Abstract
Plasmodium vivax is now the predominant species causing malarial infection and disease in most non-African areas, but little is known about its transmission efficiency from human to mosquitoes. Because the majority of Plasmodium infections in endemic areas are low density and asymptomatic, it is important to evaluate how well these infections transmit. Using membrane feeding apparatus, we fed Anopheles dirus with blood samples from 94 individuals who had natural P. vivax infection with parasitemias spanning four orders of magnitude. We found that the mosquito infection rate is positively correlated with blood parasitemia and that infection begins to rise when parasitemia is >10 parasites/μl. Below this threshold, mosquito infection is rare and associated with very few oocysts. These findings provide useful information for assessing the human reservoir of transmission and for establishing diagnostic sensitivity required to identify individuals who are most infective to mosquitoes.
Keywords: Malaria, Transmission, Plasmodium vivax, Anopheles dirus, Infectivity, Membrane Feeding assay
1. Introduction
Over the past decade, malaria incidence has steadily declined in various parts of the worlds. In many places where Plasmodium falciparum and Plasmodium vivax coexist, including South America, Southeast Asia and the Western Pacific region, the latter has now become the predominant species (Oliveira-Ferreira et al., 2010; Rodriguez et al., 2011; Imwong et al., 2015; Waltmann et al., 2015). The resilience of P. vivax relative to P. falciparum against malaria controls can be attributed, at least partially, to the parasite’s ability to remain dormant as hypnozoites in the host’s liver (Krotoski et al., 1982; White et al., 2014; Robinson et al., 2015) and its greater transmission efficiency (Boyd, 1937; Pethleart et al., 2004). Plasmodium vivax thus poses a great challenge for malaria eradication. Due to the lack of an in vitro culture system that produces infectious gametocytes, information about P. vivax transmission efficiency is limited, and has mostly relied on direct or membrane feeding experiments using blood from human malaria infections (Sattabongkot et al., 1991, 2003; Gamage-Mendis et al., 1993; Zollner et al., 2006; Rios-Velasquez et al., 2013; Vallejo et al., 2016).
Several studies have reported that the majority of P. vivax infections in the endemic areas of Asia, South America, and Oceania are asymptomatic (Harris et al., 2010; Baum et al., 2015; Imwong et al., 2015; Waltmann et al., 2015; Vasquez-Jimenez et al., 2016) and submicroscopic (Cheng et al., 2015), even in areas where malaria transmission intensity has declined. Previous studies have also shown that blood from both P. vivax-infected patients and asymptomatic carriers can infect Anopheles dirus, a Southeast Asian vector (Sattabongkot et al., 1991, 2003; Coleman et al., 2004; Pethleart et al., 2004), but the relationship between P. vivax parasitemia and the mosquito infection rate is often described as weak if not absent (Graves et al., 1988; Gamage-Mendis et al., 1991; Bharti et al., 2006; Coleman et al., 2004). At present, the relative contributions to transmission of asymptomatic and symptomatic P. vivax-infected populations remain unclear. Such information is important for improving the current disease control and elimination programs. If asymptomatic carriers are contributing substantially to transmission, then malaria interventions will need to also target these carriers to be effective.
To determine how well P. vivax transmits from humans to mosquitoes and to assess the contribution of asymptomatic carriers to transmission, we performed membrane feeding experiments on An. dirus using blood samples from both P. vivax malaria patients and asymptomatic carriers. These samples covered a broad range of parasitemias, from submicroscopic to 10,000 parasites/μl.
2. Materials and methods
2.1. Study sites
The study was conducted in Tha Song Yang District of Tak Province and Sai Yok District of Kanchanaburi Province in western Thailand between 2014 and 2015. Both areas were mountainous and populations were composed mainly of Thai and Karen ethnicities. The main occupation of the study participants was farming. Malaria in the study areas was seasonal, with the major peak season lasting from May to August, and a secondary peak in November to December. The prevalence of P. vivax and P. falciparum in Tha Song Yang in 2011-2012 was approximately 10% and 3.7% by PCR (Parker et al., 2015). Four anopheline species were recently found to be infected by Plasmodium in this area, including Anophele. maculatus, Anopheles minimus, Anophel. annularis and Anopheles barbirostris (Sriwichai et al., 2016). The prevalence of P. vivax and P. falciparum in our study site in Sai Yok was 3.8% and 1.4 % by PCR in 2012 (Nguitragool et al., unpublished data). No information is currently available about the vectors in this area.
2.2. Enrollment for membrane feeding experiments
Enrollment was limited to local residents ≥ 13 years old. Participants were enrolled all year round either at local malaria clinics where P. vivax was first identified by light microscopy, or through mass blood surveys of the general village population by genus-specific loop-mediated isothermal amplification (LAMP) (Han et al., 2007) or quantitative reverse transcription (qRT)-PCR (Wampfler et al., 2013). During the enrollment process, the body temperature of each participant was recorded using an infrared thermometer. A history of recent malaria infection was obtained through interview. Symptomatic individuals were classified as those who felt sick with malaria-like symptoms (fever, headache or chill) or had a temperature > 37.5°C at the time of the blood survey. Asymptomatic carriers were Plasmodium-positive individuals whose temperature at the time of the blood survey was < 37.5°C and reported an absence of fever during the preceding 2 weeks. Informed consent or assent was obtained from each participant as well as his/her legal guardian if the participant was < 18 years old. This study was approved by the Ethics Committees of Mahidol University, Thailand and Pennsylvania State University, USA.
In total, 222 individuals were included in the final data analysis. Individuals who were evidently infected with P. falciparum were excluded. The 222 participants comprised 93 individuals who were infected with P. vivax (70 symptomatic, 23 asymptomatic), 22 individuals who were infected with Plasmodium parasites of unknown species, and 107 individuals whose blood was virtually free of Plasmodium by genus-specific LAMP and qRT-PCR (see Sections 2.5, 2.6).
