148. doi: 10.1038/mp.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guven-Ozkan T, Busto GU, Schutte SS, Cervantes-Sandoval I, O’Dowd DK, Davis RL. MiR-980 is a memory suppressor microRNA that regulates the autism-susceptibility gene A2bp1. Cell Rep. 2016;14:1698–1709. doi: 10.1016/j.celrep.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 28.Iii RJK, Bear MF. The autistic neuron: troubled translation? Cell. 2008;135:401–406. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 29.Ashley CT, Jr, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- 30.Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, et al. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/S0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 31.Ascano M, Jr, Mukherjee N, Bandaru P, Miller JB, Nusbaum JD, Corcoran DL, et al. FMRP targets distinct mRNA sequence elements to regulate protein expression. Nature. 2012;492:382–386. doi: 10.1038/nature11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Ann Rev Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- 33.Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile-X gene, FMR1, has characteristics of an rna-binding protein. Cell. 1993;74:291–298. doi: 10.1016/0092-8674(93)90420-U. [DOI] [PubMed] [Google Scholar]
- 34.Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-I. [DOI] [PubMed] [Google Scholar]
- 36.Kanellopoulos AK, Semelidou O, Kotini AG, Anezaki M, Skoulakis EM. Learning and memory deficits consequent to reduction of the fragile X mental retardation protein result from metabotropic glutamate receptor-mediated inhibition of cAMP signaling in Drosophila. J Neurosci. 2012;32:13111–13124. doi: 10.1523/JNEUROSCI.1347-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 2000;20:8536–8547. doi: 10.1128/MCB.20.22.8536-8547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz TL. Transmitter release at the neuromuscular junction. Int Rev Neurobiol. 2006;75:105–144. doi: 10.1016/S0074-7742(06)75006-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/S0092-8674(01)00589-X. [DOI] [PubMed] [Google Scholar]
- 40.Pan L, Zhang YQ, Woodruff E, Broadie K. The Drosophila fragile X gene negatively regulates neuronal elaboration and synaptic differentiation. Curr Biol. 2004;14:1863–1870. doi: 10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 41.Gatto CL, Broadie K. Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure. Development. 2008;135:2637–2648. doi: 10.1242/dev.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development. 2003;130:5543–5552. doi: 10.1242/dev.00792. [DOI] [PubMed] [Google Scholar]
- 43.Xu XL, Li Y, Wang F, Gao FB. The steady-state level of the nervous-system-specific microRNA-124a is regulated by dFMR1 in Drosophila. J Neurosci. 2008;28:11883–11889. doi: 10.1523/JNEUROSCI.4114-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dockendorff TC, Su HS, McBride SM, Yang Z, Choi CH, Siwicki KK, et al. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34:973–984. doi: 10.1016/S0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 45.Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, et al. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–972. doi: 10.1016/S0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 46.Tessier CR, Broadie K. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 2008;135:1547–1557. doi: 10.1242/dev.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Michel CI, Kraft R, Restifo LL. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci. 2004;24:5798–5809. doi: 10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu R, Wang H, Liang Z, Ku L, O’Donnell WT, Li W, et al. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101:15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeve SP, Bassetto L, Genova GK, Kleyner Y, Leyssen M, Jackson FR, et al. The Drosophila fragile X mental retardation protein controls actin dynamics by directly regulating profilin in the brain. Curr Biol. 2005;15:1156–1163. doi: 10.1016/j.cub.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 50.Bhogal B, Jepson JE, Savva YA, Pepper AS, Reenan RA, Jongens TA. Modulation of dADAR-dependent RNA editing by the Drosophila fragile X mental retardation protein. Nat Neurosci. 