2.3. Blood collection
Five milliliters of venous blood were collected from each participant in a heparinized tube. From each whole blood sample, 200 μl of blood were collected for parasite species identification by species-specific LAMP assays (Han et al., 2007). A second portion of 200 μl was mixed with 1 ml of RNAprotect Cell Reagent (Qiagen, Germany) and stored at -80°C for RNA extraction and purification. Thick and thin blood smears were prepared in duplicate using 1 μl of whole blood for each spot for microscopic examination. The rest of the sample was kept at 37°C and used in the membrane feeding experiment within 4 h of collection.
2.4. Microscopic examination of blood smears
Thick and thin smears were prepared from whole blood. The thin smear was fixed with methanol, while the thick smear was left unfixed, before being stained with 10% Giemsa solution for 10 min and examined for Plasmodium spp. and developmental stages. Parasite and gametocyte densities were determined by counting the entire 1 μl of blood spots.
2.5. LAMP assays
Genus-specific as well as species-specific (P. falciparum or P. vivax) LAMP assays (Han et al., 2007) were performed to detect Plasmodium parasite infection in blood samples from the cross-sectional surveys. To perform these LAMP assays, 150 μl of distilled water was added to 50 μl of blood and boiled for 5 min. The sample was then centrifuged for 3 min at 13,000 g. A total volume of 5.5 μl of the supernatant containing genomic DNA was used as the template in a 25 μl LAMP reaction, using the Loopamp DNA amplification kit (Eiken Chemical Co, Japan) and an appropriate primer set as previously described (Han et al., 2007; Sattabongkot et al., 2014). Only samples that were free of P. falciparum were included in the final data analysis. The limits of the LAMP assays for P. falciparum and P. vivax were 100 copies/reaction (Han et al., 2007).
2.6. qRT-PCR analyses
RNA was extracted from 100 μl of whole blood in 500 μl of RNAprotect Cell Reagent and eluted with 50 μl of elution buffer, following the instruction of the RNeasy Plus 96 kit (Qiagen). A genus-specific quantitative PCR (qPCR) assay, QMAL (Wampfler et al., 2013), using 4 μl of purified RNA as template, was performed on all RNA samples to ensure there was no contamination of genomic DNA.
For blood samples from cross-sectional surveys, 4 μl of purified RNA were subjected to genus-specific, as well as P. vivax-specific, qRT-PCR assays targeting the 18S rRNA transcripts. The primer and probe sequences for this assay have been described previously (Rosanas-Urgell et al., 2010).
To detect P. vivax gametocytes, 4 μl of purified RNA were used as the template in qRT-PCRs to amplify the pvs25 transcripts (Wampfler et al., 2013). Copy numbers were determined from an in-plate standard curve prepared by 10-fold serial dilution of a plasmid harboring the target sequence. Values are reported as copies per μl of equivalent blood volume. To ensure that oocysts in mosquitoes were not due to P. falciparum co-infection, qRT-PCRs for pfs25 transcripts were also performed on all samples (Wampfler et al., 2013). Samples positive for pfs25 transcripts were excluded from the final analysis. The detection limits of qRT-PCR for pvs25 and pfs25 were 12 copies/reaction (Nguitragool et al., unpublished data).
2.7. Mosquito rearing
A colony of An. dirus was maintained in the insectary of Mahidol Vivax Research Unit, Faculty of Tropical Medicine, Mahidol University. The colony was established in 2011 from the original colony at Armed Force Research Institute of Medical Sciences, Bangkok, Thailand. The mosquito has been genetically typed and formally identified as An. dirus (originally dirus A, Bangkok strain). The mosquitoes were reared at 27°C (± 1°C), 80% (± 10%) relative humidity, and with a 12 h day/night cycle. For membrane feeding experiments, 200 female mosquitoes/carton were placed inside an insulated plastic cooler and ground transported from Bangkok to the field sites in Tha Song Yang and Sai Yok. Only 5-7 day-old mosquitoes were used for membrane feeding assays. Mosquitoes were starved of sugar for 6 h before blood feeding. Approximately 400 starved mosquitoes were used for each blood sample.
2.8. Membrane feeding assays
Each blood sample was prepared for membrane feeding assays (Sattabongkot et al., 2015) with and without serum replacement. Serum replacement was performed to evaluate the effect of plasma components on mosquito infection. To replace the serum, the original blood was centrifuged at 500 g for 3 min, and the pellet was washed once with RPMI medium before mixing with an equal volume of human AB serum from a malaria-naïve donor. To feed 200 female mosquitoes, 1 ml of blood was added to a water-jacketed glass membrane feeder covered with a Baudruche membrane and maintained at a constant 37°C with a circulating-water system to prevent the transition of gametocytes to gametes. Mosquitoes were allowed to feed for 30 min. All unfed mosquitoes were removed and killed by freezing. Engorged mosquitoes were maintained with 10% sucrose solution until transportation back to the insectary of Mahidol Vivax Research Unit in Bangkok.
Mosquito infection with Plasmodium oocysts was determined 7-9 days after blood feeding. At least 30 mosquito midguts were dissected, stained with 0.05% mercurochrome, and examined for oocysts under a microscope. Each dissected midgut was counted once by an experienced microscopist. The distribution of oocyst counts from infective cases of P. vivax can be found in Supplementary Fig. S1.
2.9. Data analysis
Data were analyzed with R using the mgcv package (https://cran.r-project.org/web/packages/mgcv/index.html), PASW Statistics v.18 (http://www.spss.com.hk/statistics/), and SigmaPlot v.13 (https://systatsoftware.com/products/sigmaplot/). For each blood sample, the infection rate (% mosquito infected) = 100 × number of mosquitoes with at least one oocyst/total dissected. Oocyst density (oocysts/mosquito) = total number of oocysts found in all mosquitoes/total mosquitoes dissected. A paired Wilcoxon signed rank test was used to compare the median of the mosquito infection rates or of the mean oocyst densities between the feeding assays with and without AB serum replacement. Spearman’s rank correlation coefficient (ρ) was used to determine the correlation between parasite infection rates or oocyst density with parasite densities, gametocyte density, and pvs25 transcript abundance. P-values under 0.05 was considered significant. Trend lines in Fig. 2 represent 3-parameter logistic regression. Trend lines in Fig. 3 are thin plate regression splines whose smoothing parameters were estimated with the generalized cross validation criteria.
Fig. 2.
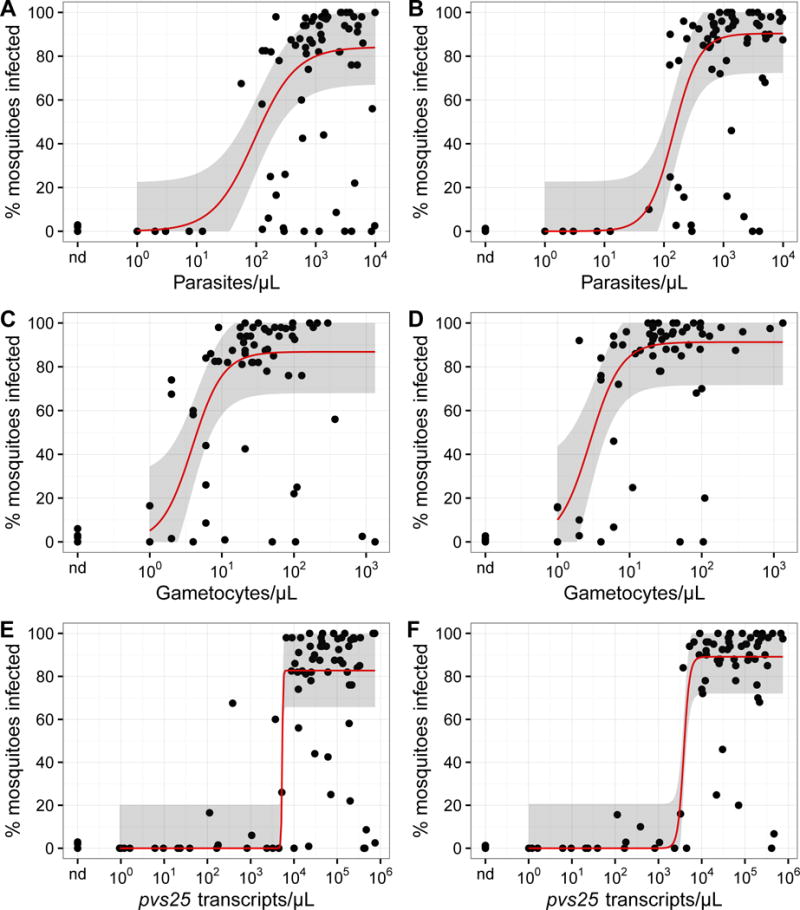
Relationship between the mosquito infection rate and Plasmodium vivax parasite, gametocyte, and pvs25 transcript densities in blood (using loop-mediated isothermal amplification). Circles represent the infection rates of individual feeding experiments. Trend lines are three parameter logistic regression; the shaded areas represent the 95% confidence intervals. (A, C, E) Experiments without human AB-serum replacement. (B, D, F) Experiments with AB malaria-naïve serum replacement. nd, not detected.
Fig. 3.
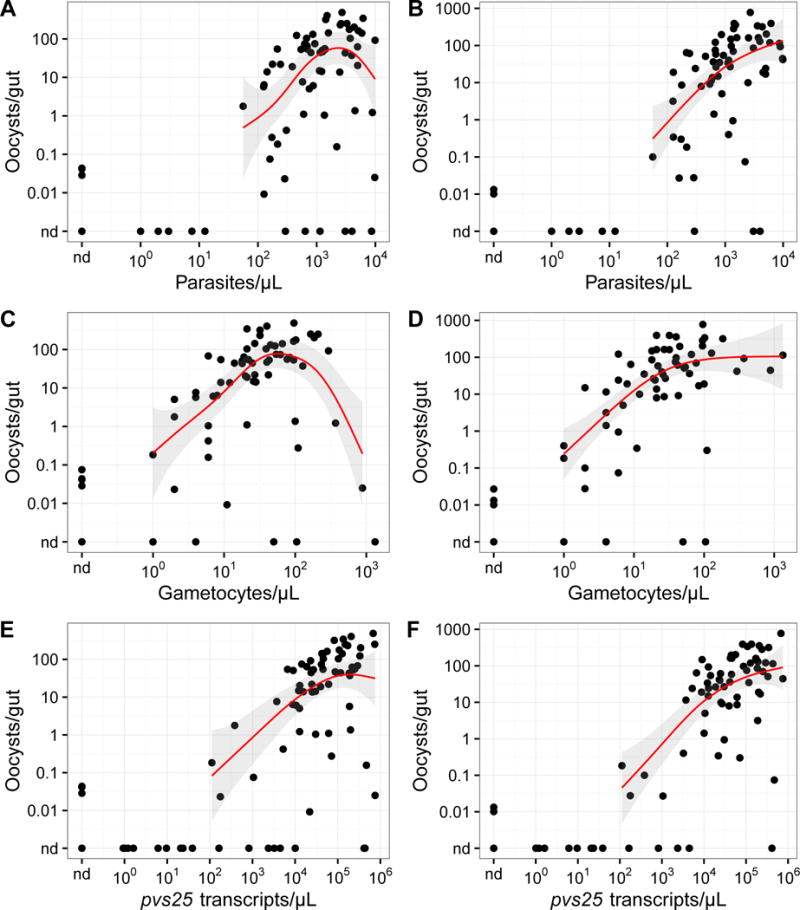
Relationship between the mean oocyst density of each membrane feeding experiment and Plasmodium vivax parasite, gametocyte, and pvs25 transcript densities in blood. Circles represent the mean oocyst densities of individual experiments. Trend lines are regression splines; the shaded areas represent the 95% confidence intervals. (A, C, E) Experiments without human AB-serum replacement. (B, D, F) Experiments with AB malaria-naïve serum replacement. nd, not detected.