2011;14:1517–1524. doi: 10.1038/nn.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, et al. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 52.Friedman SH, Dani N, Rushton E, Broadie K. Fragile X mental retardation protein regulates trans-synaptic signaling in Drosophila. Dis Model Mech. 2013;6:1400–1413. doi: 10.1242/dmm.012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kashima R, Roy S, Ascano M, Martinez-Cerdeno V, Ariza-Torres J, Kim S, et al. Augmented noncanonical BMP type II receptor signaling mediates the synaptic abnormality of fragile X syndrome. Sci Signal. 2016;9:ra58. doi: 10.1126/scisignal.aaf6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan D, Zhang ZC, Zhang X, Li Q, Han J. X chromosome-linked intellectual disability protein PQBP1 associates with and regulates the translation of specific mRNAs. Hum Mol Genet. 2015;24:4599–4614. doi: 10.1093/hmg/ddv191. [DOI] [PubMed] [Google Scholar]
- 55.Zhang XY, Qi J, Shen YQ, Liu X, Liu A, Zhou Z, et al. Mutations of PQBP1 in Renpenning syndrome promote ubiquitin-mediated degradation of FMRP and cause synaptic dysfunction. Hum Mol Genet 2017 (in press). [DOI] [PubMed]
- 56.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bateman JM, McNeill H. Insulin/IGF signalling in neurogenesis. Cell Mol Life Sci. 2006;63:1701–1705. doi: 10.1007/s00018-006-6036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fishwick KJ, Li RA, Halley P, Deng P, Storey KG. Initiation of neuronal differentiation requires PI3-kinase/TOR signalling in the vertebrate neural tube. Dev Biol. 2010;338:215–225. doi: 10.1016/j.ydbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Orlova KA, Crino PB. The tuberous sclerosis complex. Vienna: Springer; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avet-Rochex A, Carvajal N, Christoforou CP, Yeung K, Maierbrugger KT, Hobbs C, et al. Unkempt is negatively regulated by mTOR and uncouples neuronal differentiation from growth control. PLoS Genet. 2014;10:e1004624. doi: 10.1371/journal.pgen.1004624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bateman JM. Mechanistic insights into the role of mTOR signaling in neuronal differentiation. Neurogenesis (Austin, TX) 2015;2:e1058684. doi: 10.1080/23262133.2015.1058684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Natarajan R, Trivedi-Vyas D, Wairkar YP. Tuberous sclerosis complex regulates Drosophila neuromuscular junction growth via the TORC2/Akt pathway. Hum Mol Genet. 2013;22:2010–2023. doi: 10.1093/hmg/ddt053. [DOI] [PubMed] [Google Scholar]
- 63.Moreno-De-Luca D, Sanders SJ, Willsey AJ, Mulle JG, Lowe JK, Geschwind DH, et al. Using large clinical data sets to infer pathogenicity for rare copy number variants in autism cohorts. Mol Psychiatr. 2013;18:1090–1095. doi: 10.1038/mp.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- 65.Li W, Yao A, Zhi H, Kaur K, Zhu YC, Jia M, et al. Angelman syndrome protein Ube3a regulates synaptic growth and endocytosis by inhibiting BMP signaling in drosophila. PLoS Genet. 2016;12:e1006062. doi: 10.1371/journal.pgen.1006062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valdez C, Scroggs R, Chassen R, Reiter LT. Variation in Dube3a expression affects neurotransmission at the Drosophila neuromuscular junction. Biol Open. 2015;4:776–782. doi: 10.1242/bio.20148045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu Y, Wang F, Li Y, Ferris J, Lee JA, Gao FB. The Drosophila homologue of the Angelman syndrome ubiquitin ligase regulates the formation of terminal dendritic branches. Hum Mol Genet. 2009;18:454–462. doi: 10.1093/hmg/ddn373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chakraborty M, Paul BK, Nayak T, Das A, Jana NR, Bhutani S. The E3 ligase ube3a is required for learning in Drosophila melanogaster. Biochem Biophys Res Commun. 2015;462:71–77. doi: 10.1016/j.bbrc.2015.04.110. [DOI] [PubMed] [Google Scholar]