2.10. Modeling the relationship between the mosquito infection rate and the oocyst density
To describe the relationship between the infection rate and the oocyst density, we assumed that the number of oocysts in each midgut ( ) arises from two types of ookinetes: i) ones that breach the peritrophic matrix (PM) and mature to oocysts ( ) and ii) the ‘free riders’ that cross the PM through an opening generated by the first type of parasites ( ). We further assume that is proportional to the ookinete density in the blood meal, and that scales linearly with the square of the ookinete density, following an empirical second order rate of collision between ookinetes and holes in the PM. Therefore, the number of oocysts in each midgut is: where is an arbitrary constant. If the relationship between the infection rate (percent) and follows Poisson statistics, that is , then the relationship between and is .
3. Results
We performed membrane feeding experiments on 222 blood samples (Table 1). The demographic information of the blood donors as well as the raw membrane feeding data are available in Supplementary Table S1. In total, 94 of the samples were P. vivax-positive by light microscopy, P. vivax-specific LAMP (Pv-LAMP), or qRT-PCR. Twenty-one samples were Plasmodium-positive by genus-specific LAMP or qRT-PCR, but the species were indeterminate due to low parasitemias. The last 107 samples were parasite-negative by all diagnostics used (Table 1). None of the 222 samples was positive for P. falciparum by Pf-LAMP or pfs25 qRT-PCR. Of the 94 P. vivax-positive samples, 70 were from symptomatic individuals and 24 were from asymptomatic individuals. Only four of the 24 P. vivax asymptomatic infections were positive by light microscopy, all having very low parasitemias in the range 1 – 8 parasites/μl; the other 20 asymptomatic infections were positive for P. vivax only by LAMP and/or qRT-PCR.
Table 1.
Summary of the mosquito membrane feeding assays
| Blood infection | % Infective cases (infective cases/total fed) |
% Mosquitoes infected (infected mosquitoes/total dissected) |
||
|---|---|---|---|---|
|
| ||||
| WB | AB | WB | AB | |
| P. vivax, symptomatica | 84 (59/70) |
88 (59/67) |
49.33 (2,165/4,389) |
55.51 (2,352/4,237) |
| P. vivax, asymptomaticb | 0 (0/24) |
0 (0/19) |
0 (0/2,678) |
0 (0/2,157) |
| Indeterminate Plasmodiumc | 0 (0/21) |
0 (0/20) |
0 (0/2,277) |
0 (0/2,182) |
| Parasite not detectedd | 4 (4/107) |
5 (5/99) |
0.07 (8/11,681) |
0.05 (6/11,334) |
Plasmodium vivax was detected by light microscopy or quantitative reverse transcription (qRT)-PCR.
Plasmodium vivax was detected by loop-mediated isothermal amplification (LAMP) or qRT-PCR.
Plasmodium parasite was detected by genus-specific LAMP or qRT-PCR, but the species of the parasite could not be identified due to low parasitemia. All samples were negative by light microscopy.
Parasite was not detected by LAMP or qRT-PCR.
WB, original whole human blood; AB, blood whose plasma had been replaced with AB-serum from a malaria-naïve donor.
Because the original blood plasma may contain components that interfere with transmission (Sattabongkot et al., 2003), we performed two feeding experiments on the majority of samples, one with the original plasma and the other after plasma had been replaced with AB blood group serum from a malaria-naïve person. Due to the limited blood volumes, we could only perform the feeding assay without serum replacement for some samples.
Without serum replacement, 84% (59/70) of symptomatic P. vivax blood samples were infective (i.e. at least one mosquito became infected with oocysts), and nearly 50% of all 4,389 mosquitoes became infected. In contrast, none of the 23 asymptomatic P. vivax samples, and none of the 22 indeterminate Plasmodium samples, led to infection in the 4,955 mosquitoes dissected. Interestingly, 4% (4/107) of whole blood samples and 5% (5/99) of serum-replaced blood samples which were negative for Plasmodium by both LAMP and qRT-PCR resulted in mosquito infection. However, the infection rate (0.07%) was vastly lower than that of symptomatic blood (49.33%) and all infected mosquitoes only had 1 – 2 oocysts.
AB serum replacement had little impact on the number of infective cases or on the proportion of mosquitoes infected (Table 1). At the level of individual blood samples, AB serum replacement had variable effects (Fig. 1). It increased the infection rate (% mosquito infected) and/or the mean oocyst density for some samples, but decreased these parameters for others. In line with our findings here, similar blocking and enhancing effects of natural immunity against sexual stages on transmission have been reported (Mendis et al., 1990). When data from all paired samples were compared, the median of the infection rates (P = 0.003, N = 86) as well as that of the mean oocyst densities (P = 0.018, N = 86) increased slightly.
Fig. 1.
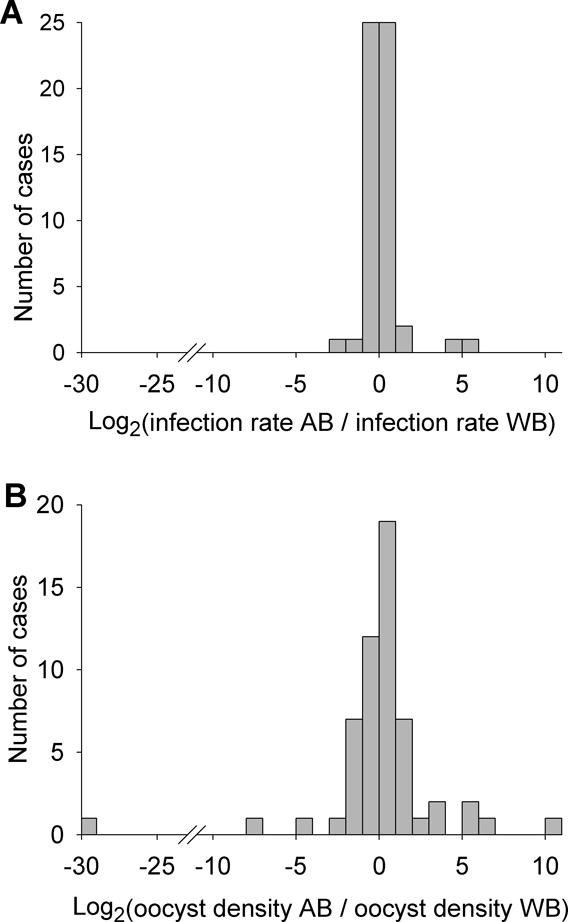
Distribution of fold changes (log2 transformed) in (A) the infection rate and (B) the mean oocyst density between experiments with AB malaria-naïve serum replacement (AB) and the original whole blood (WB). A positive value indicates enhancement by serum replacement. A negative value indicates inhibition by serum replacement. Only experiments with non-zero oocysts in both experimental arms were included.
3.1. Anopheles dirus infection as a function of P. vivax density in blood
Among the blood samples with microscopically patent parasites, the blood parasite density was positively correlated with the infection rate (ρ = 0.386, P = 0.001, N = 70; Fig. 2A). Positive correlation was also found between the infection rate and gametocyte density (ρ = 0.362, P = 0.003, N = 65; Fig. 2C) or pvs25 transcript abundance (ρ = 0.499, P = 5 × 10−6, N = 75; Fig. 2E). Despite the large sample-to-sample variation, the relationships between the infection rate and the parasite, gametocyte, and pvs25 transcript abundance are apparent when the moving median is plotted against these density measures. The relationship appears to be sigmoidal, with the infection rate beginning to rise sharply at >10 parasites/μl or >1 gametocyte/μl. The trends also became smoother after AB serum replacement (Fig. 2B, D, F).
The mean oocyst density calculated from all dissected mosquitoes (infected and uninfected) generally increased with blood parasite density (ρ = 0.432, P = 2 × 10−4, N = 70; Fig. 3A), gametocyte density (ρ = 0.424, P = 4 × 10−4, N = 65; Fig. 3C), and pvs25 transcript abundance (ρ = 0.602, P = 1 × 10−8, N = 75; Fig. 3E). However, in the highest decade of parasitemia or gametocytemia, a reduction in oocyst density was observed (Fig. 3A, C, E), perhaps reflecting inhibition by plasma components. Consistently, AB serum replacement abrogated this inhibition, leading to monotonically increasing relationships (Fig. 3B, D, F).
3.2. Relationship between the mosquito infection rate and the mean oocyst density
The infection rate was strongly correlated with the mean oocyst density (ρ = 0.825, P = 9 × 10−16, N = 59; Fig. 4). At low parasite densities, the relationship closely followed the prediction of independent formations of individual oocysts. However, at higher parasite loads, the data deviated from this Poissonian prediction – the mean oocyst density was higher than expected. These results thus suggest some form of parasite-parasite interaction that becomes more prominent at higher parasite density.
Fig. 4.
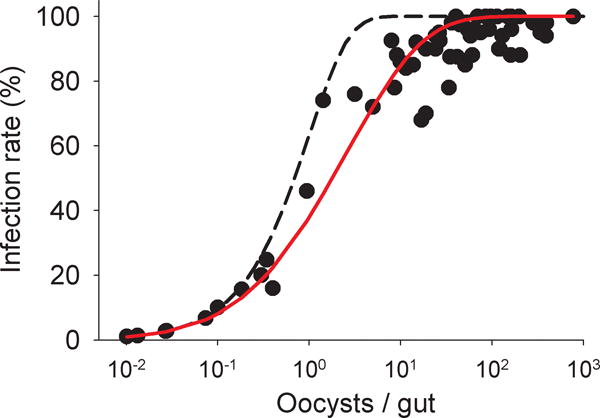
Relationship between the mean oocyst density and the infection rate of Plasmodium vivax. Circles represent values from individual feeding experiments with AB serum replacement. Dashed line, , depicts the prediction of the Poisson infection process. Solid red line is the best fit ( = 2.29) of the same infection process but allowing ‘free rider’ parasites.
4. Discussion
We used membrane feeding assays to examine mosquito infectivity of blood samples from acute P. vivax patients, asymptomatic carriers, and individuals living in the endemic areas who appeared to be free of blood-stage parasites. Although the relationship between P. vivax parasitemias and infection rates has often been described as weak or absent (Graves et al., 1988; Gamage-Mendis et al., 1991; Coleman et al., 2004; Bharti et al., 2006), we could detect significant positive correlation between P. vivax parasitemia and infection in An. dirus. We also found that parasitemia is as good a predictor of infectivity as gametocytemia, consistent with a previous report that for P. vivax parasitemia and gametocytemia are tightly linked (Koepfli et al., 2015). That gametocyte counts did not yield a stronger correlation with mosquito infectivity may also be due to our inability to differentiate fully mature gametocytes from early non-infectious stages of gametocytes. The probability that a single An. dirus would become infected with P. vivax from a symptomatic blood meal was approximately 50%, similar to the previous report of 43% when the mosquito was allowed to feed directly from the skin of symptomatic adults in Thailand (Sattabongkot et al., 1991), alleviating the concern that the membrane feeding assay may not represent natural infection. Similar infection rates (45-60%) were also measured in Anopheles aquasalis and Anopheles albitarsis in Brazillian Amazon and Anopheles albimanus in Colombia by membrane feeding using patients’ blood (Solarte et al., 2011; Rios-Velasquez et al., 2013; Vallejo et al., 2016). However, direct comparison between membrane feeding and skin feeding remains an important step in the future to fully assess how well the membrane feeding assay reflects natural infection, especially for asymptomatic carriers whose parasitemias are generally very low and whose parasite distributions in the skin may have great impacts on mosquito infections.