- 69.Reiter LT, Seagroves TN, Bowers M, Bier E. Expression of the Rho-GEF Pbl/ECT2 is regulated by the UBE3A E3 ubiquitin ligase. Hum Mol Genet. 2006;15:2825–2835. doi: 10.1093/hmg/ddl225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferdousy F, Bodeen W, Summers K, Doherty O, Wright O, Elsisi N, et al. Drosophila ube3a regulates monoamine synthesis by increasing GTP cyclohydrolase I activity via a non-ubiquitin ligase mechanism. Neurobiol Dis. 2011;41:669–677. doi: 10.1016/j.nbd.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tauber JM, Vanlandingham PA, Zhang B. Elevated levels of the vesicular monoamine transporter and a novel repetitive behavior in the Drosophila model of fragile X syndrome. PLoS ONE. 2011;6:e27100. doi: 10.1371/journal.pone.0027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wan HI, DiAntonio A, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron. 2000;26:313–329. doi: 10.1016/S0896-6273(00)81166-6. [DOI] [PubMed] [Google Scholar]
- 73.Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 74.Tian X, Li J, Valakh V, DiAntonio A, Wu C. Drosophila Rae1 controls the abundance of the ubiquitin ligase Highwire in post-mitotic neurons. Nat Neurosci. 2011;14:1267–1275. doi: 10.1038/nn.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen YC, Lin YQ, Banerjee S, Venken K, Li J, Ismat A, et al. Drosophila neuroligin 2 is required presynaptically and postsynaptically for proper synaptic differentiation and synaptic transmission. J Neurosci. 2015;32:16018–16030. doi: 10.1523/JNEUROSCI.1685-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W. Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS Lett. 2007;581:2509–2516. doi: 10.1016/j.febslet.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55:741–755. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun MK, Xing GL, Yuan LD, Gan GM, Knight D, With SI, et al. Neuroligin 2 is required for synapse development and function at the Drosophila neuromuscular junction. J Neurosci. 2011;31:687–699. doi: 10.1523/JNEUROSCI.3854-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xing G, Gan G, Chen D, Sun M, Yi J, Lv H, et al. Drosophila neuroligin3 regulates neuromuscular junction development and synaptic differentiation. J Biol Chem. 2014;289:31867–31877. doi: 10.1074/jbc.M114.574897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y, Zhou ZK, Zhang XW, Tong HW, Li PP, Zhang ZC, et al. Drosophila neuroligin 4 regulates sleep through modulating GABA transmission. J Neurosci. 2013;33:15545–15554. doi: 10.1523/JNEUROSCI.0819-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tong H, Li Q, Zhang ZC, Li Y, Han J. Neurexin regulates nighttime sleep by modulating synaptic transmission. Sci Rep. 2016;6:38246. doi: 10.1038/srep38246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Larkin A, Chen MY, Kirszenblat L, Reinhard J, Swinderen BV, Claudianos C. Neurexin-1 regulates sleep and synaptic plasticity in Drosophila melanogaster. Eur J Neurosci. 2015;42:2455–2466. doi: 10.1111/ejn.13023. [DOI] [PubMed] [Google Scholar]
- 83.Li T, Tian Y, Li Q, Chen H, Lv H, Xie W, et al. The neurexin/N-ethylmaleimide-sensitive factor (NSF) interaction regulates short term synaptic depression. J Biol Chem. 2015;290:17656–17667. doi: 10.1074/jbc.M115.644583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu L, Tian Y, Zhang XY, Zhang X, Li T, Xie W, et al. Neurexin restricts axonal branching in columns by promoting ephrin clustering. Dev Cell. 2017;41(94–106):e104. doi: 10.1016/j.devcel.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Poliak S. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 2000;24:1037–1047. doi: 10.1016/S0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 86.Sun MK, Liu LJ, Zeng XK, Xu M, Liu L, Fang M, et al. Genetic interaction between Neurexin and CAKI/CMG is important for synaptic function in Drosophila neuromuscular junction. Neurosci Res. 2009;64:362–371. doi: 10.1016/j.neures.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 87.Grice SJ, Liu JL, Webber C. Synergistic interactions between Drosophila orthologues of genes spanned by de novo human CNVs support multiple-hit models of autism. PLoS Genet. 2015;11:e1004998. doi: 10.1371/journal.pgen.1004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Howlett E, Lin CJ, Lavery W, Stern M. A PI3-Kinase–mediated negative feedback regulates neuronal excitability. PLoS Genet. 2008;4:212–220. doi: 10.1371/journal.pgen.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 90.Nakamura K, Anitha A, Yamada K, Tsujii M, Iwayama Y, Hattori E, et al. Genetic and expression analyses reveal elevated expression of syntaxin 1A (STX1A) in high functioning autism. Int J Neuropsychopharmacol. 2008;11:1073–1084. doi: 10.1017/S1461145708009036. [DOI] [PubMed] [Google Scholar]