We did not detect any infection among the 4,835 mosquitoes that had fed on the 23 asymptomatic P. vivax blood samples. This is likely due to the very low parasitemias of these samples; 19 of those had a submicroscopic parasite density (i.e. <1 parasite/μl for this study) and four had only 1-8 parasites/μl. However, 5% of samples from qRT-PCR/LAMP-negative participants resulted in mosquito infections. These observations are consistent with the 10-100 parasites/μl threshold for robust mosquito infection (Fig. 1A). Due to the limited sample size, as well as the skewed age distribution towards adults (13-59 years), these 23 samples may not represent the true distribution of asymptomatic P. vivax parasitemias in the population. Interestingly, a recent membrane feeding study from Colombia using An. albimanus found that 57% (8/14) of submicroscopic asymptomatic blood samples led to mosquito infection at 2.5-5% infection rates (Vallejo et al., 2016). These values are much higher than those presented here. We do not know the reason for this discrepancy, but it is possible that different mosquito species differ in their susceptibility to the parasite. Several anopheline species have been found in our study area including, but not limited to, An. maculatus, An. minimus, An. annularis, An. barbirostris, and An. dirus (Sriwichai et al., 2016). However, it still remains unclear which species are the main drivers of malaria transmission. Anopheles dirus was used in this study because it has high vectorial competence and wide geographical distribution in Southeast Asia (Manguin et al., 2008). Our infectivity data should therefore be used judiciously in the context of malaria control programs where a different or multiple mosquito species contribute to transmission.
Through a series of cross-sectional malaria surveys conducted in several endemic villages in north-western Thailand and western Cambodia, Imwong and coworkers (2016) recently estimated the overall prevalence of P. vivax to be ~17% (Imwong et al., 2016). They also determined the P. vivax parasitemia distribution in these populations to be unimodal and log normal, peaking at 5 parasites/μl. Assuming this distribution, 14% of all asymptomatically infected individuals are predicted to have >100 parasites/μl, the density at which efficient An. dirus infection was observed (Fig. 2). This translates to 2.4% of the population (i.e. 14% of the 17% asymptomatic P. vivax prevalence) being able to transmit the parasite well. This number of infective asymptomatic individuals exceeds that of the symptomatic malaria patients at any given time. It thus follows that, if An. dirus is representative of Southeast Asian vectors, asymptomatic P. vivax carriers should contribute substantially to transmission. The outdoor biting behaviors of An. dirus (Tananchai et al., 2012), which favor transmission from physically active individuals, can only enhance the contribution of the asymptomatic carriers relative to that of the symptomatic patients.
Although the threshold for P. vivax transmission in An. dirus appears to be 10-100 parasites/μl, we believe that it is important for targeted elimination approaches to employ a diagnostic tool that can detect lower parasite densities. This is because i) parasitemia in asymptomatic carriers can fluctuate and reach the transmissible densities if left untreated and ii) other local mosquito species may be more susceptible to infection that An. dirus. Our data suggested that microscopy and commercial malaria rapid diagnostic tests, which typically have a limit of detection of approximately 50 and 100-200 parasites/μl, respectively (Ochola et al., 2006; World Health Organization, 2012), will likely miss a significant portion of the infectious reservoir. Molecular diagnostics such as low volume LAMP and PCR, which can detect a few parasites/μl (Cordray and Richards-Kortum, 2012), should fare much better. They should be able to identify the large majority of individuals currently transmitting the parasite. However, even people with no detectable P. vivax blood stage infection can harbor hypnozoites and may thus experience relapsing P. vivax infection and contribute to transmission (Robinson et al., 2015). Programs aimed at vivax malaria elimination will thus not only need to identify people who can currently transmit the infection, but also address the silent hypnozoite reservoir and reintroduction of the parasite through migration.
Supplementary Material
Acknowledgments
This study was supported by the TransEPI consortium funded by the Bill & Melinda Gates Foundation, USA (www.gatesfoundation.org) and National Institutes of Health, USA, International Centers of Excellence in Malaria Research grant (U19 AI089672, www.niaid.nih.gov). We greatly appreciate the contribution to microscopic examinations of blood samples by Mrs. Nongnuj Maneechai. IM is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship. WN is supported by a UK Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine.
References
- Baum E, Sattabongkot J, Sirichaisinthop J, Kiattibutr K, Davies DH, Jain A, Lo E, Lee MC, Randall AZ, Molina DM, Liang X, Cui L, Felgner PL, Yan G. Submicroscopic and asymptomatic Plasmodium falciparum and Plasmodium vivax infections are common in western Thailand - molecular and serological evidence. Malar J. 2015;14:95. doi: 10.1186/s12936-015-0611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AR, Chuquiyauri R, Brouwer KC, Stancil J, Lin J, Llanos-Cuentas A, Vinetz JM. Experimental infection of the neotropical malaria vector Anopheles darlingi by human patient-derived Plasmodium vivax in the Peruvian Amazon. Am J Trop Med Hyg. 2006;75:610–616. [PMC free article] [PubMed] [Google Scholar]
- Boyd MF, K SF. On the Infectiousness of Patients Infected with Plasmodium vivax and Plasmodium falciparum. Am J Trop Med Hyg. 1937;s1-17:253–262. [Google Scholar]
- Cheng Q, Cunningham J, Gatton ML. Systematic review of sub-microscopic P. vivax infections: prevalence and determining factors. PLoS Negl Trop Dis. 2015;9:e3413. doi: 10.1371/journal.pntd.0003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE, Kumpitak C, Ponlawat A, Maneechai N, Phunkitchar V, Rachapaew N, Zollner G, Sattabongkot J. Infectivity of asymptomatic Plasmodium-infected human populations to Anopheles dirus mosquitoes in western Thailand. J Med Entomol. 2004;41:201–208. doi: 10.1603/0022-2585-41.2.201. [DOI] [PubMed] [Google Scholar]
- Cordray MS, Richards-Kortum RR. Emerging nucleic acid-based tests for point-of-care detection of malaria. Am J Trop Med Hyg. 2012;87:223–230. doi: 10.4269/ajtmh.2012.11-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage-Mendis AC, Rajakaruna J, Carter R, Mendis KN. Infectious reservoir of Plasmodium vivax and Plasmodium falciparum malaria in an endemic region of Sri Lanka. Am J Trop Med Hyg. 1991;45:479–487. doi: 10.4269/ajtmh.1991.45.479. [DOI] [PubMed] [Google Scholar]
- Gamage-Mendis AC, Rajakaruna J, Weerasinghe S, Mendis C, Carter R, Mendis KN. Infectivity of Plasmodium vivax and P. falciparum to Anopheles tessellatus; relationship between oocyst and sporozoite development. Trans R Soc Trop Med Hyg. 1993;87:3–6. doi: 10.1016/0035-9203(93)90396-8. [DOI] [PubMed] [Google Scholar]
- Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, Brabin BJ, Gibson FD, Bradley DJ, Alpers MP. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology. 1988;96(Pt 2):251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- Han ET, Watanabe R, Sattabongkot J, Khuntirat B, Sirichaisinthop J, Iriko H, Jin L, Takeo S, Tsuboi T. Detection of four Plasmodium species by genus- and species-specific loop-mediated isothermal amplification for clinical diagnosis. J Clin Microbiol. 2007;45:2521–2528. doi: 10.1128/JCM.02117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris I, Sharrock WW, Bain LM, Gray KA, Bobogare A, Boaz L, Lilley K, Krause D, Vallely A, Johnson ML, Gatton ML, Shanks GD, Cheng Q. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J. 2010;9:254. doi: 10.1186/1475-2875-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong M, Nguyen TN, Tripura R, Peto TJ, Lee SJ, Lwin KM, Suangkanarat P, Jeeyapant A, Vihokhern B, Wongsaen K, Van Hue D, Dong le T, Nguyen TU, Lubell Y, von Seidlein L, Dhorda M, Promnarate C, Snounou G, Malleret B, Renia L, Keereecharoen L, Singhasivanon P, Sirithiranont P, Chalk J, Nguon C, Hien TT, Day N, White NJ, Dondorp A, Nosten F. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand-Myanmar border areas, Cambodia, and Vietnam. Malar J. 2015;14:381. doi: 10.1186/s12936-015-0906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imwong M, Stepniewska K, Tripura R, Peto TJ, Lwin KM, Vihokhern B, Wongsaen K, von Seidlein L, Dhorda M, Snounou G, Keereecharoen L, Singhasivanon P, Sirithiranont P, Chalk J, Nguon C, Day NP, Nosten F, Dondorp A, White NJ. Numerical Distributions of Parasite Densities During Asymptomatic Malaria. J Infect Dis. 2016;213:1322–1329. doi: 10.1093/infdis/jiv596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepfli C, Robinson LJ, Rarau P, Salib M, Sambale N, Wampfler R, Betuela I, Nuitragool W, Barry AE, Siba P, Felger I, Mueller I. Blood-Stage Parasitaemia and Age Determine Plasmodium falciparum and P. vivax Gametocytaemia in Papua New Guinea. PLoS One. 2015;10:e0126747. doi: 10.1371/journal.pone.0126747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotoski WA, Collins WE, Bray RS, Garnham PC, Cogswell FB, Gwadz RW, Killick-Kendrick R, Wolf R, Sinden R, Koontz LC, Stanfill PS. Demonstration of hypnozoites in sporozoite-transmitted Plasmodium vivax infection. Am J Trop Med Hyg. 1982;31:1291–1293. doi: 10.4269/ajtmh.1982.31.1291. [DOI] [PubMed] [Google Scholar]
- Manguin S, Garros C, Dusfour I, Harbach RE, Coosemans M. Bionomics, taxonomy, and distribution of the major malaria vector taxa of Anopheles subgenus Cellia in Southeast Asia: an updated review. Infect Genet Evol. 2008;8:489–503. doi: 10.1016/j.meegid.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Mendis KN, David PH, Carter R. Human immune responses against sexual stages of malaria parasites: considerations for malaria vaccines. Int J Parasitol. 1990;20:497–502. doi: 10.1016/0020-7519(90)90197-u. [DOI] [PubMed] [Google Scholar]
- Ochola LB, Vounatsou P, Smith T, Mabaso ML, Newton CR. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect Dis. 2006;6:582–588. doi: 10.1016/S1473-3099(06)70579-5. [DOI] [PubMed] [Google Scholar]