- 91.Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, et al. De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatr. 2013;18:1315–1323. doi: 10.1038/mp.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iossifov I, O’Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Neale BM, Kou Y, Liu L, Ma’ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cartier E, Hamilton PJ, Belovich AN, Shekar A, Campbell NG, Saunders C, et al. Rare autism-associated variants implicate syntaxin 1 (STX1 R26Q) phosphorylation and the dopamine transporter (hDAT R51W) in dopamine neurotransmission and behaviors. EBioMedicine. 2015;2:135–146. doi: 10.1016/j.ebiom.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gauthier M. State of the art on insect nicotinic acetylcholine receptor function in learning and memory. Adv Exp Med Biol. 2010;683:97–115. doi: 10.1007/978-1-4419-6445-8_9. [DOI] [PubMed] [Google Scholar]
- 96.Valles AS, Barrantes FJ. Chaperoning alpha 7 neuronal nicotinic acetylcholine receptors. Biochim Biophys Acta. 2012;1818:718–729. doi: 10.1016/j.bbamem.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 97.Fayyazuddin A, Zaheer MA, Hiesinger PR, Bellen HJ. The nicotinic acetylcholine receptor Dα7 is required for an escape behavior in Drosophila. PLoS Biol. 2006;4:e63. doi: 10.1371/journal.pbio.0040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hosie AM, Aronstein K, Sattelle DB. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997;20:578–583. doi: 10.1016/S0166-2236(97)01127-2. [DOI] [PubMed] [Google Scholar]
- 99.D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, et al. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 100.Gatto CL, Pereira D, Broadie K. GABAergic circuit dysfunction in the Drosophila Fragile X syndrome model. Neurobiol Dis. 2014;65:142–159. doi: 10.1016/j.nbd.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kinstrie R, Lochhead PA, Sibbet G, Morrice N, Cleghon V. dDYRK2 and Minibrain interact with the chromatin remodelling factors SNR1 and TRX. Biochem J. 2006;398:45–54. doi: 10.1042/BJ20060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parrish JZ, Kim MD, Jan LY, Jan YN. Genome-wide analyses identify transcription factors required for proper morphogenesis of Drosophila sensory neuron dendrites. Genes Dev. 2006;20:820–835. doi: 10.1101/gad.1391006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen CK, Bregere C, Paluch J, Lu JF, Dickman DK, Chang KT. Activity-dependent facilitation of Synaptojanin and synaptic vesicle recycling by the Minibrain kinase. Nat Commun. 2014;5:4246. doi: 10.1038/ncomms5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kurshan PT, Oztan A, Schwarz TL. Presynaptic α2δ-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang TT, Jones RT, Whippen JM, Davis GW. α2δ-3 is required for rapid transsynaptic homeostatic signaling. Cell Rep. 2016;16:2875–2888. doi: 10.1016/j.celrep.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Betancur C, Buxbaum JD. SHANK3 haploinsufficiency: a “common” but underdiagnosed highly penetrant monogenic cause of autism spectrum disorders. Mol Autism. 2013;4:17–19. doi: 10.1186/2040-2392-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kolevzon A, Cai G, Soorya L, Takahashi N, Grodberg D, Kajiwara Y, et al. Analysis of a purported SHANK3 mutation in a boy with autism: clinical impact of rare variant research in neurodevelopmental disabilities. Brain Res. 2011;1380:98–105. doi: 10.1016/j.brainres.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 110.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/S0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 111.Meyer G, Varoqueaux F, Neeb A, Oschlies M, Brose N. The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology. 2004;47:724–733. doi: 10.1016/j.neuropharm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 112.Arons MH, Thynne CJ, Grabrucker AM, Li D, Schoen M, Cheyne JE, et al. Autism-associated mutations in ProSAP2/Shank3 impair synaptic transmission and neurexin-neuroligin-mediated transsynaptic signaling. J Neurosci. 2012;32:14966–14978. doi: 10.1523/JNEUROSCI.2215-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liebl FL, Featherstone DE. Identification and investigation of Drosophila postsynaptic density homologs. Bioinform Biol Insights. 2007;2:375–387. doi: 10.4137/bbi.s2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harris KP, Akbergenova Y, Cho RW, Baas-Thomas MS, Littleton JT. Shank modulates postsynaptic Wnt signaling to regulate synaptic development. J Neurosci. 2016;36:5820–5832. doi: 10.1523/JNEUROSCI.4279-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castermans D, Wilquet V, Parthoens E, Huysmans C, Steyaert J, Swinnen L, et al. The neurobeachin gene is disrupted by a translocation in a patient with idiopathic autism. J Med Genet. 2003;40:352–356. doi: 10.1136/jmg.40.5.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Savelyeva L, Sagulenko E, Schmitt JG, Schwab M. The neurobeachin gene spans the common fragile site FRA13A. Hum Genet. 2006;118:551–558. doi: 10.1007/s00439-005-0083-z. [DOI] [PubMed] [Google Scholar]
- 117.Wise A, Tenezaca L, Fernandez RW, Schatoff E, Flores J, Ueda A, et al. Drosophila mutants of the autism candidate gene neurobeachin (rugose) exhibit neuro-developmental disorders, aberrant synaptic properties, altered locomotion, and impaired adult social behavior and activity patterns. J Neurogenet. 2015;29:135–143. doi: 10.3109/01677063.2015.1064916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yao A, Jin S, Li X, Liu Z, Ma X, Tang J, et al. Drosophila FMRP regulates microtubule network formation and axonal transport of mitochondria. Hum Mol Genet. 2011;20:51–63. doi: 10.1093/hmg/ddq431. [DOI] [PubMed] [Google Scholar]
- 119.Estes PS, O’Shea M, Clasen S, Zarnescu DC. Fragile X protein controls the efficacy of mRNA transport in Drosophila neurons. Mol Cell Neurosci. 2008;39:170–179. doi: 10.1016/j.mcn.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 120.Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M, Aberle H. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008;58:210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 121.Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A novel presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and trans-synaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iqbal Z, Vandeweyer G, van der Voet M, Waryah AM, Zahoor MY, Besseling JA, et al. Homozygous and heterozygous disruptions of ANK3: at the crossroads of neurodevelopmental and psychiatric disorders. Hum Mol Genet. 2013;22:1960–1970. doi: 10.1093/hmg/ddt043. [DOI] [PubMed] [Google Scholar]
- 123.Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 124.Park SM, Littleton JT, Park HR, Lee JH. Drosophila homolog of human KIF22 at the autism-linked 16p11.2 loci influences synaptic connectivity at larval neuromuscular junctions. Exp Neurobiol 2016, 25: 33–39. [DOI] [PMC free article] [PubMed]
- 125.Burns JG, Mery F. Transgenerational memory effect of ageing in Drosophila. J Evol Biol. 2010;23:678–686. doi: 10.1111/j.1420-9101.2010.01932.x. [DOI] [PubMed] [Google Scholar]
- 126.Cock MD, Maas YGH, Bor MVD. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatr. 2012;101:811–818. doi: 10.1111/j.1651-2227.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- 127.Hirth F, Reichert H. Conserved genetic programs in insect and mammalian brain development. BioEssays. 1999;21:677–684. doi: 10.1002/(SICI)1521-1878(199908)21:8<677::AID-BIES7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 128.O’Kane CJ. Drosophila as a model organism for the study of neuropsychiatric disorders. Curr Top Behav Neurosci. 2011;7:37–60. doi: 10.1007/7854_2010_110. [DOI] [PubMed] [Google Scholar]