- Oliveira-Ferreira J, Lacerda MV, Brasil P, Ladislau JL, Tauil PL, Daniel-Ribeiro CT. Malaria in Brazil: an overview. Malar J. 2010;9:115. doi: 10.1186/1475-2875-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DM, Matthews SA, Yan G, Zhou G, Lee MC, Sirichaisinthop J, Kiattibutr K, Fan Q, Li P, Sattabongkot J, Cui L. Microgeography and molecular epidemiology of malaria at the Thailand-Myanmar border in the malaria pre-elimination phase. Malar J. 2015;14:198. doi: 10.1186/s12936-015-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pethleart A, Prajakwong S, Suwonkerd W, Corthong B, Webber R, Curtis C. Infectious reservoir of Plasmodium infection in Mae Hong Son Province, north-west Thailand. Malar J. 2004;3:34. doi: 10.1186/1475-2875-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Velasquez CM, Martins-Campos KM, Simoes RC, Izzo T, dos Santos EV, Pessoa FA, Lima JB, Monteiro WM, Secundino NF, Lacerda MV, Tadei WP, Pimenta PF. Experimental Plasmodium vivax infection of key Anopheles species from the Brazilian Amazon. Malar J. 2013;12:460. doi: 10.1186/1475-2875-12-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, Hofmann NE, Kinboro B, Waltmann A, Brewster J, Lorry L, Tarongka N, Samol L, Silkey M, Bassat Q, Siba PM, Schofield L, Felger I, Mueller I. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12:e1001891. doi: 10.1371/journal.pmed.1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JC, Uribe GA, Araujo RM, Narvaez PC, Valencia SH. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):114–122. doi: 10.1590/s0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanas-Urgell A, Mueller D, Betuela I, Barnadas C, Iga J, Zimmerman PA, del Portillo HA, Siba P, Mueller I, Felger I. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar J. 2010;9:361. doi: 10.1186/1475-2875-9-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattabongkot J, Kumpitak C, Kiattibutr K. Membrane Feeding Assay to Determine the Infectiousness of Plasmodium vivax Gametocytes. Methods Mol Biol. 2015;1325:93–99. doi: 10.1007/978-1-4939-2815-6_8. [DOI] [PubMed] [Google Scholar]
- Sattabongkot J, Maneechai N, Phunkitchar V, Eikarat N, Khuntirat B, Sirichaisinthop J, Burge R, Coleman RE. Comparison of artificial membrane feeding with direct skin feeding to estimate the infectiousness of Plasmodium vivax gametocyte carriers to mosquitoes. Am J Trop Med Hyg. 2003;69:529–535. [PubMed] [Google Scholar]
- Sattabongkot J, Maneechai N, Rosenberg R. Plasmodium vivax: gametocyte infectivity of naturally infected Thai adults. Parasitology. 1991;102(Pt 1):27–31. doi: 10.1017/s0031182000060303. [DOI] [PubMed] [Google Scholar]
- Sattabongkot J, Tsuboi T, Han ET, Bantuchai S, Buates S. Loop-mediated isothermal amplification assay for rapid diagnosis of malaria infections in an area of endemicity in Thailand. J Clin Microbiol. 2014;52:1471–1477. doi: 10.1128/JCM.03313-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solarte Y, Manzano MR, Rocha L, Hurtado H, James MA, Arevalo-Herrera M, Herrera S. Plasmodium vivax sporozoite production in Anopheles albimanus mosquitoes for vaccine clinical trials. Am J Trop Med Hyg. 2011;84:28–34. doi: 10.4269/ajtmh.2011.09-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwichai P, Samung Y, Sumruayphol S, Kiattibutr K, Kumpitak C, Payakkapol A, Kaewkungwal J, Yan G, Cui L, Sattabongkot J. Natural human Plasmodium infections in major Anopheles mosquitoes in western Thailand. Parasit Vectors. 2016;9:17. doi: 10.1186/s13071-016-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tananchai C, Tisgratog R, Juntarajumnong W, Grieco JP, Manguin S, Prabaripai A, Chareonviriyaphap T. Species diversity and biting activity of Anopheles dirus and Anopheles baimaii (Diptera: Culicidae) in a malaria prone area of western Thailand. Parasit Vectors. 2012;5:211. doi: 10.1186/1756-3305-5-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AF, Garcia J, Amado-Garavito AB, Arevalo-Herrera M, Herrera S. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J. 2016;15:48. doi: 10.1186/s12936-016-1104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez-Jimenez JM, Arevalo-Herrera M, Henao-Giraldo J, Molina-Gomez K, Arce-Plata M, Vallejo AF, Herrera S. Consistent prevalence of asymptomatic infections in malaria endemic populations in Colombia over time. Malar J. 2016;15:70. doi: 10.1186/s12936-016-1124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltmann A, Darcy AW, Harris I, Koepfli C, Lodo J, Vahi V, Piziki D, Shanks GD, Barry AE, Whittaker M, Kazura JW, Mueller I. High Rates of Asymptomatic, Sub-microscopic Plasmodium vivax Infection and Disappearing Plasmodium falciparum Malaria in an Area of Low Transmission in Solomon Islands. PLoS Negl Trop Dis. 2015;9:e0003758. doi: 10.1371/journal.pntd.0003758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, Beck HP, Mueller I, Felger I. Strategies for detection of Plasmodium species gametocytes. PLoS One. 2013;8:e76316. doi: 10.1371/journal.pone.0076316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MT, Karl S, Battle KE, Hay SI, Mueller I, Ghani AC. Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. Elife. 2014;3 doi: 10.7554/eLife.04692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Malaria Rapid Diagnostic Test Performance. Results of WHO Product Testing of Malaria RDTs: Round. 2012;4 [Google Scholar]
- Zollner GE, Ponsa N, Garman GW, Poudel S, Bell JA, Sattabongkot J, Coleman RE, Vaughan JA. Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes. Malar J. 2006;5:68. doi: 10.1186/1475-2875-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


